Abstract
The world is amid a pandemic caused by severe acute respiratory syndrome-coronavirus 2. Severe acute respiratory syndrome-coronavirus causes serious respiratory tract infections that can lead to viral pneumonia, acute respiratory distress syndrome, and death. Some patients with coronavirus disease 2019 (COVID-19) have an activated coagulation system characterized by elevated plasma levels of d-dimer—a biomarker of fibrin degradation. Importantly, high levels of D-dimer on hospital admission are associated with increased risk of mortality. Venous thromboembolism is more common than arterial thromboembolism in hospitalized COVID-19 patients. Pulmonary thrombosis and microvascular thrombosis are observed in autopsy studies, and this may contribute to the severe hypoxia observed in COVID-19 patients. It is likely that multiple systems contribute to thrombosis in COVID-19 patients, such as activation of coagulation, platelet activation, hypofibrinolysis, endothelial cell dysfunction, inflammation, neutrophil extracellular traps, and complement. Targeting these different pathways may reduce thrombosis and improve lung function in COVID-19 patients.
Keywords: coronavirus, fibrin, orthomyxoviridae, pandemics, thrombosis
Highlights.
Coronavirus disease 2019 (COVID-19) patients have an increased risk of arterial and venous thrombosis.
Elevated levels of D-dimer are associated with increased thrombosis and mortality.
Multiple pathways likely contribute to thrombosis in COVID-19 patients.
Pandemic Respiratory Viruses
In the last century, several new viruses have emerged, including different strains of influenza virus A virus (IAV), severe acute respiratory syndrome-coronavirus (SARS-CoV), Middle East respiratory syndrome-coronavirus (MERS-CoV), and most recently, SARS-CoV-2, that have caused epidemics and pandemics. IAV transmission from zoonotic reservoirs into humans has caused the last 4 influenza pandemics: 1918 H1N1 Spanish flu, 1957 H2N2 Asian flu, 1968 H3N2 Hong Kong flu, and 2009 H1N1.1–3 The SARS-CoV epidemic occurred between 2002 and 2004 and infected ≈8000 people with at least 774 deaths worldwide.4–6 MERS-CoV appeared in 2012 and infected ≈2500 people with over ≈850 deaths, and cases still occur.7–9 In December 2019, SARS-CoV-2 emerged in China and quickly spread throughout the world. As of June 17, 2020, there have been over 8.2 million diagnosed cases of coronavirus disease 2019 (COVID-19) with >445 000 deaths worldwide (Johns Hopkins Coronavirus Resource Center, https://coronavirus.jhu.edu/map.html).
Influenza viruses and coronaviruses are enveloped viruses with a single-stranded RNA genome (either a positive- or negative-sense RNA). Influenza viruses enter cells via endocytosis that requires binding and proteolytic cleavage of hemagglutinin on epithelial cells.10,11 SARS-CoV, MERS-CoV, and SARS-CoV-2 all belong to the coronavirus family. They are called coronaviruses because they have large spike proteins on the capsid surface that create a crown-like shape. Entry of coronaviruses into host cells involves binding of the spike proteins with host receptors, followed by proteolytic cleavage of the spike protein to expose the S2 fusion domain with subsequent membrane fusion.12,13 MERS-CoV uses DPP4 (dipeptidyl peptidase 4) as a cellular receptor, whereas SARS-CoV and SARS-CoV-2 use ACE2 (angiotensin-converting enzyme 2) as entry receptors.14–18 Importantly, SARS-CoV-2 has a stronger binding to ACE2 compared with SARS-CoV.19 ACE2 is predominantly expressed in epithelial cells of subsegmental bronchial branches.20 Interestingly, one study found low levels of ACE2 in alveolar epithelial cells and endothelial cells in uninfected control lungs but an increased expression of ACE2 in both cell types in the lungs of COVID-19 patients.21 In a physiological setting, ACE2 cleaves and inactivates angiotensin I and angiotensin II and, therefore, plays a critical role in regulating the renin-angiotensin system.22 Differences in tissue expression of these receptors and activating proteases may contribute to unique aspects of the pathophysiology of each virus.
Acute Respiratory Distress Syndrome Associated With Pandemic Respiratory Viruses
Super pandemic viruses, such as IAV H1N1, SARS-CoV, MERS-CoV, and SARS-CoV-2, cause serious respiratory tract infections that can lead to viral pneumonia and acute respiratory distress syndrome (ARDS).23–25 ARDS is a type of respiratory failure characterized by widespread local and systemic inflammation.26 Both viral infection of cells and the host response to infection damage the epithelial-endothelial cell barrier that separates the alveoli from capillaries. This injury compromises the lung’s ability to exchange oxygen and carbon dioxide.26 Lung stillness, fluid-filled alveoli, and a rise in carbon dioxide levels lead to hypoxemia and respiratory distress.27 The primary treatment for ARDS is mechanical ventilation and supportive treatment in an intensive care unit.28 IAV patients with classic ARDS that requires mechanical ventilation have decreased lung compliance with elevated plateau pressures.29 One study reported that 46 (23%) of 199 patients hospitalized with SARS developed ARDS, and these patients had a mortality rate of 37% at 28 days.30 Similarly, 20% of hospitalized COVID-19 patients in New York required mechanical ventilation.31 Surprisingly, some COVID-19 patients with ARDS have well-preserved lung mechanics despite severe hypoxia.32,33 This has led to the suggestion that microvascular thrombosis rather than decreased lung compliance contributes to the impaired oxygenation in COVID-19 patients.
Cytokine Storm Is Associated With Pandemic Respiratory Viruses
Infection with pandemic respiratory viruses can lead to an overproduction of numerous cytokines that is termed the cytokine storm.34–36 This hyperinflammatory response contributes to disease severity and death. TNFα (tumor necrosis factor-alpha), IL (interleukin)-1β, and IL-6 orchestrate the inflammatory response.34,37 Both IAV and SARS-CoV infection are associated with a cytokine storm.38,39 Davey et al40 reported the results of 2 international cohort studies that measured the association of 25 plasma biomarkers with disease progression in 837 IAV(H1N1)pdm09 patients. Seven biomarkers, including IL-6, were associated with disease progression in outpatients and inpatients, whereas 5 biomarkers, including TNFα and IL-8, were associated with disease progression among hospitalized patients. Critically ill patients with IAV(H1N1)pdm09 also exhibited higher levels of IL-6 compared with patients with bacterial pneumonia.41 A recent study suggested that monocytes and macrophages play a key role in the hyperinflammatory response in COVID-19 patients.42 Indeed, severe SARS-CoV-2 infection is associated with increased circulating levels of various inflammatory mediators, including IL-6 and CRP (C-reactive protein).43–47 We observed increased levels of CRP in severe IAV H1N1 patients.48 Zhou et al47 observed a serial increase in IL-6 in nonsurviving patients but not in surviving patients. Accordingly, a pilot study analyzed the effect of the IL-6 receptor antagonist tocilizumab on survival of 129 hospitalized COVID-19 patients with moderate or severe viral pneumonia. Tocilizumab significantly reduced the number of life-support interventions and deaths compared with the control group (https://www.clinicaltrialsarena.com/news/french-early-trial-tocilizumab-covid-19/). This preliminary study led the Food and Drug Administration to approve a phase 3 trial of tocilizumab for the treatment of severe COVID-19 patients (https://www.clinicaltrials.gov; unique identifier: NCT04361552), with additional tocilizumab clinical trials underway. However, it remains unclear whether IL-6 targeting alone will be adequate to improve outcomes caused by a plethora of cytokines. In addition, it is unclear whether tocilizumab will mitigate the thrombotic propensity in COVID-19 patients, although the expected reduction of IL-6–dependent CRP expression has been observed.49,50
Thrombosis Associated With Pandemic Respiratory Viruses
Critically ill patients exhibit a rate of venous thromboembolism (VTE; deep vein thrombosis or pulmonary embolism [PE]), of VTE 5% to 10% despite thromboprophylaxis.51 VTE, pulmonary microvascular thrombosis, and arterial thrombosis have been associated with IAV and pandemic coronavirus infections. One study of hospitalized H1N1 patients observed 7 (5.9%) thrombotic vascular events (4 venous and 3 arterial) in 119 patients.52 Another study observed a higher rate of VTE (44%) in hospitalized H1N1 patients (n=36) with severe ARDS compared with 29% in non-H1N1 patients with ARDS.24 Thrombotic complications have also been observed in SARS-CoV patients.53 A study from a single hospital in Singapore reported that one-third of SARS-CoV patients experienced VTE despite the use of low-molecular-weight heparin at doses to achieve anti-Xa levels of 0.5 to 1.0 IU/mL54; however, no additional details of the VTE events were provided. Arterial ischemic stroke was observed in a small number of SARS-CoV patients.54 Surprisingly, perhaps, there are no reports of thrombosis in MERS-CoV patients.
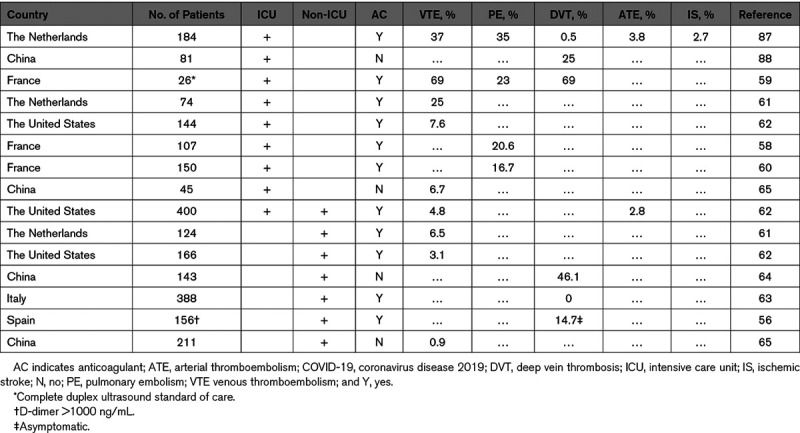
Recently, several studies have reported VTE rates ranging from 0.9% to 6.5% for noncritically ill hospitalized COVID-19 patients and 8% to 69% in COVID-19 patients in the intensive care unit (Table 1).55–64 Rates of PE were between 16.7% and 35% in severely ill COVID-19 patients, and rates of deep vein thrombosis were between 0% and 46.1% for nonseverely ill COVID-19 patients (Table 1). Rates of arterial thrombotic events were between 2.8% and 3.8%.57,62 There are several factors that could explain the wide variation in thrombosis rates in the different studies that include differences in clinical practice, such as if venous ultrasound is performed as a screening strategy or if thromboprophylaxis is routinely used, reporting of symptomatic versus asymptomatic VTE, and also differences in patient populations. Notably, however, several groups have reported that VTE may occur despite standard thromboprophylaxis (Table 1), which is like what was observed in SARS-CoV infection. Although initial reports suggested that COVID-19 patients had higher rates of thrombosis compared with patients with other types of pneumonia, a recent study found that the rate of VTE in COVID-19 patients was 2% compared with 3.6% in patients with non–COVID-19 community-associated pneumonia.65 Furthermore, it is important to note that a recent study reported a VTE rate of 4.8% and a rate of overall bleeding of 4.8% in COVID-19 patients.62 Another study of 353 COVID-19 patients in Boston found that the cumulative incidence of thrombotic events was 10.2% and major or fatal bleeding of 20.8% in hospitalized COVID-19 patients (J. Zwicker, unpublished data, 2020). At present, the optimal antithrombotic prophylactic strategy for patients with COVID-19 is unclear. A new clinical trial (IMPROVE [Intermediate or Prophylactic Dose Anticoagulation for Venous or Arterial Thromboembolism in Severe COVID-19]; https://www.clinicaltrials.gov; unique identifier: NCT04367831) will hopefully shed light on this question.
Table 1.
Incidence of Thrombosis in COVID-19
Several studies have reported pathological findings from autopsies of patients infected with pandemic coronaviruses. Pulmonary thromboemboli within the main pulmonary artery or segmental pulmonary arteries, thrombi in small vessels, and fibrin within pulmonary vessels were observed in SARS-CoV patients.66–68 A recent series of studies have described the findings of autopsies of COVID-19 patients.33,69–71 Fibrin-rich thrombi were found in small vessels and capillaries in the lung, as well as foci of hemorrhages.33,70 Interestingly, CD61+ megakaryocytes were observed within alveolar capillaries.70 Some fibrin and platelets within small vessels were also associated with neutrophils. Intra-alveolar fibrin deposition was observed in a subset of severe COVID-19 patients consistent with a loss of vascular integrity.69 One autopsy study found that 7 of 12 (58%) COVID-19 patients had a deep vein thrombosis that was not suspected antemortem, and PE was the direct cause of death in 4 of these patients.72 A recent study performed autopsies on 7 COVID-19 patients and compared the findings to 7 H1N1 patients.21 There was widespread thrombosis and microangiopathy in the lungs of COVID-19 patients, and capillary microthrombi were 9× more prevalent than in H1N1, which suggested a different pathological process.21
Pandemic Respiratory Viruses Activate the Coagulation System
The innate immune response is activated in response to invading pathogens to counteract the infection. This is generally accompanied by activation of coagulation that, in part, serves to localize the infection.73,74 However, excessive and widespread activation of coagulation can lead to disseminated intravascular coagulation (DIC), defined as fulminant activation of coagulation, consumption of coagulation factors, and bleeding.75,76 Classic DIC caused by bacterial sepsis is associated with prolonged activated partial thromboplastin time, prothrombin time (PT), thrombocytopenia, elevated D-dimer, and microangiopathic thrombosis in multiple organs.75,76 D-dimer is a product of plasmin-mediated degradation of cross-linked fibrin.
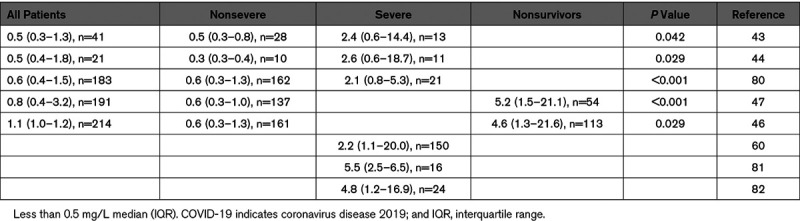
Elevated plasma D-dimer is associated with a higher risk of disease progression in hospitalized IAV(H1N1)pdm09-infected patients.40 Two other studies of patients with probable IAV H1N1 infection found that D-dimer predicted disease progression.77,78 Elevated plasma levels of D-dimer have also been reported in SARS-CoV–infected patients.79 D-dimer has attracted attention as a prognostic marker in COVID-19 patients.43,44,46,47,60,80–82 As expected, COVID-19 patients with VTE had higher D-dimer levels than non-VTE patients.55 A series of articles from China reported higher D-dimer levels in severely affected patients compared with those with a nonsevere disease and higher D-dimer levels in nonsurvivors compared with survivors (Table 2).43–47,80 Similarly, studies from France and Italy found high D-dimer levels in COVID-19 patients in the intensive care unit (Table 2).60,81 Two studies found that a higher D-dimer level on admission was associated with increased mortality.47,64 One study used 2.0 μg/mL as a cutoff for D-dimer and found a mortality rate of 0.37% (1 of 267 COVID-19 patients, <2.0 μg/mL) versus 17.9% (12 of 67 COVID-19 patients, ≥2.0 μg/mL).64 In contrast, a study from France observed a less impressive separation of mortality rates based on the same D-dimer cutoff (10.4% [8 of 77 COVID-19 patients], <2.0 μg/mL versus 18.3% [17 of 93 COVID-19 patients], ≥2.0 μg/mL) and suggested that the Chinese study had selection bias.83
Table 2.
d-Dimer Levels in Patients With COVID-19
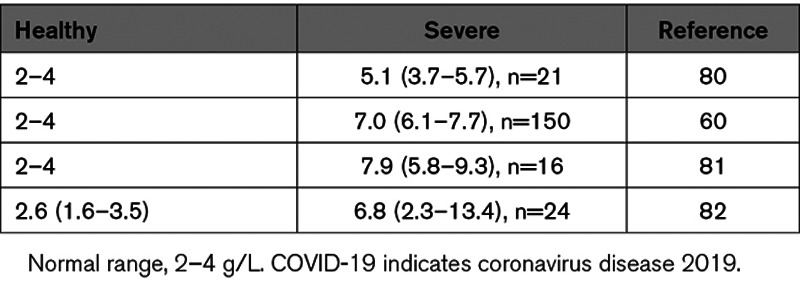
Thrombocytopenia was observed in 45% to 55% of SARS-CoV patients, but overt DIC was rarely observed.53,79,84 Thrombocytopenia was also found to be evident in a subset of MERS-CoV patients.85–88 Similarly, thrombocytopenia and an elevated PT was observed in 2 fatal cases of MERS-CoV patients, consistent with a diagnosis of DIC.86 Many COVID-19 patients have mild thrombocytopenia (100−150×109/L) at most and do not exhibit an increase in PT or decrease in AT (antithrombin) levels.33,43–46,60,80–82,89 These results suggested the absence of a consumptive coagulopathy in most patients. However, several studies found that nonsurviving patients had slightly prolonged PT and a further decrease in platelet count.46,47,80 Interestingly, patients with severe SARS-CoV-2 infection also have elevated levels of fibrinogen ranging from 1.3 to 2.0× above the normal range (2–4 g/L; Table 3).60,80–82 We observed increased levels of fibrinogen in severe IAV H1N1 patients.48 Ranucci et al81 showed an association between IL-6 and fibrinogen levels. In addition, FVIII (factor VIII) and VWF (von Willebrand Factor) levels were increased in COVID-19 patients by 2- to 2.3-fold and 3- to 4.1-fold above the normal range, respectively.60,82
Table 3.
Fibrinogen Levels in Severe COVID-19 Patients
Taken together, these results indicate that most COVID-19 patients have an activated coagulation system that is associated with increased levels of D-dimer; however, it is unlike classic DIC since there is little change in PT and the thrombocytopenia is generally mild. Elevated levels of FVIII and fibrinogen likely contribute to the prothrombotic state in COVID-19 patients. Elevated FVIII and VWF may reflect activated/infected endothelium, whereas elevated fibrinogen likely reflects enhanced production by hepatocytes as part of the host’s acute phase responses driven by IL-6. In the later stages of disease, nonsurviving COVID-19 patients may develop classic DIC with prolongation of the PT, moderate-to-severe thrombocytopenia (platelet count, <50×109/L), and decreased fibrinogen (<1.0 g/L).
Mouse Models of Pandemic Virus Infection
Several mouse models have been developed to study the pathological changes in the lung associated with infection with IAV H1N1, SARS-CoV, and MERS-CoV. One study reported that over 3500 genes were differentially regulated in the lungs of mice following SARS-CoV infection.90 Importantly, mice infected with 1918 and 2009 IAV H1N1 strains exhibited similar transcriptional signatures, which suggested a common mechanism of lung injury.90 Infection with IAV H1N1, SARS-CoV, and MERS-CoV is associated with lung hemorrhages.90–92 Infection of mice with different coronavirus mouse hepatitis virus strains also caused severe pneumonia and lung hemorrhage.93 However, thrombosis has also been observed in the lungs of mice expressing human DPP4 infected with MERS-CoV.94
Possible Mechanisms Driving Thrombosis in Pandemic Virus Infection
At this time, we can only speculate about the mechanisms of thrombosis in COVID-19 patients based on the available plasma biomarkers and clinical presentation. Several recent comments/reviews have described the coagulation abnormalities and thrombosis occurring in COVID-19 patients.95–99 There is clear evidence for activation of different cell types, such as lung epithelial cells, macrophages, neutrophils, endothelial cells, and platelets, as well as different systems, such as coagulation, inflammation, and complement, in the lungs of COVID-19 patients (Figure). We will briefly summarize some of these pathways and refer to reviews that cover some of the pathways in more detail.
Figure.
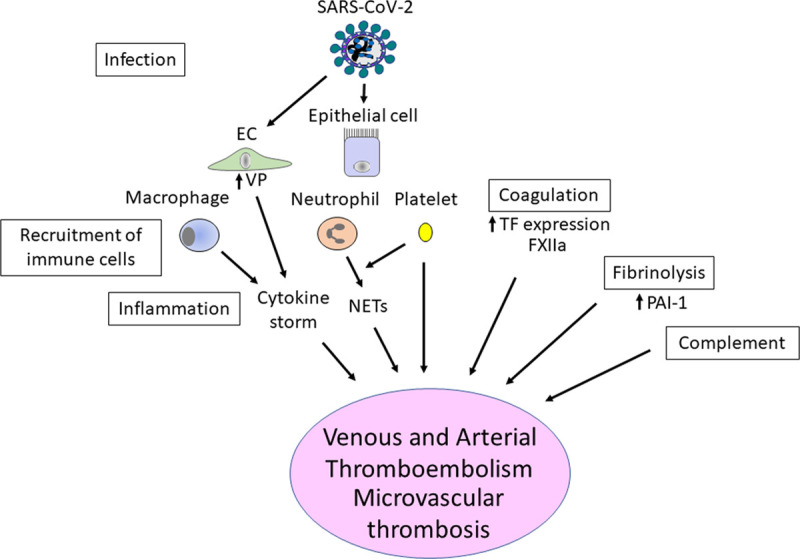
Potential pathways that drive thrombosis in coronavirus disease 2019 (COVID-19) patients. Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infects lung epithelial cells and endothelial cells (ECs), which leads to the recruitment of a variety of immune cells, such as macrophages and neutrophils. Activated macrophages and ECs contribute to the cytokine storm. EC activation also increases vascular permeability (VP). Neutrophils release neutrophil extracellular traps (NETs). Activated platelets likely contribute to thrombosis and NET formation. TF (tissue factor) expression is likely to be increased in activated epithelial cells, macrophages, and ECs and will activate the coagulation system. Similarly, FXIIa (factor XIIa) can increase coagulation. SARS-CoV-2 infection also activates the fibrinolytic system and may increase PAI-1 (plasminogen activator inhibitor 1), which would reduce fibrin degradation. Finally, the complement system is activated in COVID-19 patients, and cellular damage would increase the activation of the coagulation system.
TF Pathway
Aberrant TF (tissue factor) expression is associated with most forms of thrombosis.100 Importantly, TF is a key mediator of activation of coagulation in different forms of ARDS.101–104 Viral infection of a variety of cell types, including lung epithelial cells, endothelial cells, and monocytes induces TF expression.74,91,105 In addition, TF expression in endothelial cells is induced by activation of TLR3 (toll-like receptor 3)—a pattern-recognition receptor that detects single-stranded RNA.106,107 Interestingly, TLR3 was shown to protect mice from SARS-CoV infection.108 Cytokines produced during the cytokine storm (TNFα, IL-1β, and IL-6) induce TF expression in endothelial cells, and IL-6 induces TF expression in mononuclear cells.109–111 Angiotensin II can also induce TF expression in vascular smooth muscle cells and endothelial cells.112,113 Herpes simplex virus infection of endothelial cells increases TF expression.114 Similarly, one would expect that SARS-CoV-2 infection of the endothelium would increase TF expression and microvascular thrombosis. Therefore, there are a variety of mechanisms for increasing TF expression in different cell types in the lung during viral infections. We found that plasma levels of extracellular vesicle TF activity were increased in severe influenza virus patients and were associated with mortality.48 Increased TF is also observed after infection of mice with IAV H1N1, SARS-CoV, and MERS-CoV90,91 (T. Sheahan, unpublished data). We found that IAV H1N1 infection of mice increases TF expression in lung epithelial cells and activates coagulation.91 Furthermore, both a genetic reduction of TF in epithelial cells and administration of anticoagulants to wild-type mice was associated with increased alveolar hemorrhage.91,115 This suggests that TF-dependent activation of coagulation is part of the host innate immune response to viral infection that helps protect against intrapulmonary hemorrhage. However, a complication of this response is thrombosis. Therefore, it seems likely that TF plays a central role in thrombosis in COVID-19 patients. Two recent articles have discussed the role of TF in thrombosis in COVID-19 patients.116,117
Contact Activation Pathway
Activation of the contact system leads to thrombin generation and upregulation of the kallikrein-kinin system.118 Kallikrein induces the generation of bradykinin, which increases vascular permeability. In addition, bradykinin interacts with the renin-angiotensin system and increases inflammation, fibrinolysis, and complement activation.119 The effect of targeting the contact system has been studied in animal models of bacterial sepsis. An early study showed that administration of an anti-FXII (factor XII) antibody C6B7 prevented hypotension and extended the life of baboons challenged with Escherichia coli but did not prevent DIC.120 In a second study, C6B7 reduced complement activation, neutrophil activation, and the fibrinolytic response (reduced tissue plasminogen activator and plasmin-α2-antiplasmin complexes) but increased PAI-1 (plasminogen activator inhibitor 1) in septic baboons.121 More recently, the effect of blocking the contact pathway using an antibody 3G3 that prevents FXIIa activation of FXI (factor XI) was evaluated in a lethal Staphylococcus aureus baboon model.122 Pretreatment of the baboons with 3G3 reduced the activation of coagulation, fibrin deposition in tissues, inflammation, neutrophil activation, complement activation, and increased survival.122 An anti-FXII antibody 3F7 also reduced bradykinin generation and edema in mice.123 Acquired ACE2 deficiency also leads to more bradykinin via an unknown mechanism, which would increase vascular permeability. A recent review discussed the potential benefits of targeting the contact activation pathway in COVID-19 patients.124
Fibrinolysis
The fibrinolytic system is activated in ARDS.104,125,126 Elevated levels of PAI-1 in ARDS create a hypofibrinolytic state that leads to increased fibrin deposition within the vasculature and within the alveolar space. High plasma PAI-1 levels are associated with mortality in ARDS patients.127,128 One study reported that the plasma PAI-1 level was higher in 16 SARS-CoV patients than 19 patients with other infectious pneumonias and healthy controls.129 PAI-1 expression was increased in SARS-CoV–infected mice, and PAI-1−/− mice exhibited increased lung hemorrhage and increased mortality.90 This study suggested that PAI-1–dependent inhibition of fibrinolysis is protective against intrapulmonary hemorrhage. A recent review described the fibrinolytic abnormalities associated with ARDS and discussed the use of thrombolytic drugs to treat COVID-19.130 It was proposed that nebulized plasminogen activators could be used to degrade fibrin in the alveoli and improve oxygenation in COVID-19 patients.130 Indeed, a recent study reported that intravenous administration of tissue plasminogen activator temporally improved the respiratory status of 3 patients with severe COVID-19 respiratory failure.131
Platelets
Platelets play an essential role in maintaining vascular integrity but also contribute to thrombosis. More recently, platelets have been found to participate in the immune response to viruses.132 Interestingly, IAV particles were observed within platelets from patients with acute influenza infection.133 In addition, IAV engulfment by platelets led to TLR7-dependent release of C3 and subsequent activation of neutrophils and neutrophil extracellular trap (NET) release.133 Therefore, platelets participate in the host response to IAV infection. However, platelet activation during viral infection may also increase the risk of thrombosis. One study in COVID-19 patients found an association between thrombocytopenia and risk of in-hospital mortality.134 A recent review discussed the potential role of platelets in thrombosis in COVID-19.135
Activation of the Endothelium
Under normal conditions, the endothelium maintains vascular integrity, limits binding and activation of immune cells and platelets, and inhibits coagulation by expression of anticoagulant proteins. However, during infection, the endothelium becomes activated, resulting in a loss of barrier function, expression of adhesion proteins that facilitate the recruitment of immune cells, release of VWF that allows binding of platelets, and expression of TF that activates the coagulation system. One study found that soluble ICAM-1 (intercellular adhesion molecule 1) and soluble VCAM-1 (vascular cell adhesion molecule 1) were associated with disease progression among hospitalized IAV(H1N1)pdm09 patients.40 These biomarkers indicate that the endothelium is activated possibly by circulating inflammatory mediators. Although some IAV strains have been shown to replicate in human lung microvascular endothelial cells, only avian IAV H5N1 has been shown to infect lung microvascular endothelial cells in vivo.136,137 Importantly, one study found that blocking replication of the highly pathogenic IAV strain H5N1 in the endothelium reduced systemic viral spread and mortality without affecting viral replication in the lungs of infected mice.3 A recent study found that human capillary organoids derived from induced pluripotent stems cells could be infected with SARS-CoV-2, and this infection was blocked with recombinant, soluble human ACE2.138 Interestingly, deceased COVID-19 patients had increased ACE2 expression in endothelial cells in the lungs compared with noninfected controls.21 Two studies found evidence for direct infection of the endothelium by SARS-CoV-2 and diffuse endothelial inflammation in the lung, heart, kidney, and liver.21,139 SARS-CoV-2 infection of endothelial cells may lead to apoptosis or pyroptosis. Recent reviews have discussed the potential role of the endothelium in COVID-19.140,141
Neutrophils and NETs
Hematopoietic changes are observed in SARS-CoV and MERS-CoV patients.53 For instance, SARS-CoV patients often present with neutrophilia, which is associated with poor outcome.79,84 Other studies have observed neutrophilia in MERS-CoV–infected patients.85–87 COVID-19 patients generally have increased numbers of circulating neutrophils, and an elevated neutrophil count has been associated with poor outcome.43–47
Neutrophils play a key role in clearing viruses in the lung by phagocytosing viral particles and by releasing NETs.142–144 However, activated neutrophils can also damage host cells.145–148 Neutrophils also play a key role in immunothrombosis—a term that has been used to describe the activation of coagulation that accompanies host innate immune defense.149 Importantly, NETs may contribute to thrombosis and vascular occlusion.150,151 There are several biomarkers used to measure the levels of NETs in plasma, including MPO (myeloperoxidase)-DNA complexes and citrullinated histone H3.151 However, many of these assays have low specificity for NETs.151 One study in IAV H1N1 and H7N9 patients reported elevated levels of MPO-DNA complexes at hospital admission that correlated with disease severity.152 Similarly, serum from severe COVID-19 patients contained elevated levels of MPO-DNA complexes and citrullinated histone H3.153 These results suggest that NETs may contribute to impairment of blood flow in the lungs of COVID-19 patients.151,154
Complement
The complement system plays a key role in the host immune response to viruses by opsonization of viral particles, recruitment of inflammatory cells, and lysis of infected cells.155 However, complement activation can also damage host cells. SARS-CoV infection in mice activates the complement system.156 C3−/− mice exhibited reduced recruitment of neutrophils and inflammatory monocytes into the lung and less respiratory dysfunction after SARS-CoV infection compared with controls.156 Similarly, inhibition of the C5a receptor reduced lung injury in hDPP4 mice infected with MERS-CoV.157 These results indicate that the complement system contributed to the lung pathology after SARS-CoV and MERS-CoV infection in mice. Importantly, significant deposits of terminal complement components have been noted in the lung microvasculature of COVID-19 patients.33 Complement system inhibition with eculizumab, which binds to C5, might be beneficial for COVID-19—a hypothesis that is currently investigated in a clinical trial (https://www.clinicaltrials.gov; unique identifier: NCT04288713).158 A recent review discussed complement as a target in COVID-19.159
Conclusions
Further studies are needed to understand the molecular basis of thrombosis in COVID-19 patients and how this contributes to morbidity and mortality. Measurement of additional circulating biomarkers of different systems, such coagulation, fibrinolysis, and complement, as well as markers of endothelial cell activation, will provide much needed information on the pathology of COVID-19. When optimizing antithrombotic treatment for COVID-19 patients, it is important to balance the risk of thrombosis and the risk of bleeding, especially as bleeding has been observed in the lungs of COVID-19 patients. It will be also interesting to know whether any of the proposed treatments for COVID-19 patients, such as blocking the IL-6 receptor or inhibiting complement activation, will reduce thrombosis.
Acknowledgments
We would like to acknowledge colleagues in the Division of Hematology (Drs Stephan Moll, Steven Grover, and Yohei Hisada), in the Division of Pulmonary and Critical Care Medicine (Drs C. Adrian Austin and Robert Hagan), and in the Department of Epidemiology (Dr Lisa Gralinski) at the University of North Carolina at Chapel Hill and Dr Alvin Schmaier (Case Western Reserve University) for helpful discussions and comments. Webinars from the International Society on Thrombosis and Haemostasis were also very helpful (https://academy.isth.org/isth/2020/covid-19/291581/marcel.levi.26.beverley.jane.hunt.thrombosis.thromboprophylaxis.26.coagulopathy.html?f=menu%3D8%2Abrowseby%3D8%2Asortby%3D2%2Alabel%3D19794; https://academy.isth.org/isth/2020/covid-19/293464/doctor.jerrold.h.levy.and.doctor.nicole.p.juffermans.html?f=menu%3D8%2Ac_id%3D293464%2Afeatured%3D16772).
Sources of Funding
Funding was from grants from the National Institutes of Health (HL119523, N.M. and HL142799, S.A.).
Disclosures
None.
Footnotes
Nonstandard Abbreviations and Acronyms
- ACE2
- angiotensin-converting enzyme 2
- ARDS
- acute respiratory distress syndrome
- AT
- antithrombin
- COVID-19
- coronavirus disease 2019
- CRP
- C-reactive protein
- DIC
- disseminated intravascular coagulation
- DPP4
- dipeptidyl peptidase 4
- FVIII
- factor VIII
- FXI
- factor XI
- FXII
- factor XII
- IAV
- influenza A virus
- ICAM-1
- intercellular adhesion molecule 1
- IL
- interleukin
- IMPROVE
- Intermediate or Prophylactic Dose Anticoagulation for Venous or Arterial Thromboembolism in Severe COVID-19
- MERS-CoV
- Middle East respiratory syndrome-coronavirus
- MPO
- myeloperoxidase
- NET
- neutrophil extracellular trap
- PAI-1
- plasminogen activator inhibitor 1
- PE
- pulmonary embolism
- PT
- prothrombin time
- SARS-CoV
- severe acute respiratory syndrome-coronavirus
- TF
- tissue factor
- TLR3
- toll-like receptor 3
- TNFα
- tumor necrosis factor-alpha
- VCAM-1
- vascular cell adhesion molecule 1
- VTE
- venous thromboembolism
- VWF
- von Willebrand factor
For Sources of Funding and Disclosures, see page 2040.
References
- 1.Taubenberger JK, Morens DM. The 1918 influenza pandemic and its legacy. Cold Spring Harb Perspect Med. 2019:a038695. doi: 10.1101/cshperspect.a038695. doi: 10.1101/cshperspect.a038695. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson LJ, Rutter PD, Ellis BM, Greaves FE, Mytton OT, Pebody RG, Yardley IE. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:b5213. doi: 10.1136/bmj.b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tundup S, Kandasamy M, Perez JT, Mena N, Steel J, Nagy T, Albrecht RA, Manicassamy B. Endothelial cell tropism is a determinant of H5N1 pathogenesis in mammalian species. PLoS Pathog. 2017;13:e1006270. doi: 10.1371/journal.ppat.1006270. doi: 10.1371/journal.ppat.1006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, et al. ; SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 20033481953–1966doi: 10.1056/NEJMoa030781 [DOI] [PubMed] [Google Scholar]
- 5.Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 2003362263–270doi: 10.1016/S0140-6736(03)13967-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 20033481967–1976doi: 10.1056/NEJMoa030747 [DOI] [PubMed] [Google Scholar]
- 7.de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish ZA, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 2013877790–7792doi: 10.1128/JVI.01244-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 20123671814–1820doi: 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- 9.Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A. The Middle East Respiratory Syndrome (MERS). Infect Dis Clin North Am 201933891–905doi: 10.1016/j.idc.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol 200212159–166doi: 10.1002/rmv.352 [DOI] [PubMed] [Google Scholar]
- 11.Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 19992581–20doi: 10.1006/viro.1999.9716 [DOI] [PubMed] [Google Scholar]
- 12.Reinke LM, Spiegel M, Plegge T, Hartleib A, Nehlmeier I, Gierer S, Hoffmann M, Hofmann-Winkler H, Winkler M, Pöhlmann S. Different residues in the SARS-CoV spike protein determine cleavage and activation by the host cell protease TMPRSS2. PLoS One. 2017;12:e0179177. doi: 10.1371/journal.pone.0179177. doi: 10.1371/journal.pone.0179177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo M. Influenza virus entry. Adv Exp Med Biol 2012726201–221doi: 10.1007/978-1-4614-0980-9_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh SK. Middle east respiratory syndrome virus pathogenesis. Semin Respir Crit Care Med 201637572–577doi: 10.1055/s-0036-1584796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003426450–454doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020181271–280.e8doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 20201261456–1474doi: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 202027325–328doi: 10.1016/j.chom.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39:e105114. doi: 10.15252/embj.20105114. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Eng J Med. 2020383120–128doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 200227714838–14843doi: 10.1074/jbc.M200581200 [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, Zhang J, Zhao C. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis [published online April 15, 2020]. J Med Virol. doi: 10.1002/jmv.25884. doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obi AT, Tignanelli CJ, Jacobs BN, Arya S, Park PK, Wakefield TW, Henke PK, Napolitano LM. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord 20197317–324doi: 10.1016/j.jvsv.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 25.Nguyen T, Kyle UG, Jaimon N, Tcharmtchi MH, Coss-Bu JA, Lam F, Teruya J, Loftis L. Coinfection with Staphylococcus aureus increases risk of severe coagulopathy in critically ill children with influenza A (H1N1) virus infection. Crit Care Med 2012403246–3250doi: 10.1097/CCM.0b013e318260c7f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA 2018319698–710doi: 10.1001/jama.2017.21907 [DOI] [PubMed] [Google Scholar]
- 27.Fanelli V, Ranieri VM. Mechanisms and clinical consequences of acute lung injury. Ann Am Thorac Soc 201512suppl 1S3–S8doi: 10.1513/AnnalsATS.201407-340MG [DOI] [PubMed] [Google Scholar]
- 28.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 20171951253–1263doi: 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 29.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 20003421301–1308doi: 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 30.Lew TW, Kwek TK, Tai D, Earnest A, Loo S, Singh K, Kwan KM, Chan Y, Yim CF, Bek SL, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA 2003290374–380doi: 10.1001/jama.290.3.374 [DOI] [PubMed] [Google Scholar]
- 31.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, et al. ; the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 20203232052–2059doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 20202011299–1300doi: 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 20202201–13doi: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010140805–820doi: 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 35.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teijaro JR. Cytokine storms in infectious diseases. Semin Immunopathol 201739501–503doi: 10.1007/s00281-017-0640-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 20168959–70doi: 10.2217/imt-2016-0020 [DOI] [PubMed] [Google Scholar]
- 38.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 201739529–539doi: 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo XJ, Thomas PG. New fronts emerge in the influenza cytokine storm. Semin Immunopathol 201739541–550doi: 10.1007/s00281-017-0636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davey RT, Jr, Lynfield R, Dwyer DE, Losso MH, Cozzi-Lepri A, Wentworth D, Lane HC, Dewar R, Rupert A, Metcalf JA, et al. ; INSIGHT FLU 002 & 003 Study Groups The association between serum biomarkers and disease outcome in influenza A(H1N1)pdm09 virus infection: results of two international observational cohort studies. PLoS One 20138e57121.doi: 10.1371/journal.pone.0057121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rondina MT, Brewster B, Grissom CK, Zimmerman GA, Kastendieck DH, Harris ES, Weyrich AS. In vivo platelet activation in critically ill patients with primary 2009 influenza A(H1N1). Chest 20121411490–1495doi: 10.1378/chest.11-2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 202020355–362doi: 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020395497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 20201302620–2629doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 20203231061–1069doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 20203951054–1062doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rondina MT, Tatsumi K, Bastarache JA, Mackman N. Microvesicle tissue factor activity and interleukin-8 levels are associated with mortality in patients with influenza A/H1N1 infection. Crit Care Med 201644e574–8doi: 10.1097/CCM.0000000000001584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alattar R, Ibrahim TBH, Shaar SH, Abdalla S, Shukri K, Daghfal JN, Khatib MY, Aboukamar M, Abukhattab M, Alsoub HA, et al. Tocilizumab for the treatment of severe coronavirus disease 2019 [published online May 5, 2020]. J Med Virol. doi: 10.1002/jmv.25964. doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 202011710970–10975doi: 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook DJ, Crowther MA. Thromboprophylaxis in the intensive care unit: focus on medical-surgical patients. Crit Care Med 201038suppl 2S76–S82doi: 10.1097/CCM.0b013e3181c9e344 [DOI] [PubMed] [Google Scholar]
- 52.Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD. Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis 201152e14–e17doi: 10.1093/cid/ciq125 [DOI] [PubMed] [Google Scholar]
- 53.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Umapathi T, Kor AC, Venketasubramanian N, Lim CC, Pang BC, Yeo TT, Lee CC, Lim PL, Ponnudurai K, Chuah KL, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol 20042511227–1231doi: 10.1007/s00415-004-0519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020181421–1424doi: 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, Parra-Virto A, Toledano-Macías M, Toledo-Samaniego N, García-García A, García-Fernández-Bravo I, Ji Z, de-Miguel-Diez J, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res 202019223–26doi: 10.1016/j.thromres.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020191148–150doi: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S; Lille ICU Haemostasis COVID-19 Group Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence [published online April 24, 2020]. Circulation doi: 10.1161/CIRCULATIONAHA.120.047430 [DOI] [PubMed] [Google Scholar]
- 59.Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020181743–1746doi: 10.1111/jth.14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020461089–1098doi: 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19 [published online May 5, 2020]. J Thromb Haemost. doi: 10.1111/jth.14888. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JC, Fogerty AE, Waheed A, Goodarzi K, Bendapudi P, Bornikova L, Gupta S, et al. COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection [published online June 3, 2020]. Blood. doi: 10.1182/blood.2020006520. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, Muscarella G, Orlandi M. Pulmonary embolism or pulmonary thrombosis in COVID-19? is the recommendation to use high-dose heparin for thromboprophylaxis justified? [published online April 29, 2020]. Thromb Haemost. doi: 10.1055/s-0040-1712097. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost 2020181324–1329doi: 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mei F, Fan J, Yuan J, Liang Z, Wang K, Sun J, Guan W, Huang M, Li Y, Zhang WW. Comparison of venous thromboembolism risks between COVID-19 pneumonia and community-acquired pneumonia patients. Arterioscler Thromb Vasc Biol 2020402332–2337doi: 10.1161/ATVBAHA.120.314779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chong PY, Chui P, Ling AE, Franks TJ, Tai DY, Leo YS, Kaw GJ, Wansaicheong G, Chan KP, Ean Oon LL, et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med 2004128195–204doi: 10.1043/1543-2165(2004)128<195:AODDTS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 67.Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet 20033611773–1778doi: 10.1016/s0140-6736(03)13413-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, Cai J, Li X, Kang W, Weng D, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 2003200282–289doi: 10.1002/path.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 202015700–704doi: 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 20208681–686doi: 10.1016/S2213-2600(20)30243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li G, Fox SE, Summa B, Hu B, Wenk C, Akmatbekov A, Harbert JL, Vander Heide RS, Brown JQ. Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy. Biorxiv. 2020 doi: 10.1101/2020.04.11.037473. [Google Scholar]
- 72.Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schroder AS, et al. Autopsy findings and venous thromboembolism in patients with COVID-19 [published online May 6, 2020]. Ann Intern Med. doi: 10.7326/M20-2003. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antoniak S. The coagulation system in host defense. Res Pract Thromb Haemost 20182549–557doi: 10.1002/rth2.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antoniak S, Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood 20141232605–2613doi: 10.1182/blood-2013-09-526277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001861327–1330 [PubMed] [Google Scholar]
- 76.Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M; Scientific and Standardization Committee on DIC, and the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost 2019171989–1994doi: 10.1111/jth.14578 [DOI] [PubMed] [Google Scholar]
- 77.Kiliç H, Kanbay A, Karalezli A, Hasanoğlu HC, Ateş C. Clinical characteristics of 75 pandemic H1N1 influenza patients from Turkey; risk factors for fatality. Turk J Med Sci 201545562–567doi: 10.3906/sag-1401-111 [DOI] [PubMed] [Google Scholar]
- 78.Wang ZF, Su F, Lin XJ, Dai B, Kong LF, Zhao HW, Kang J. Serum D-dimer changes and prognostic implication in 2009 novel influenza A(H1N1). Thromb Res 2011127198–201doi: 10.1016/j.thromres.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 79.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 20033481986–1994doi: 10.1056/NEJMoa030685 [DOI] [PubMed] [Google Scholar]
- 80.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 202018844–847doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020181747–1751doi: 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020181738–1742doi: 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gris JC, Quere I, Perez-Martin A, Lefrant JY, Sotto A. Uncertainties on the prognostic value of D-dimers in COVID-19 patients [published online April 28, 2020]. J Thromb Haemost. doi: 10.1111/jth.14876. doi: 10.1111/jth.14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong RS, Wu A, To KF, Lee N, Lam CW, Wong CK, Chan PK, Ng MH, Yu LM, Hui DS, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 20033261358–1362doi: 10.1136/bmj.326.7403.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drosten C. Is MERS another SARS? Lancet Infect Dis 201313727–728doi: 10.1016/S1473-3099(13)70159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Abdallat MM, Payne DC, Alqasrawi S, Rha B, Tohme RA, Abedi GR, Al Nsour M, Iblan I, Jarour N, Farag NH, et al. ; Jordan MERS-CoV Investigation Team Hospital-associated outbreak of middle east respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis 2014591225–1233doi: 10.1093/cid/ciu359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van den Brand JM, Smits SL, Haagmans BL. Pathogenesis of middle east respiratory syndrome coronavirus. J Pathol 2015235175–184doi: 10.1002/path.4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hwang SM, Na BJ, Jung Y, Lim HS, Seo JE, Park SA, Cho YS, Song EH, Seo JY, Kim SR, et al. Clinical and laboratory findings of middle east respiratory syndrome coronavirus infection. Jpn J Infect Dis 201972160–167doi: 10.7883/yoken.JJID.2018.187 [DOI] [PubMed] [Google Scholar]
- 89.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020395507–513doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gralinski LE, Bankhead A, III, Jeng S, Menachery VD, Proll S, Belisle SE, Matzke M, Webb-Robertson BJ, Luna ML, Shukla AK, et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio 20134e00271–13doi: 10.1128/mBio.00271-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antoniak S, Tatsumi K, Hisada Y, Milner JJ, Neidich SD, Shaver CM, Pawlinski R, Beck MA, Bastarache JA, Mackman N. Tissue factor deficiency increases alveolar hemorrhage and death in influenza A virus-infected mice. J Thromb Haemost 2016141238–1248doi: 10.1111/jth.13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Albuquerque N, Baig E, Ma X, Zhang J, He W, Rowe A, Habal M, Liu M, Shalev I, Downey GP, et al. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J Virol 20068010382–10394doi: 10.1128/JVI.00747-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, Gibson-Corley KN, Meyerholz DK, McCray PB., Jr. Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 2016213712–722doi: 10.1093/infdis/jiv499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 20207e438–e440doi: 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020752950–2973doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Becker RC. COVID-19 update: COVID-19-associated coagulopathy. J Thromb Thrombolysis 20205054–67doi: 10.1007/s11239-020-02134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 20201352033–2040doi: 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bikdeli B, Madhavan MV, Gupta A, Jimenez D, Burton JR, Der Nigoghossian C, Chuich T, Nouri SN, Dreyfus I, Driggin E, et al. ; Global COVID-19 Thrombosis Collaborative Group. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research [published online May 30, 2020]. Thromb Haemost doi: 10.1055/s-0040-1713152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 201838709–725doi: 10.1161/ATVBAHA.117.309846 [DOI] [PubMed] [Google Scholar]
- 101.Welty-Wolf KE, Carraway MS, Ortel TL, Piantadosi CA. Coagulation and inflammation in acute lung injury. Thromb Haemost 20028817–25 [PubMed] [Google Scholar]
- 102.Welty-Wolf KE, Carraway MS, Idell S, Ortel TL, Ezban M, Piantadosi CA. Tissue factor in experimental acute lung injury. Semin Hematol 2001384 suppl 1235–38doi: 10.1053/shem.2001.29505 [DOI] [PubMed] [Google Scholar]
- 103.Sebag SC, Bastarache JA, Ware LB. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol 2011121481–1496doi: 10.2174/138920111798281171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Idell S, Gonzalez K, Bradford H, MacArthur CK, Fein AM, Maunder RJ, Garcia JG, Griffith DE, Weiland J, Martin TR. Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome. Contribution of tissue factor associated with factor VII. Am Rev Respir Dis 19871361466–1474doi: 10.1164/ajrccm/136.6.1466 [DOI] [PubMed] [Google Scholar]
- 105.Bastarache JA, Wang L, Geiser T, Wang Z, Albertine KH, Matthay MA, Ware LB. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax 200762608–616doi: 10.1136/thx.2006.063305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shibamiya A, Hersemeyer K, Schmidt Wöll T, Sedding D, Daniel JM, Bauer S, Koyama T, Preissner KT, Kanse SM. A key role for toll-like receptor-3 in disrupting the hemostasis balance on endothelial cells. Blood 2009113714–722doi: 10.1182/blood-2008-02-137901 [DOI] [PubMed] [Google Scholar]
- 107.Beutler BA. TLRs and innate immunity. Blood 20091131399–1407doi: 10.1182/blood-2008-07-019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Totura AL, Whitmore A, Agnihothram S, Schäfer A, Katze MG, Heise MT, Baric RS. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio 20156e00638–e00615doi: 10.1128/mBio.00638-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neumann FJ, Ott I, Marx N, Luther T, Kenngott S, Gawaz M, Kotzsch M, Schömig A. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol 1997173399–3405doi: 10.1161/01.atv.17.12.3399 [DOI] [PubMed] [Google Scholar]
- 110.Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res 2005961233–1239doi: 10.1161/01.RES.0000171805.24799.fa [DOI] [PubMed] [Google Scholar]
- 111.Parry GC, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler Thromb Vasc Biol 199515612–621doi: 10.1161/01.atv.15.5.612 [DOI] [PubMed] [Google Scholar]
- 112.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, et al. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation 20001012382–2387doi: 10.1161/01.cir.101.20.2382 [DOI] [PubMed] [Google Scholar]
- 113.Müller DN, Mervaala EM, Dechend R, Fiebeler A, Park JK, Schmidt F, Theuer J, Breu V, Mackman N, Luther T, et al. Angiotensin II (AT(1)) receptor blockade reduces vascular tissue factor in angiotensin II-induced cardiac vasculopathy. Am J Pathol 2000157111–122doi: 10.1016/S0002-9440(10)64523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Key NS, Vercellotti GM, Winkelmann JC, Moldow CF, Goodman JL, Esmon NL, Esmon CT, Jacob HS. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci USA 1990877095–7099doi: 10.1073/pnas.87.18.7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tatsumi K, Antoniak S, Subramaniam S, Gondouin B, Neidich SD, Beck MA, Mickelson J, Monroe DM, III, Bastarache JA, Mackman N. Anticoagulation increases alveolar hemorrhage in mice infected with influenza A. Physiol Rep. 2016;4:e13071. doi: 10.14814/phy2.13071. doi: 10.14814/phy2.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bautista-Vargas M, Bonilla-Abadia F, Canas CA. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19 [published online June 9, 2020]. J Thromb Thrombolysis. doi: 10.1007/s11239-020-02172-x. doi: 10.1007/s11239-020-02172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DiNicolantonio JJ, McCarty M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart. 2020;7:e001337. doi: 10.1136/openhrt-2020-001337. doi: 10.1136/openhrt-2020-001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gailani D, Renné T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost 200751106–1112doi: 10.1111/j.1538-7836.2007.02446.x [DOI] [PubMed] [Google Scholar]
- 119.Schmaier AH. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am J Physiol Regul Integr Comp Physiol 2003285R1–13doi: 10.1152/ajpregu.00535.2002 [DOI] [PubMed] [Google Scholar]
- 120.Pixley RA, De La Cadena R, Page JD, Kaufman N, Wyshock EG, Chang A, Taylor FB, Jr, Colman RW. The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. In vivo use of a monoclonal anti-factor XII antibody to block contact activation in baboons. J Clin Invest 19939161–68doi: 10.1172/JCI116201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jansen PM, Pixley RA, Brouwer M, de Jong IW, Chang AC, Hack CE, Taylor FB, Jr, Colman RW. Inhibition of factor XII in septic baboons attenuates the activation of complement and fibrinolytic systems and reduces the release of interleukin-6 and neutrophil elastase. Blood 1996872337–2344 [PubMed] [Google Scholar]
- 122.Silasi R, Keshari RS, Lupu C, Van Rensburg WJ, Chaaban H, Regmi G, Shamanaev A, Shatzel JJ, Puy C, Lorentz CU, et al. Inhibition of contact-mediated activation of factor XI protects baboons against S aureus-induced organ damage and death. Blood Adv 20193658–669doi: 10.1182/bloodadvances.2018029983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Björkqvist J, de Maat S, Lewandrowski U, Di Gennaro A, Oschatz C, Schönig K, Nöthen MM, Drouet C, Braley H, Nolte MW, et al. Defective glycosylation of coagulation factor XII underlies hereditary angioedema type III. J Clin Invest 20151253132–3146doi: 10.1172/JCI77139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van de Veerdonk FL, Netea MG, van Deuren M, van der Meer JW, de Mast Q, Bruggemann RJ, van der Hoeven H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife. 2020;9:e57555. doi: 10.7554/eLife.57555. doi: 10.7554/eLife.57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ozolina A, Sarkele M, Sabelnikovs O, Skesters A, Jaunalksne I, Serova J, Ievins T, Bjertnaes LJ, Vanags I. Activation of coagulation and fibrinolysis in acute respiratory distress syndrome: a prospective pilot study. Front Med (Lausanne) 2016;3:64. doi: 10.3389/fmed.2016.00064. doi: 10.3389/fmed.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Idell S, Peters J, James KK, Fair DS, Coalson JJ. Local abnormalities of coagulation and fibrinolytic pathways that promote alveolar fibrin deposition in the lungs of baboons with diffuse alveolar damage. J Clin Invest 198984181–193doi: 10.1172/JCI114139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003285L20–L28doi: 10.1152/ajplung.00312.2002 [DOI] [PubMed] [Google Scholar]
- 128.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med 2007351821–1828doi: 10.1097/01.CCM.0000221922.08878.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu YP, Wei R, Liu ZH, Chen B, Lisman T, Ren DL, Han JJ, Xia ZL, Zhang FS, Zhang FS, et al. Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb Haemost 200696100–101doi: 10.1160/TH05-12-0827 [DOI] [PubMed] [Google Scholar]
- 130.Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost 2020181548–1555doi: 10.1111/jth.14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost 2020181752–1755doi: 10.1111/jth.14828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res 2018122337–351doi: 10.1161/CIRCRESAHA.117.310795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koupenova M, Corkrey HA, Vitseva O, Manni G, Pang CJ, Clancy L, Yao C, Rade J, Levy D, Wang JP, et al. The role of platelets in mediating a response to human influenza infection. Nat Commun. 2019;10:1780. doi: 10.1038/s41467-019-09607-x. doi: 10.1038/s41467-019-09607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost 2020181469–1472doi: 10.1111/jth.14848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koupenova M. Potential role of platelets in COVID-19: implications for thrombosis. Res Pract Thromb Haemost 20204737–740doi: 10.1002/rth2.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Armstrong SM, Darwish I, Lee WL. Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection. Virulence 20134537–542doi: 10.4161/viru.25779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feldmann A, Schäfer MK, Garten W, Klenk HD. Targeted infection of endothelial cells by avian influenza virus A/FPV/Rostock/34 (H7N1) in chicken embryos. J Virol 2000748018–8027doi: 10.1128/jvi.74.17.8018-8027.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020181905–913.e7doi: 10.1016/j.cell.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 20203951417–1418doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gustafson D, Raju S, Wu R, Ching C, Veitch S, Rathnakumar K, Boudreau E, Howe KL, Fish JE. Overcoming barriers: the endothelium as a linchpin of coronavirus disease 2019 pathogenesis? Arterioscler Thromb Vasc Biol. 2020401818–1829doi: 10.1161/ATVBAHA.120.314558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol 202020389–391doi: 10.1038/s41577-020-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol 20091837441–7450doi: 10.4049/jimmunol.0902497 [DOI] [PubMed] [Google Scholar]
- 143.Camp JV, Jonsson CB. A role for neutrophils in viral respiratory disease. Front Immunol. 2017;8:550. doi: 10.3389/fimmu.2017.00550. doi: 10.3389/fimmu.2017.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peiró T, Patel DF, Akthar S, Gregory LG, Pyle CJ, Harker JA, Birrell MA, Lloyd CM, Snelgrove RJ. Neutrophils drive alveolar macrophage IL-1β release during respiratory viral infection. Thorax 201873546–556doi: 10.1136/thoraxjnl-2017-210010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell 2013154197–212doi: 10.1016/j.cell.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 201818134–147doi: 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 147.Kulkarni U, Zemans RL, Smith CA, Wood SC, Deng JC, Goldstein DR. Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality. Mucosal Immunol 201912545–554doi: 10.1038/s41385-018-0115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hornick EE, Banoth B, Miller AM, Zacharias ZR, Jain N, Wilson ME, Gibson-Corley KN, Legge KL, Bishop GA, Sutterwala FS, et al. Nlrp12 mediates adverse neutrophil recruitment during influenza virus infection. J Immunol 20182001188–1197doi: 10.4049/jimmunol.1700999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 20131334–45doi: 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- 150.Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renné C, Renné T, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 20173581202–1206doi: 10.1126/science.aam8897 [DOI] [PubMed] [Google Scholar]
- 151.Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol 2019391724–1738doi: 10.1161/ATVBAHA.119.312463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu L, Liu L, Zhang Y, Pu L, Liu J, Li X, Chen Z, Hao Y, Wang B, Han J, et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza a infection. J Infect Dis 2018217428–437doi: 10.1093/infdis/jix475 [DOI] [PubMed] [Google Scholar]
- 153.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Dassler-Plenker J, Guerci P, Huynh C, Knight JS, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mastellos DC, Ricklin D, Lambris JD. Clinical promise of next-generation complement therapeutics. Nat Rev Drug Discov 201918707–729doi: 10.1038/s41573-019-0031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, Whitmore A, Heise MT, Baric RS. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 20189e01753–18doi: 10.1128/mBio.01753-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang Y, Zhao G, Song N, Li P, Chen Y, Guo Y, Li J, Du L, Jiang S, Guo R, et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7:77. doi: 10.1038/s41426-018-0063-8. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19-related systemic thrombosis? Circulation 20201411739–1741doi: 10.1161/CIRCULATIONAHA.120.047419 [DOI] [PubMed] [Google Scholar]
- 159.Risitano AM, Mastellos DC, Huber-Lang M, Yancopoulou D, Garlanda C, Ciceri F, Lambris JD. Complement as a target in COVID-19? Nat Rev Immunol 202020343–344doi: 10.1038/s41577-020-0320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


