INTRODUCTION:
Although current literature has addressed gastrointestinal presentations including nausea, vomiting, diarrhea, abnormal liver chemistries, and hyperlipasemia as possible coronavirus disease 2019 (COVID-19) manifestations, the risk and type of gastrointestinal bleeding (GIB) in this population is not well characterized.
METHODS:
This is a matched case-control (1:2) study with 41 cases of GIB (31 upper and 10 lower) in patients with COVID-19 and 82 matched controls of patients with COVID-19 without GIB. The primary objective was to characterize bleeding etiologies, and our secondary aim was to discuss outcomes and therapeutic approaches.
RESULTS:
There was no difference in the presenting symptoms of the cases and controls, and no difference in severity of COVID-19 manifestations (P > 0.05) was observed. Ten (32%) patients with upper GIB underwent esophagogastroduodenoscopy and 5 (50%) patients with lower GIBs underwent flexible sigmoidoscopy or colonoscopy. The most common upper and lower GIB etiologies were gastric or duodenal ulcers (80%) and rectal ulcers related to rectal tubes (60%), respectively. Four of the esophagogastroduodenoscopies resulted in therapeutic interventions, and the 3 patients with rectal ulcers were referred to colorectal surgery for rectal packing. Successful hemostasis was achieved in all 7 cases that required interventions. Transfusion requirements between patients who underwent endoscopic therapy and those who were conservatively managed were not significantly different. Anticoagulation and rectal tube usage trended toward being a risk factor for GIB, although it did not reach statistical significance.
DISCUSSION:
In COVID-19 patients with GIB, compared with matched controls of COVID-19 patients without GIB, there seemed to be no difference in initial presenting symptoms. Of those with upper and lower GIB, the most common etiology was peptic ulcer disease and rectal ulcers from rectal tubes, respectively. Conservative management seems to be a reasonable initial approach in managing these complex cases, but larger studies are needed to guide management.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2, a highly transmissible respiratory virus, has become a worldwide pandemic with over 2,649,000 confirmed cases globally (1). As of March 2, 2020, there are over 1,092,815 cases in the United States leading to more than 13,114 hospitalizations (1). Although respiratory symptoms are the most common manifestation, there have been rapidly emerging data implicating the gastrointestinal (GI) tract as a key site for extrapulmonary involvement (2–4). GI manifestations include nausea, vomiting, diarrhea, abdominal pain, and GI hemorrhage (2). Gastrointestinal bleeding (GIB) has been described in 2%–13% of patients hospitalized with coronavirus disease 2019 (COVID-19) (2–4). As the clinical course of the disease evolves, with many patients having protracted hospital stays, an increase in GIB has created new challenges for the endoscopist.
In acute GIB, endoscopy remains the first-line therapy after adequate resuscitation. Current guidelines suggest that endoscopy should be performed within 24 hours for patients with acute upper GIB and within 24 hours after adequate bowel preparation in patients with acute lower GIB (5,6). In patients with COVID-19, the risk-benefit analysis for luminal evaluation is made more complex by concerns for provider safety, a need to preserve personal protective equipment (PPE), and increasing prevalence of critical illness such as respiratory compromise. There are currently limited data on the diagnostic and therapeutic benefits of endoscopy in this cohort, leaving endoscopists with inadequate information to guide the decision of when the risk of endoscopy may outweigh the benefits. In a case series by Cavaliere et al.(7), 6 patients with COVID-19–related pneumonia and upper GIB were treated conservatively with blood transfusions and proton pump inhibitors (PPIs) alone. Although cessation of bleeding was achieved in all 6 patients without the need for endoscopic management, the exact cause of GIB was not determined, the follow-up duration was not specified, and patients with lower GI bleeding were not included. Another study reported findings of esophageal erosions and ulcerations in a patient intubated with COVID-19 and concomitant upper GIB, although no endoscopic intervention was reported (2).
Whether GIB in these patients is primarily because of direct COVID-19 disease, indirectly from treatment-related effects, or a combination of both is yet to be clarified. In this study, our primary aim was to characterize bleeding etiologies in COVID-19 positive patients with upper and lower GIBs. Our secondary aim was to discuss outcomes and potential preventative and therapeutic management approaches.
MATERIALS AND METHODS
This was a retrospective, multicenter, matched 1:2 case-control study that reviewed all confirmed COVID-19 cases with GIB admitted to an academic tertiary care center and a smaller nonteaching hospital in the United States between March 4, 2020, and April 23, 2020. Patients had a documented clinical diagnosis and laboratory confirmation using real-time reverse transcription polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). GIB was defined as evidence of hematemesis, coffee-ground emesis, melena, maroon stools, or hematochezia. The study was approved by our institutional review board.
A total of 987 patients were admitted with COVID-19 diagnosis, all of whose inpatient charts from March 4 to April 23, 2020, were reviewed in our electronic medical record for signs and symptoms of GIB on admission or throughout their hospital course. To confirm that these patients with GIB were clinically significant, all were noted to have either a 2 g/dL decline in hemoglobin (Hgb) from peak to nadir values, heart rate >100 beats per minute, systolic blood pressure <100 mm Hg, or a dedicated plan to address the suspected GI bleed. In addition, the GI consultation service page logs from March 4 to April 23, 2020, were screened for COVID-19 patients consulted for GIB, and our endoscopy database (ProVation) was used to identify all patients from March 4 to April 23, 2020, who underwent endoscopy during that time period. After cross-referencing all 3 sources, 41 confirmed cases of COVID-19 with GIB were identified.
Bleeding was characterized according to its clinical presentation, anatomical location, and etiology. Endoscopic findings and therapeutic outcomes were described for both upper and lower GIB. Cases and controls were compared regarding their past medical history, laboratory findings, clinical course, and in-hospital mortality.
Matching
The cases comprised hospitalized COVID-19 positive patients with GIB. Hospitalized COVID-19 positive patients who did not have GIB were randomly chosen from March 4 to April 23 and were matched to cases for decades of age, sex, and presence of coronary artery disease in a 1:2 case-to-control ratio. Bleeding etiology, endoscopic intervention, and therapeutic outcomes were described.
STATISTICAL ANALYSIS
Descriptive statistics were reported as means (SD) or counts and proportions. Variables were compared using χ2 and Fisher exact tests and logistic regressions. Univariable and multivariable conditional logistic regressions were used to compare cases and controls. All analyses were based on nonmissing data, and missing data were not imputed. All tests were 2-tailed with a significance level of alpha = 0.05. All analyses were performed with Stata 13.0 for Windows, StataCorp LP (College Station, TX).
RESULTS
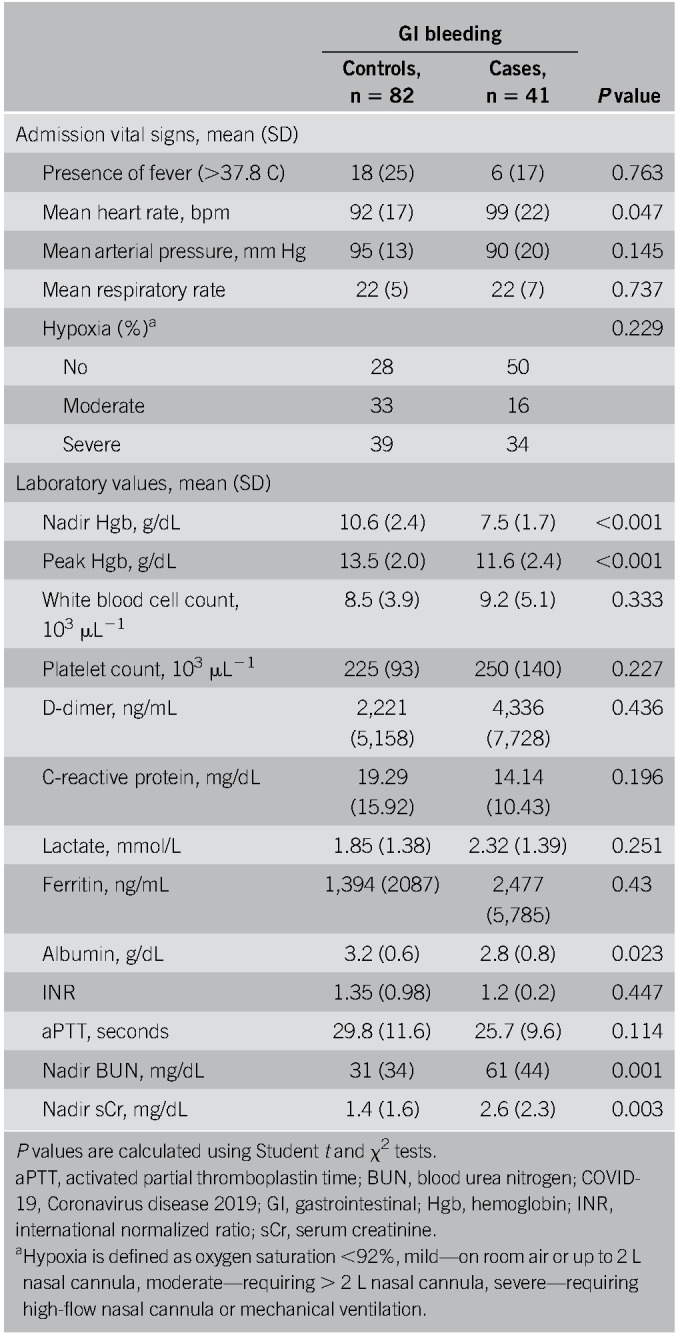
A total of 41 COVID-19 positive inpatients with GIB were evaluated (27 male patients, mean age ± SD, 68.7 ± 15.1 years) and 82 COVID-19 positive inpatient controls without GIB (54 male patients, mean age ± SD, 67.6 ± 14.3 years) were used for comparison, and baseline characteristics of both groups are shown in Table 1. COVID-19 patients with a history of GIB had a higher incidence of bleeding events (27% vs 5%, P = 0.004) (Table 1). The average nadir Hgb was statistically lower, and the average blood urea nitrogen and serum creatinine were statistically higher in the GIB group (7.5 ± 1.7, 61 ± 44, 2.6 ± 2.3, respectively) compared with the matched controls (10.6 ± 2.4, 31 ± 34, 2.6 ± 2.3, respectively) (Table 2). Other than a lower albumin, values of platelets, international normalized ratio, C-reactive protein, and D-dimer were not statistically different between both groups (Table 2). A higher percentage of GIB patients required blood transfusions (73% vs 14%, P < 0.001). The overall rate of in-hospital mortality was not statistically different between GIB cases and controls (20% vs 16%, P = 0.611). Among the 41 COVID-19 positive patients with GIB, 39% were on therapeutic anticoagulation, 15% were on aspirin (ASA), and 17% were on nonsteroidal anti-inflammatory drugs (NSAIDs), all of which were not significantly different than the controls (Table 1). The average heart rate was 99 beats/min (± 22 beats/min), and the average mean arterial pressure was 90 mm Hg (± 20 mm Hg) (Table 2). Sixty-three percent of patients (n = 26) developed GIB after at least 24 hours of admission, whereas 37% (n = 15) presented with bleeding within the first 24 hours of admission. Compared with those who bled after 24 hours of hospitalization, patients presenting with GI hemorrhage were more likely to have a history of GIB (53% vs 12%, P = 0.007, respectively) and had a lower mean peak Hgb (10.3 g/dL vs 12.3 g/dL respectively, P = 0.01). Forty-two percent of patients who bled while hospitalized had received anticoagulation compared with only 7% in those who presented to the hospital with a GIB (P < 0.001). Patient demographics, comorbidities, in-hospital death, and medication use such as antiplatelets, NSAIDs, steroids, and PPI use were not statistically different between groups.
Table 1.
Demographic and clinical findings of patients with COVID-19 and GI bleeding and matched controls
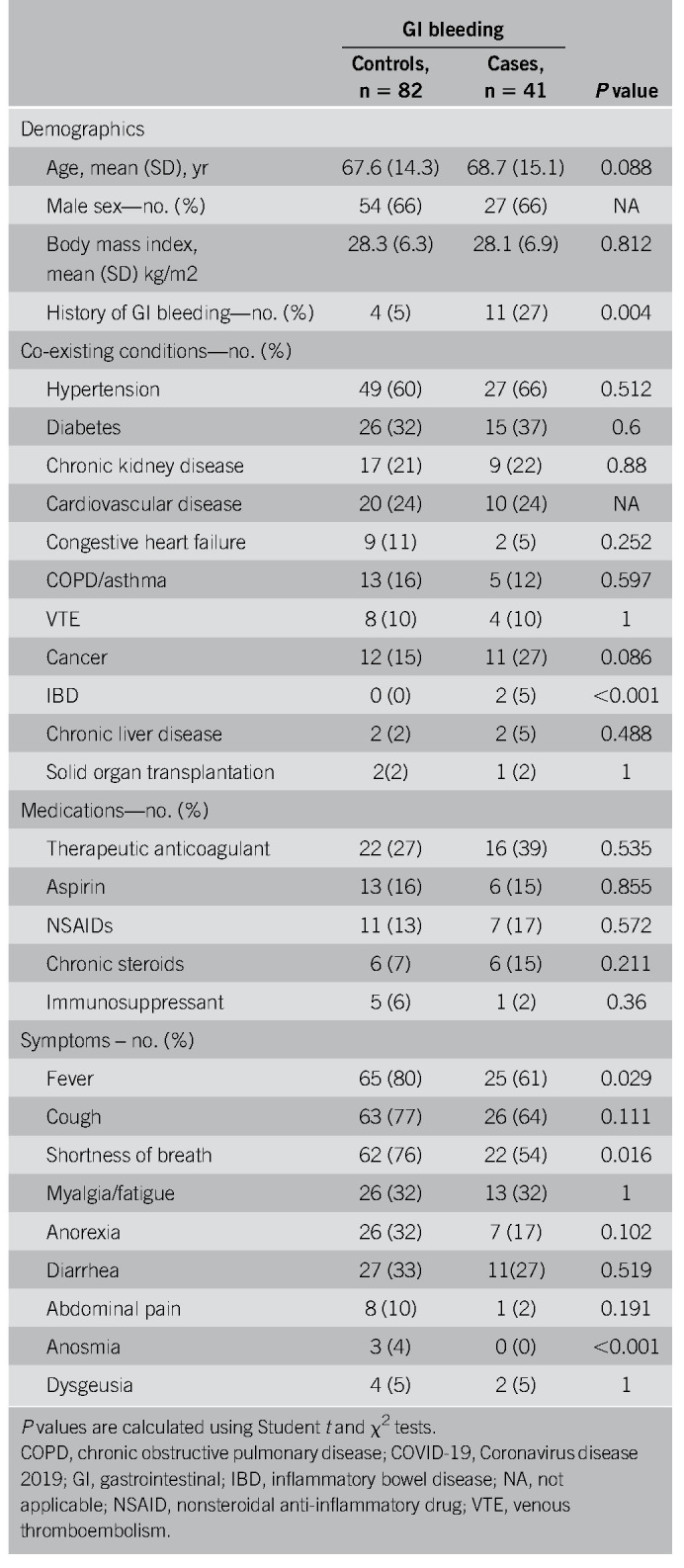
Table 2.
Vital signs and laboratory findings of patients with COVID-19 and GI bleeding and matched controls
Upper GIB
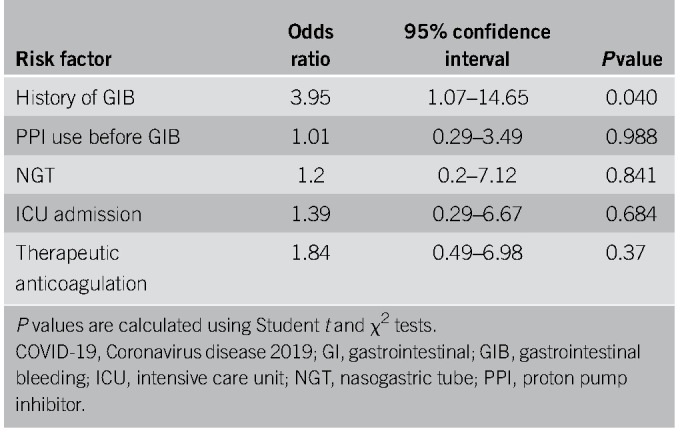
Thirty-one patients had symptoms of upper GIB described as hematemesis, coffee-ground emesis, melena, or maroon stools (Table 3). Melena was the most common bleeding manifestation, accounting for 68% of upper GIB. Eight patients (26%) were on PPIs before bleeding, 4 (13%) were on an H2 blocker, 13 (42%) were on ASA, 12 (39%) were on therapeutic inpatient anticoagulation before onset of bleeding (Table 4), and 3 (10%) had previous NSAID use. All 31 patients received PPI therapy at the onset of bleeding (Table 4). Upper endoscopies were performed in 10 patients (32%) with an average delay of 2.4 days (± 2) (Tables 3 and 5). Endoscopy was generally performed in patients with melena or hematemesis seen on examination with ongoing hemodynamic instability or severe anemia not responsive to packed red blood cells (pRBCs) transfusions. The decision for luminal evaluation was based on individual gastroenterologists' clinical judgment and required approval from our chief of endoscopy. Of the patients who received endoscopy, the most common bleeding etiology was gastric or duodenal ulcers (80%) despite 2 of these cases being on a PPI before bleeding onset. Forty percent (4/10) of esophagogastroduodenoscopies performed resulted in single or combined endoscopic therapy with epinephrine injection, endoclips, and cautery. Two of those patients had Forrest class 1b ulcers and 2 had Forrest class IIa ulcers. Hemostasis was achieved in all patients with no immediate postprocedural complications. In this cohort, the average nadir Hgb was 7.6 g/dL (± 1.88). Twenty-two of 31 patients received packed pRBCs, and the transfusion requirement between those who underwent endoscopic interventions and those who were conservatively managed was not statistically significant (3.75 ± 3.40 vs 3.33 ± 2.19 units, P = 0.777). Four patients had recurrence of bleeding during this hospitalization, and there were 8 in-hospital deaths (26%), although none were associated with GIB (Table 5). There were no interventional radiology or surgical procedures performed in this cohort. A multivariable analysis was performed to evaluate potential predictors for upper GI hemorrhage, and a history of GIB was the only statistically significant risk factor (odds ratio [OR] 3.95, P = 0.040) (Table 6). Anticoagulation had an OR of 1.84 but was not statistically significant (P = 0.37) (Table 6).
Table 3.
Characteristics of COVID-19 patients with upper and lower GI bleeding and its management
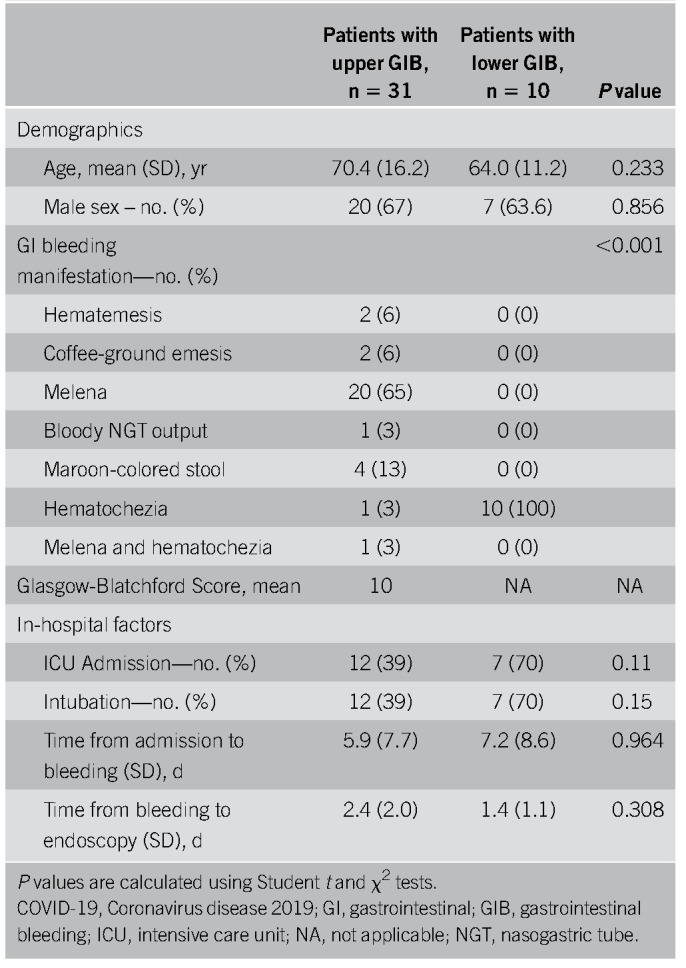
Table 4.
Potential factors affecting GI bleeding in COVID-19 patients with upper and lower GI bleeding
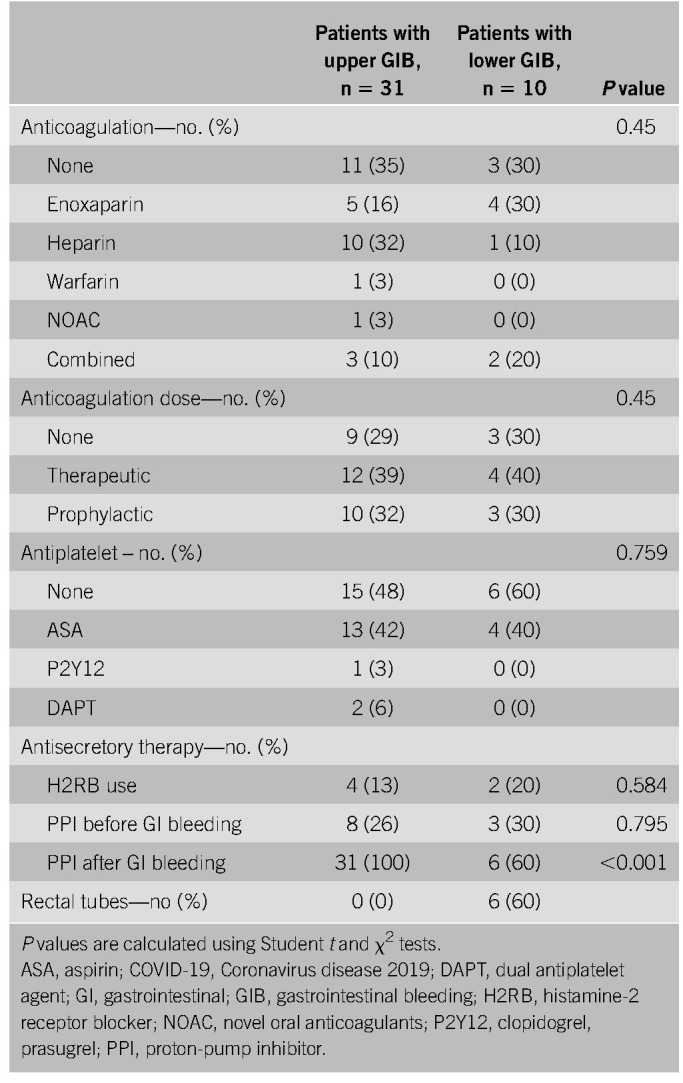
Table 5.
Endoscopic interventions and outcomes of COVID-19 patients with upper and lower GI bleeding
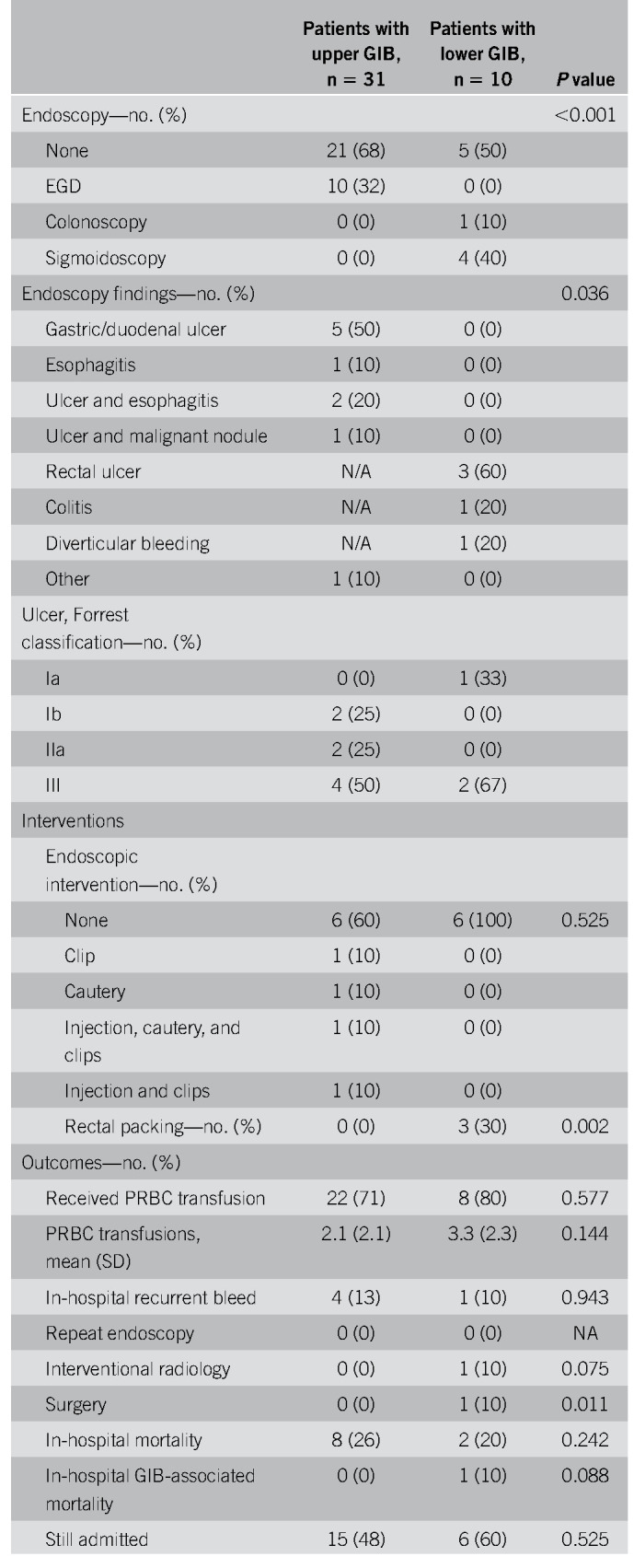
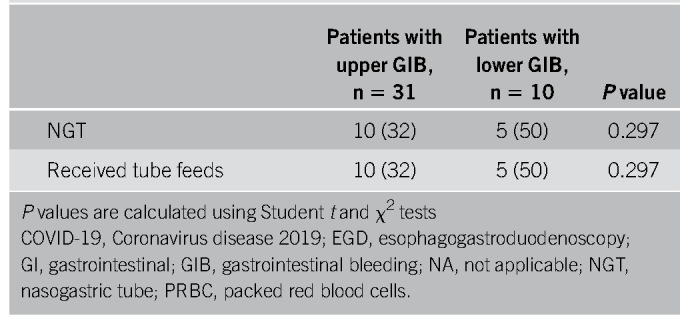
Table 6.
Multivariable analysis of potential risk factors for upper GI bleeding in patients admitted with COVID-19
Lower GIB
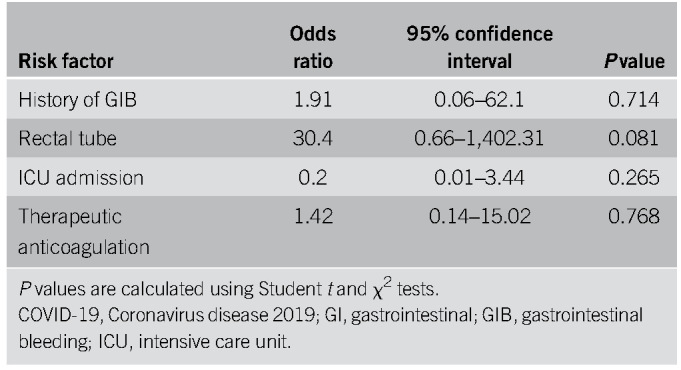
Ten patients had symptoms of lower GIB described as hematochezia (Table 3). Three patients (30%) were on NSAIDs and 4 (40%) were on ASA. Four patients (40%) were on anticoagulation during their hospitalization and 6 (60%) had rectal tubes in place (Table 4). Five patients (50%) underwent flexible sigmoidoscopy or colonoscopy, of whom 3 (60%) had rectal ulcers as the etiology, all of whom had rectal tubes and were on anticoagulation (Table 5). The 3 patients with rectal ulcers were referred to colorectal surgery for rectal packing, which successfully achieved hemostasis in all 3 cases. Another patient who did not undergo luminal evaluation because of hemodynamic instability was treated with empiric rectal packing for presumed rectal ulcer, with unsuccessful hemostasis and later died from critical illness related to COVID-19. In this cohort, the average nadir Hgb was 7.2 g/dL (± 1.0), and 8 patients (80%) required pRBCs for GIB (Table 5). One patient had recurrence of bleeding during this hospitalization, there were 2 in-hospital deaths (20%), and 1 was associated with GIB (Table 5). One patient had a computed tomography angiography that showed active extravasation at the splenic flexure. Given the unstable hemodynamic status, the patient went directly to interventional radiology and had a negative angiography and was subsequently taken to the operating room for a left hemicolectomy. On gross and microscopic examination of the resected specimen, no bleeding source was identified. There were epithelial changes microscopically that could not clearly be attributed to SARS-CoV-2 and did not seem to be ischemic in etiology. A multivariate analysis was performed to determine potential predictors of lower GIB, and although rectal tube usage trended towards being a risk factor, it was not statistically significant (OR 30.4, P = 0.081) (Table 6). Anticoagulation was also not predictive with an OR of 1.42 and a P value of 0.76.
DISCUSSION
In this multicenter matched case-control study, we demonstrated that in COVID-19 patients with GIB, the most common etiologies for upper GIB were gastric or duodenal ulceration (80%) and rectal ulceration (50%) for lower GIB. Other than a history of GIB, there does not seem to be distinctive prehospitalization characteristics of patients who had GIB vs those who did not. Although in-hospital, therapeutic anticoagulation and rectal tube usage showed a nonsignificant trend toward increased risk of upper and lower GIB, respectively. Of those patients who underwent endoscopic evaluation, 4 upper GIB patients received therapeutic endoscopic interventions and the 3 patients with rectal ulcers were treated with rectal packing, with successful hemostasis in all cases. The decision for endoscopy was based on the clinical judgement of the gastroenterologist but was generally performed in the setting of ongoing hemodynamic instability or severe anemia not responsive to transfusions. Compared with the matched controls, COVID-19 patients with GIB were noted to have higher transfusion requirements, but the in-hospital mortality rates were similar. Ultimately, one of the 10 patients with lower GIB died with GI hemorrhage as a contributing cause of death. Given the small sample size, further studies are needed to better determine predictors of bleeding and outcome measures in this cohort.
It is worth considering whether GIB is related to the direct effect of COVID-19 on the integrity of the GI mucosa. The virus is known to bind to angiotensin-converting enzyme 2 that is expressed in absorptive intestinal epithelial cells in the upper esophagus, ileum, and colon (8). Recent studies also reported SARS-CoV-2 nucleic acid in the stool of infected patients, although the clinical relevance of this is uncertain (4,9). It is plausible that the GI tract is potentially a route of transmission, but whether COVID-19 directly causes GI inflammation or mucosal injury remains unclear. Interestingly, a study in China reported that an endoscopy for upper GIB in a COVID-19 patient showed esophageal erosions and ulcerations with SARS-CoV-2 RNA detected in mucosal biopsy specimens at the bleeding site and esophagus, stomach, duodenum, and rectum (2). Another study described a COVID-19 patient with coffee-ground emesis and esophageal mucosal injury on esophagogastroduodenoscopy but with no evidence of mucosal damage on biopsied specimens (4). The etiology of bleeding in this patient population is likely multifactorial. However, our study showed that most bleeding occurred during the hospitalization, rather than on initial presentation, suggesting that bleeding is more likely treatment-related or secondary to factors related to critical illness rather than primarily viral-induced mucosal injury. As both anticoagulation and rectal tubes are increasingly being used in the management of COVID-19 patients, we postulate that these factors may be contributing to GI hemorrhage.
An emerging concept with COVID-19 has been the increased risk of coagulopathy and thromboembolic events, including deep venous thrombi, pulmonary emboli, and cerebrovascular events. Thus, higher doses of anticoagulation are being recommended, with the speculation that COVID-19 can induce a prothrombotic state with micro- and macro-vascular thrombi (10,11). Of our 41 patients with GIB, 39% were on therapeutic anticoagulation as an inpatient before onset of bleeding (Table 4). Our data did not demonstrate anticoagulation as a statistically significant risk factor for GIB (Tables 6 and 7). However, given our small sample size, short follow-up duration and that a more standardized COVID-19 anticoagulation protocol was not implemented until 3 weeks into our study, the effects of anticoagulation among this cohort still remains unclear. In particular, 26% of the patients with upper GIB were on pre-existing PPI as an outpatient and whether prophylactic PPIs can mitigate the effects of bleeding in these anticoagulated patients is yet to be determined (Table 4). Even in non–COVID-19 patients, there have been no randomized trials looking at the effect of PPIs with anticoagulants in the prevention of upper GIB. That said, large cohort studies and a meta-analysis suggest a protective role for concomitant acid suppression in high-risk patients requiring anticoagulation (12,13). Larger controlled trials are needed to understand the implications of PPI as a preventative measure in the COVID-19 population, although the rapid pace of clinical development for this disease has led to an expert consensus recommendation at our institution of judicious use of prophylactic PPI in patients on higher-intensity DVT prophylaxis.
Table 7.
Multivariable analysis of potential risk factors for lower GI bleeding in patients admitted with COVID-19
All 31 patients with upper GI hemorrhage received PPI therapy at the onset of bleeding (Table 4). Only 32% underwent endoscopy with an average delay of 2.4 days, which is longer than the recommended guidelines. As an epicenter during the COVID-19 pandemic, such a delay was because of concerns for the patient's respiratory status or illness severity, providers' safety, PPE conservation, and the preservation of ventilators by avoiding procedural-related intubations. All patients ultimately had cessation of bleeding with no difference in pRBC transfusion requirements when comparing endoscopic treatment vs conservative management. These findings suggest that because we make challenging management decisions weighing patient and provider safety and resource utilization, it may be reasonable to consider managing GIB in COVID-19 patients conservatively and reconsider endoscopy if the patient does not respond within 24 hours. Cavaliere et al. (7) observed similar findings in 6 patients with COVID-19 pneumonia and upper GIB. All 6 patients had resolution of bleeding within 24 hours of conservative management. However, this case series is also limited because of small sample size and lack of adequate follow-up (7). Although several of our patients have been hospitalized for less than 30 days, we observed no in-hospital upper GIB-associated deaths despite our prolonged procedural delay times (Table 5). Four of 31 patients rebled, one of whom had previously undergone endoscopy with therapeutic intervention. Ultimately, larger, more controlled trials are needed to determine the safety of this management approach.
In addition to anticoagulation, intrarectal catheters (“rectal tubes”) may increase the risk of lower GIB. These catheters are widely used as a means to divert liquid or semiliquid stool to mitigate the risk of perianal skin breakdown, nosocomial infections, and decrease labor-intensive nursing care (14). They have been particularly valuable in COVID-19 patients with diarrhea, in which they can minimize nursing exposure and conserve limited PPE. However, there have been several published reports of rectal bleeding, rectal ulceration, and life-threatening hemorrhage from indwelling intrarectal catheters (14–17). Nevertheless, bleeding complications in this setting of prolonged indwelling intrarectal catheters are likely an underreported phenomenon. Padmanabhan and colleagues published an early prospective study of intrarectal catheters, and of the 42 patients in their study cohort, the authors reported 1 episode (2.4%) of GIB from pressure ulceration (18). The underlying mechanism of injury was believed to be pressure necrosis mediated by the inflated balloon that results in mucosal ulceration (17). In our study, all 3 confirmed cases of rectal ulcers occurred in the setting of rectal tubes and concurrent anticoagulation. Although not statistically significant, rectal tube usage trended toward an association with GIB with an OR of 30.4. With only 10 lower GIB patients in this cohort, larger studies are needed to better evaluate the association between anticoagulation, rectal tube usage, and GI hemorrhage in this population. Taken together, it is imperative that clinicians exercise caution when using these devices in COVID-19 patients particularly in those on anticoagulants, known large internal hemorrhoids or a history of lower GIB. If other methods for skin protection are not possible, the indications for rectal catheter use should be reassessed daily with strict avoidance of overinflation of the balloon (>45 mL) and limited to 29 days, given the potential consequences (19).
There are limitations that should be considered when interpreting the results. The data were collected in a retrospective fashion without randomization. We relied on documentation of GIB by healthcare providers and cases of GIB may have been missed, particularly more mild cases. In addition, because there can be significant interobserver variability in descriptions of GIB such as melena, it is possible that patients with dark brown or green stool may have been included. Nonetheless, the chart review was performed to ensure that patients included had significant GIB. Misclassification of upper or lower GIB may have also occurred because patients with melena sometimes have lower GI etiologies, and those with hematochezia may have a brisk upper GIB. Maroon stools in this study were characterized as upper GIB, but this could have represented a lower GIB etiology. In addition, although our study includes a larger number of patients with GIB who underwent endoscopic evaluation than previously reported, it is still an overall small number and findings of stigmata of high-risk hemorrhage were rare, limiting the ability to extrapolate or predict which patients may have these findings. An interesting comparison would have been between COVID-19 and non–COVID-19 GI bleeders during this time period; however, because our hospitals were at the epicenter of the COVID-19 pandemic, there were only 13 COVID-19 negative patients who received consultations for GIB. This was too small of a sample size for a comparative control. Our data were also not statistically significant in evaluating potential GIB risk factors including anticoagulation, intensive care unit admission, nasogastric, and rectal tube usage and is likely related to our small sample size and short follow-up duration. Finally, given that many of the patients included in our study are still admitted and/or with limited follow-up, the ultimate rates of rebleeding and mortality are likely to increase with time. Nonetheless, it should be noted that GIB among COVID-19 patients is a novel clinical phenomenon with limited existing data and has important implications for management.
CONCLUSIONS
As the COVID-19 pandemic evolves, gastroenterologists have been confronted with unique challenges, particularly understanding and managing GI sequelae of the SARS-CoV-2 virus. With increased usage of rectal tubes and anticoagulation in admitted COVID-19 patients, the incidence of GIB is likely to increase. As we continue to manage these patients, providers must keep in mind the risk of GI hemorrhage and consider preventative measures such as guidelines for prophylactic PPIs and the development of a rectal tube care protocol for nursing staff. We must continue to balance the urgency for endoscopy in these patients with the potential risk that it may pose to the patient and endoscopy team in the current environment.
CONFLICTS OF INTEREST
Guarantor of the article: David W. Wan, MD.
Specific author contributions: Study concept and design (T.A.M., D.W.W., K.H., A.K., R.Z.S.); acquisition of data (T.A.M., D.W.W., K.H., S.T., S.L.S., A.M., A.K., G.G., A.J.C., T.I.K., B.E.F.); analysis and interpreting data (T.A.M., D.W.W., K.H., S.L.S., R.Z.S.); drafting the manuscript (T.A.M., D.W.W., K.H., A.M.); critical revision of the manuscript for important intellectual content (T.A.M., D.W.W., K.H., S.T., A.K., T.I.K., C.V.C., R.Z.S.); statistical analysis (K.H.); study supervision (T.A.M., D.W.W., R.Z.S.). For each author, he or she has approved the final draft submitted.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ GI symptoms are common presenting complaints in patients with COVID-19.
✓ GIB occurs in 2%–13% of patients hospitalized with COVID-19.
WHAT IS NEW HERE
✓ In COVID-19 patients, the most common etiologies for upper GIB were peptic ulcer disease and esophagitis.
✓ Rectal ulceration secondary to intrarectal catheter use was the most common etiology of lower GIB in patients with COVID-19.
Footnotes
T.A. Martin and D.W. Wan are co-first authors.
REFERENCES
- 1.CDC COVID Data Tracker [database Online]. Atlanta GUSDoHaHS, Centers for Disease Control and Prevention. 2020. [Google Scholar]
- 2.Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020;69:997–1001. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020;158:1831–3.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau JYW, Yu Y, Tang RSY, et al. Timing of endoscopy for acute upper gastrointestinal Bleeding. N Engl J Med 2020;382:1299–308. [DOI] [PubMed] [Google Scholar]
- 6.Strate LL, Gralnek IM. ACG clinical guideline: Management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol 2016;111:755. [DOI] [PubMed] [Google Scholar]
- 7.Cavaliere K, Levine C, Wander P, et al. Management of upper GI bleeding in patients with COVID-19 pneumonia. Gastrointest Endosc 2020. [Epub ahead of print April 20, 2020.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollias A, Kyriakoulis KG, Dimakakos E, et al. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br J Haematol 2020;189:846–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang CS, Joo MK, Kim BW, et al. The role of acid suppressants in the prevention of anticoagulant-related gastrointestinal bleeding: A systematic review and meta-analysis. Gut Liver 2020;14:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA 2018;320:2221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Ssaydeh DA, Rechnitzer TW, Knowles BP, et al. Major haemorrhage associated with the Flexi-Seal(R) fecal management system. Anaesth Intensive Care 2018;46:140. [PubMed] [Google Scholar]
- 15.Deeb L, Koczka C, Andrawes S, et al. Significant rectal bleeding as a complication of a fecal collecting device: Report of a case: 891. Am J Gastroenterol 2011;106:S334. [Google Scholar]
- 16.Sparks D, Chase D, Heaton B, et al. Rectal trauma and associated hemorrhage with the use of the ConvaTec Flexi-Seal fecal management system: Report of 3 cases. Dis Colon Rectum 2010;53:346–9. [DOI] [PubMed] [Google Scholar]
- 17.Tiwari A, Sharma H, Qamar K, et al. The traumatic tube: Bleeding rectal ulcer caused by flexi-seal device. Case Rep Gastrointest Med 2017;2017:5278971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padmanabhan A, Stern M, Wishin J, et al. Clinical evaluation of a flexible fecal incontinence management system. Am J Crit Care 2007;16:384–93. [PubMed] [Google Scholar]
- 19.Whiteley I, Sinclair G, Lyons A, et al. A retrospective review of outcomes using a fecal management system in acute care patients. Ostomy Wound Manage 2014;60:37–43. [PubMed] [Google Scholar]


