Background:
COVID-19 and its social responses threaten the health of people living with HIV. We conducted a rapid-response interview to assess COVID-19 protective behaviors of people living with HIV and the impact of their responses on HIV-related health care.
Method:
Men and women living with HIV (N = 162) aged 20–37 years participating in a longitudinal study of HIV treatment and care completed routine study measures and an assessment of COVID-19–related experiences.
Results:
At baseline, most participants demonstrated HIV viremia, markers indicative of renal disorders, and biologically confirmed substance use. At follow-up, in the first month of responding to COVID-19, engaging in more social distancing behaviors was related to difficulty accessing food and medications and increased cancelation of health care appointments, both by self and providers. We observed antiretroviral therapy adherence had improved during the initial month of COVID-19 response.
Conclusions:
Factors that may pose added risk for COVID-19 severity were prevalent among people living with HIV, and those with greater risk factors did not practice more COVID-19 protective behaviors. Social distancing and other practices intended to mitigate the spread of COVID-19 interfered with HIV care, and impeded access to food and medications, although an immediate adverse impact on medication adherence was not evident. These results suggest social responses to COVID-19 adversely impacted the health care of people living with HIV, supporting continued monitoring to determine the long-term effects of co-occurring HIV and COVID-19 pandemics.
Key Words: HIV continuum of care, COVID-19, coronavirus, food insecurity
INTRODUCTION
The SARS-CoV-2 pandemic has rapidly emerged as a significant threat to public health, with the greatest degree of morbidity and mortality occurring among the elderly and individuals with underlying chronic health conditions, including people with compromised immune systems.1–3 Although the factors and combinations of underlying conditions that determine the severity of the SARS-CoV-2 disease (COVID-19) are under investigation, immune system dysfunction and other co-occurring chronic conditions raise concerns for COVID-19 severity.4 For people living with HIV, unsuppressed virus is the hallmark of HIV infection progression and may increase COVID-19 severity. In addition, indicators of renal disease that are commonly observed with HIV infection can lead to more severe COVID-19 outcomes.5 Liu et al,6 for example, found that COVID-19 severity is significantly greater in patients with urinary glucose and protein markers. Furthermore, conditions of poverty, particularly poor nutrition resulting from food insecurity, can impede immune functioning.7
Substance use also raises concerns for increased COVID-19 severity. Tobacco use, particularly cigarette smoking, is associated with COVID-19 severity,8 and tobacco use may interact with other substances to further compromise the immune system.9,10 Alcohol and other drug use also suppresses immune responses,11–13 particularly among people living with HIV.14 Along with underlying health conditions, substance use raises concerns for COVID-19 morbidity and mortality in people living with HIV.15 High prevalence of substance use and co-occurring underlying health conditions have the potential to amplify the severity of COVID-19 in people living with HIV.16,17
The increased vulnerability for COVID-19 severity in people living with HIV shines a light on the necessity of adopting COVID-19 protective behaviors, avoiding public gatherings, reducing social contacts, and physical distancing. COVID-19 protective behaviors were recommended in early March 2020 as some states (eg, California, Washington, and New York) responded to the unfolding health crisis. By contrast and in the absence of a national COVID-19 strategy, the state of Georgia was late in response. On March 13, 2020, the US government declared a national state of emergency, and on March 15, 2020, the Centers for Disease Control and Prevention issued recommendations to avoid social gatherings.18 Initial reports from HIV clinical settings indicated the potential for interruptions in essential HIV care services.19 Concerns have also been raised that stay-at-home orders and physical distancing could exacerbate what is already a high prevalence of food insecurity among people living with HIV.20
Here, we report the results of a rapid-response interview with men and women living with HIV in Atlanta, GA, conducted at the start of the US COVID-19 epidemic. There were 2397 diagnosed cases and 12 deaths in the state of GA on March 17, 2020, and 20,058 diagnosed cases and 749 deaths 1 month later.21 During the final week of data collection for the current study, the 7-day average number of COVID-19 diagnoses in the state of Georgia was greater than 600, and the average daily deaths was greater than 25. More than 40% of cases and 40% of deaths in Georgia occurred in the Atlanta metro area. The City of Atlanta acted to issue orders for protective policies before the state government. On March 16, 2020, the Mayor of Atlanta issued a state of emergency, and on March 19, 2020, all nonessential businesses closed,22 which remained in effect throughout the data collection period. We examined social responses to COVID-19 including physical distancing and reducing social contacts in response to the earliest public alerts. We specifically tested the association between COVID-19 protective actions and their impact on HIV-related care and treatment.
METHODS
Participants
Participants in the current study were men and women living with HIV in Atlanta, GA, who were between the ages of 20 and 37 years and screened positive for active substance use. The current sample was actively participating in an ongoing antiretroviral therapy (ART) adherence study at the time of the COVID-19 outbreak.
Procedures
Participants were taking part in an 18-month longitudinal HIV treatment engagement and adherence study. Men and women living with HIV were recruited through social media websites, targeted online ads, and a participant-driven adaptation of snowball-sampling techniques. Specifically, participants were encouraged to refer their HIV-positive friends to the study and were offered a modest incentive for their efforts.
After informed consent, participants completed measures of demographic and health characteristics, including audio-computer–assisted self-interviews, blood samples for HIV viral load testing, and urine samples for substance use testing and urinary health markers. After the baseline assessment, participants were contacted monthly to complete health care engagement and health behavior interviews. All interviews were conducted by telephone as part of the original study protocol using methods consistent with best practices for conducting remote research.23 During a 1-month period, median 9 months from baseline, participants due for their routine telephone interview completed questions regarding their experience with COVID-19. The University of Connecticut Institutional Review Board approved all study procedures.
Measures
Computerized Self-Interviews
Participants were asked their self-identified gender, race, age, years of education, the stability of their current housing, income, and employment status. We also administered the Centers for Epidemiological Studies Depression scale to assess symptoms of depression (alpha = 0.87) and the 3-item consumption subscale of the Alcohol Use Disorders Identification Test (AUDIT-C).24,25 To assess food insecurity, we used items adapted from the US Food Security Scale that have been validated in the past research and used by the US Census Bureau.26
History of COVID-19 Severity Risks
We constructed an unweighted index of 6 underlying health indicators for increased risk of COVID-19 morbidity and mortality. The markers include HIV viremia, renal health markers, tobacco, alcohol, cannabis, and other drug use that were collected at the baseline assessment.
HIV Viremia
To determine HIV RNA concentrations (viral load), participants provided 80 µL of fingerstick blood for dried blood spots collected in HemaSpot HF devices that were frozen before laboratory delivery. HIV-1 viral load testing was conducted using the Abbott RealTime HIV-1 assay, a reverse transcription PCR assay performed on the automated Abbott m2000 platform (Abbott Molecular Inc, Des Plaines, IL).27 The target sequence for the assay is the highly conserved pol/integrase region of the HIV-1 genome. The limit of detection of the assay is 2.92 log copies/mL, and it can quantify up to 7.0 log copies/mL.28 All samples required an upfront processing to improve assay sensitivity before subjecting to RNA extraction. Forty-nine (30%) participants were unable or unwilling to provide a blood sample and were given the option to provide a recent viral load test result obtained within 90 days of the assessment from a health care provider. Because HIV viremia indicates greater immune suppression, a detectable viral load was coded as an indicator of COVID-19 severity risk.
Renal Health Markers
Urine specimens were collected and tested on site for 4 health markers: glucose, an indicator of diabetes or renal disease; leukocytes, an indicator of urinary system inflammation29,30; protein as indicative of kidney disease; and blood as an indicator of multiple disease processes.31 We used any positive urine health indicator as a marker for a potential underlying renal condition.
Substance Use
Because any substance use is a known immune suppressant, we conducted a multipanel urine dip test to detect common drug use. The test strip uses a lateral flow chromatographic immunoassay for the qualitative detection of 12 drugs and drug metabolites (Redwood Toxicology Labs—Reditest-12). These tests are FDA approved and are reliable and valid for initial screening of drug use in the previous 72–96 hours. We considered THC as a separate marker because it is prevalent and its administration is typically smoked, posing specific risks for compromised lung function.32 For alcohol use, we used the 500 ng/mL cutoff for ethyl glucuronide from a separate dip test with detection of ethyl glucuronide up to 80 hours after drinking.33 Finally, participants reported smoked tobacco products with items adapted from the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST).34
Health Care and HIV Treatment
Telephone interviews assessed current HIV care engagement, treatment status, and ART adherence. Measures of health care engagement were adapted from the HIV Cost and Services Utilization Study consortium35 and include attending and not attending scheduled care appointments. To assess ART adherence, we used the 3-item self-report instrument for retrospective adherence (IRA) developed and validated by Wilson et al.36 The items represent the number of days medications were taken over the previous 7 days, the frequency of taking medications as directed, and a self-perception rating of how well medications were taken over the previous week. We used methods suggested by Wilson et al to convert scores for each item on a scale of 0–100 using linear transformations and calculating the mean to a single adherence score with a range from 0 to 100, interpreted as percent adherence over the past week (alpha = 0.73). Wilson et al36 found that the IRA correlates 0.74 with electronically monitored ART adherence. The IRA was administered during the phone assessment, as well as the phone assessment conducted the previous month.
COVID-19 Protective Actions
Participants reported whether they heard of the novel coronavirus/COVID-19, how much they had heard about it, whether they had been tested for COVID-19, and whether they had been diagnosed with COVID-19. We also asked participants whether they had engaged in 5 recommended actions to mitigate their risk for contracting COVID-19. All behaviors assessed were recommended by the US Federal Government and the state of GA to mitigate the spread of SARS-CoV-2 at the time the study commenced. The specific actions are shown in the results. We created an index of COVID-19 protective actions by summing the use/nonuse of the 5 behaviors. We also assessed participant concerns that they may contract COVID-19 using a 100-point rating scale in response to the question: “From 0 to 100, how concerned are you about catching the new coronavirus, with 0 = not at all concerned and 100 = extremely concerned.”
COVID-19 Interruptions to Care
We asked participants whether they had been unable to get the food they need, get to the pharmacy, and get their medicines in relation to their response to COVID-19. Participants were also asked whether HIV care providers and other service providers had canceled any of their care appointments because of COVID-19.
Data Analyses
We conducted descriptive analyses for participants identifying as male and female on demographic, health, and COVID-19 protective behaviors using contingency table χ2 tests for categorical variables and independent t tests for continuous measures. We also formed 2 groups based on relatively fewer (≤2, N = 65) and relatively greater (≥3, N = 97) COVID-19 protective behaviors and examined differences between groups using contingency table χ2 tests for categorical variables and independent t tests for continuous measures. Poisson regression was used to test a multivariable model predicting the number of COVID-19 protective behaviors from participant characteristics and the history of COVID-19 severity risks. ART adherence for the assessment period and the previous month was compared using a dependent (paired) t test. All statistical tests defined significance by P < 0.05.
RESULTS
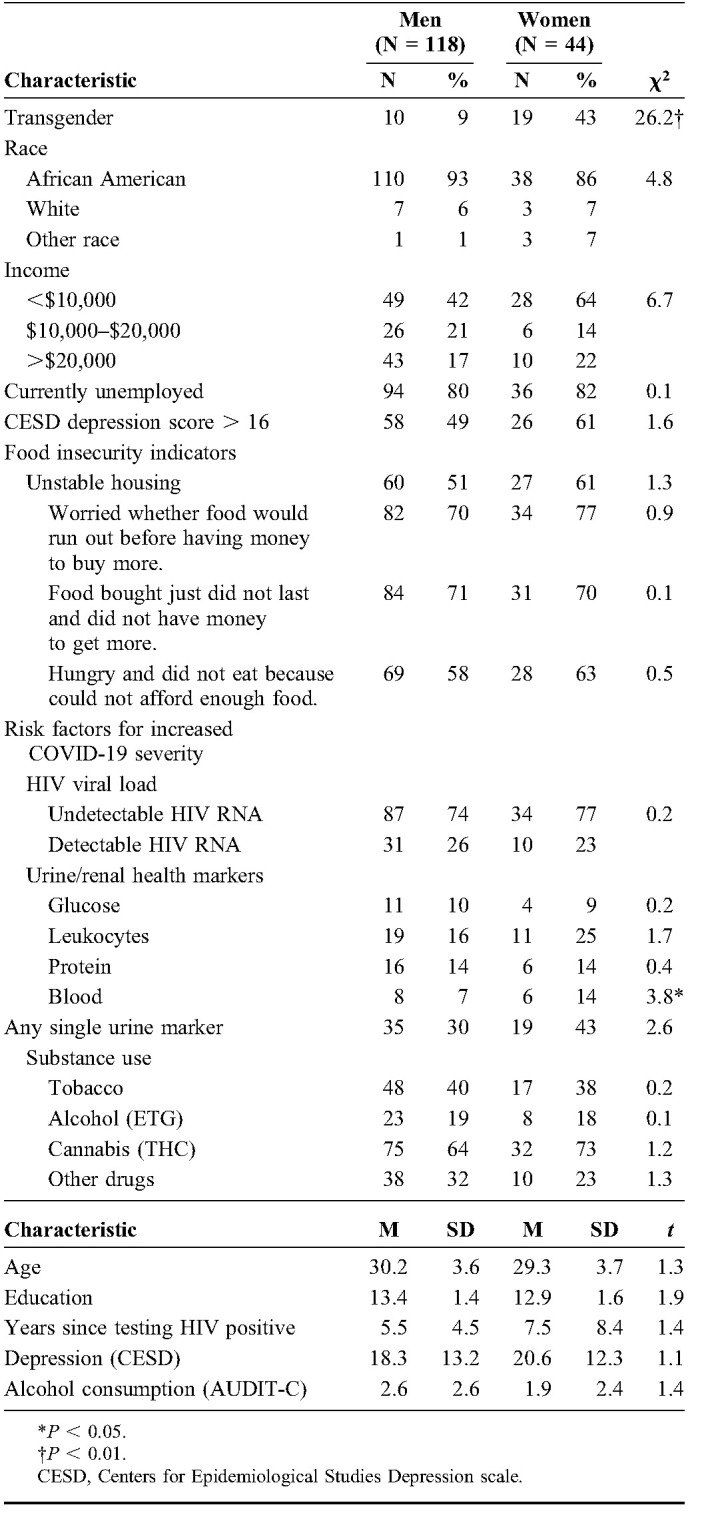
Table 1 shows the participant demographic and health characteristics. Most participants demonstrated a history of underlying health risks for severe COVID-19. As shown in Table 1, half of participants did not know their CD4 cell count, an indicator of not being fully engaged in HIV care. Among those participants who did know their most recent CD4 cell count, more than 1 in 4 indicated CD4 cell counts under 350 cells/mm3. In addition, 1 in 4 participants were not HIV suppressed and 1 in 3 tested positive for at least 1 underlying renal condition. Most participants were actively using substances, including 40% tobacco, 19% alcohol, 65% cannabis (THC), and 29% testing positive for other drugs. Overall, 90% of our participants had at least 1 indicator of immune suppression beyond their HIV status.
TABLE 1.
Participant Characteristics Among Men and Women Living With HIV
COVID-19 Protective Behaviors
Nearly all participants reported staying indoors and away from public places to avoid contracting COVID-19. Table 2 shows the frequency of COVID-19 protective behaviors in the early days of the pandemic. Most participants had canceled plans, asked others to stay away, and avoided public transportation to mitigate their risk for contracting COVID-19. On average, participants had taken multiple steps to reduce their risks. However, there were gender differences in COVID-19 protective actions, with women more likely to stay indoors and away from public places than men. Consistent with this pattern, women also rated greater concern about contracting COVID-19 than men. Poisson regression indicated that participant gender, Wald χ2 = 8.9, P < 0.01, and level of concern for contracting COVID-19, Wald χ2 = 31.2, P < 0.001, predicted the number of COVID-19 protective behaviors practiced; women engaged in more protective behaviors than men (B = −0.255, se = 0.085, 95% confidence interval: −0.422 to −0.088), and greater concern was related to engaging in more protective behaviors (B = 0.006, se = 0.001, 95% confidence interval: 0.004 to 0.008). The remaining factors in the model were not significantly associated with COVID-19 protective behaviors: years of education, Wald χ2 = 2.1, ns, years since testing HIV positive, Wald χ2 = 0.1, ns, and number of risks for COVID-19 severity, Wald χ2 = 0.6, ns (Table 2).
TABLE 2.
Physical Distancing and Other COVID-19 Protective Behaviors Among Men and Women Living With HIV

COVID-19 and Food Security
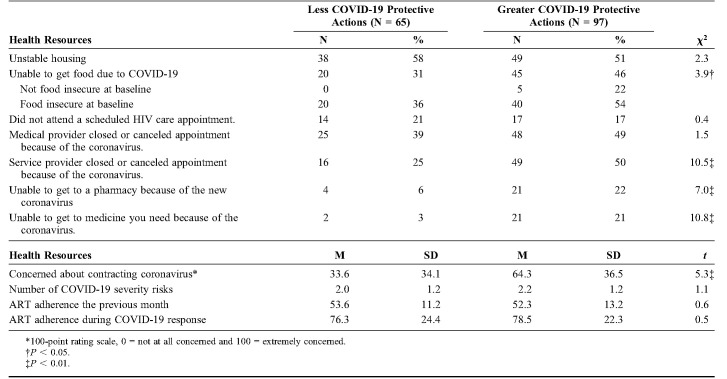
Results showed that 40% of participants reported being unable to access food because of the COVID-19 outbreak. Participants who engaged in greater protective behaviors also experienced more difficulty accessing food compared with persons who engaged in less protective behaviors. Among participants who reported being unable to access food due to COVID-19, 22% were not food insecure at baseline (Table 3).
TABLE 3.
Impacts of COVID-19 Protective Actions on Food Security, Health Care Retention, and ART Adherence
COVID-19 and Health Care
Nearly 1 in 5 participants (n = 31, 19%) indicated that they had missed a scheduled HIV care appointment in the previous 30 days. When asked the reason for their missed appointment, 14 (45%) spontaneously stated the reason was directly related to COVID-19. In addition to their missing a scheduled medical appointment, 45% of participants reported that a medical provider canceled an appointment, and 40% reported a nonmedical service provider contacted them to cancel an appointment due to COVID-19. Participants who engaged in greater COVID-19 protective behaviors also experienced more nonmedical service cancelations than participants engaging in fewer COVID-19 protective behaviors (Table 3). Practicing greater COVID-19 protective behaviors was also related to an inability to get to the pharmacy and an inability to access medications. There were no associations between COVID-19 protective actions and ART adherence in the month before, or the month during, the COVID-19 assessment. However, adherence improved significantly in the month since the onset of COVID-19 protective actions, t (159) = 17.2, P <0.01.
DISCUSSION
COVID-19 adds to what is already a complex matrix of co-occurring epidemics and health disparities facing people living with HIV.37 Participants in the current study presented multiple challenges in managing their HIV infection before the emergence of COVID-19, including substance use, mental health problems, history of comorbidities, and food insecurity. Most participants were polysubstance users, demonstrated clinical indications of depression, and experienced food insecurity as severe as hunger. We found that all participants were aware of COVID-19 at the earliest days of response by their city officials. Nearly all participants were taking some steps to mitigate their risk for COVID-19. Their immediate response, however, exacerbated food insecurity, with more than 1 in 3 participants reporting difficulty accessing food, including individuals who were not previously food insecure reporting an inability to access food. COVID-19 protective behaviors also impeded their health care, created barriers to accessing medications, and disrupted social services. Similar impacts of COVID-19 on HIV care have been reported by others1,38 and have implications for widening what are already entrenched HIV-related health disparities.39
We also observed an unexpected significant increase in ART adherence over the 1 month of response to COVID-19. Participants may have improved their ART adherence out of health concerns or because they were home more, or possibly other reasons.40,41 This positive change occurred despite interruption in accessing medications. It is possible that this increase in adherence is a blip and will subside, especially if barriers in accessing medications are not resolved. Nevertheless, the heightened and uncontrollable health concerns brought by COVID-19 may have motivated health behaviors for which individuals do have control, such as taking their medications. Monitoring ART adherence over the course of COVID-19 should be a priority in ongoing studies.
The current findings should be interpreted in light of their methodological limitations. The sample for this study was one of convenience and cannot be considered representative of people living with HIV. Although large enough for the number of variables tested, the sample was relatively small. In addition, the sample was largely male and African American and therefore limited in its generalizability. In addition, we assessed substance use with biological testing and could not discern whether some drugs, particularly methamphetamine and cocaine, we smoked, with greater implications for complicating COVID-19. The study was undertaken in immediate response to the COVID-19 public health crisis, and our findings may be transient and specific to this time period. Although the results have implications for future spikes in COVID-19 outbreaks and potential future emerging infections, they may also be unique to this time period and this pandemic. Nevertheless, these findings have implications for managing HIV infection during the response to COVID-19.
People living with HIV may be at increased risk for a more severe clinical course of COVID-19, and our sample demonstrated a history of multiple factors that would likely contribute further to their risks for greater severity of COVID-19. Of particular concern were high rates of HIV viremia, smoking,8 other substance use,32 and depression symptoms.42 We found that at baseline 1-in-3 participants had evidence of a potential underlying renal condition. Of particular concern are 10% and 15% of participants testing positive for urinary glucose and protein, respectively, both of which are associated with COVID-19 severity.6 The accumulation of risk factors for severe COVID-19 among people living with HIV, who are already immune compromised, signals an urgent need to mitigate their exposure to SARS-CoV-2. We found that women engaged in more protective behaviors than men and that protective behaviors were associated with how concerned participants were about contracting COVID-19. However, there was no association between the number of underlying risks for severe COVID-19 and the number of protective actions taken. At the time of this study, public health messaging focused on increased COVID-19 risks for the elderly and people with cardiovascular disease, diabetes, and immune suppression.43 Although people with HIV will recognize their increased risks due to an immune suppressive condition, the added burden of smoking and other substance use, as well as underlying conditions common to HIV infection, have not been included in Centers for Disease Control and Prevention reports of severe case outcomes and have not been included in public health messaging.44 Patients should be fully informed of their risks for severe COVID-19 to understand the importance of long-term mitigation.
Footnotes
Supported by the National Institute on Drug Abuse Grant R01-DA033067.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Jiang H, Zhou Y, Tang W. Maintaining HIV care during the COVID-19 pandemic. Lancet HIV. 2020;7:e308–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco JL, Ambrosioni J, Garcia F, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7:e314–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adepoju P. Tuberculosis and HIV responses threatened by COVID-19. Lancet HIV. 2020;7:e319–e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozma MA, Maroufi P, Khodadadi E, et al. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez Med. 2020;28:153–165. [PubMed] [Google Scholar]
- 6.Liu R, Ma Q, Han H, et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin Chem Lab Med. 2020;58:1121–1124. [DOI] [PubMed] [Google Scholar]
- 7.de Pee S, Semba RD. Role of nutrition in HIV infection: review of evidence for more effective programming in resource-limited settings. Food Nutr Bull. 2010;31:S313–S344. [PubMed] [Google Scholar]
- 8.Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of Covid-19: a systemic review and meta-analysis. J Med Virol. 2020. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karoly HC, Bidwell LC, Mueller RL, et al. Investigating the relationships between alcohol consumption, cannabis use, and circulating cytokines: a preliminary analysis. Alcohol Clin Exp Res. 2018;42:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoptaw S, Stall R, Bordon J, et al. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. Int J STD AIDS. 2012;23:576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr T, Helms C, Grant K, et al. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrico AW, Horvath KJ, Grov C, et al. Double jeopardy: methamphetamine use and HIV as risk factors for COVID-19. AIDS Behav. 2020. doi: 10.1007/s10461-020-02854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang W, Luo Z, Martin L, et al. Drug use is associated with anti-CD4 IgG-mediated CD4+ T cell death and poor CD4+ T cell recovery in viral-suppressive HIV-infected individuals under antiretroviral therapy. Curr HIV Res. 2018;16:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monnig MA. Immune activation and neuroinflammation in alcohol use and HIV infection: evidence for shared mechanisms. Am J Drug Alcohol Abuse. 2017;43:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaydos J, McNally A, Guo R, et al. Alcohol abuse and smoking alter inflammatory mediator production by pulmonary and systemic immune cells. Am J Physiol Lung Cell Mol Physiol. 2016;310:L507–L518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drain PK, Garrett N. SARS-CoV-2 pandemic expanding in sub-Saharan Africa: considerations for COVID-19 in people living with HIV. EClinicalMedicine. 2020:100342. doi: 10.1016/j.eclinm.2020.100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Algarin AB, Varas-Rodriguez E, Valdivia C, et al. Symptoms, stress, and HIV-related care among older people living with HIV during the COVID-19 pandemic, Miami, Florida. AIDS Behav. 2020. Available at: 10.1007/s10461-020-02869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Today U. Three Months in: A Timeline of How COVID-19 Has Unfolded in the US 2020. Available at: https://www.usatoday.com/in-depth/news/nation/2020/04/21/coronavirus-updates-how-covid-19-unfolded-u-s-timeline/2990956001/. Accessed May 1, 2020. [Google Scholar]
- 19.Ridgway J, Schmitt J, Friedman E, et al. HIV care continuum and COVID-19 outcomes among people living with HIV during the COVID-19 pandemic, Chicago. IL. AIDS Behav. 2020. doi: 10.1007/s10461-020-02905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLinden T, Stover S, Hogg R. HIV and food insecurity: a syndemic amid the COVID-19 pandemic. AIDS Behav. 2020. doi: 10.1007/s10461-020-02904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health GDoP. Georgia Department of Public Health COVID-19 Daily Status Report 2020. Available at: https://dph.georgia.gov/covid-19-daily-status-report. Accessed May 1, 2020. [Google Scholar]
- 22.Atlanta Co. City of Atlanta Coronavirus Disease 2019 (COVID-19) Response 2020. Available at: https://www.atlantaga.gov/government/mayor-s-office/city-of-atlanta-covid-19-response.Accessed May 1, 2020. [Google Scholar]
- 23.Marhefka S, Lockhart E, Turner D. Achieve research continuity during social distancing by rapidly implementing individual and group videoconferencing with participants: key considerations, best practices, and protocols. AIDS Behav. 2020. doi: 10.1007/s10461-020-02837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addictions. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 25.Maisto SA, Conigliaro J, McNeil M, et al. An empirical investigation of the factor structure of the AUDIT. Psychol Assess. 2000;12:346–353. [DOI] [PubMed] [Google Scholar]
- 26.Cook JT, Frank DA. Food security, poverty, and human development in the United States. Ann N Y Acad Sci. 2008;1136:193–209. [DOI] [PubMed] [Google Scholar]
- 27.Molecular A. RealTime HIV-1 Assay. Abbott Park, IL: Abbott Laboratories; 2020. Available at: https://www.molecular.abbott/us/en/products/infectious-disease/realtime-hiv-1-viral-load. Accessed May 1, 2020. [Google Scholar]
- 28.Tang N, Pahalawatta V, Frank A, et al. HIV-1 viral load measurement in venous blood and fingerprick blood using Abbott RealTime HIV-1 DBS assay. J Clin Virol. 2017;92:56–61. [DOI] [PubMed] [Google Scholar]
- 29.Anderson BL, Wang CC, Delong AK, et al. Genital tract leukocytes and shedding of genital HIV type 1 RNA. Clin Infect Dis. 2008;47:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laisaar KT, Uusküla A, Sharma A, et al. Developing an adherence support intervention for patients on antiretroviral therapy in the context of the recent IDU-driven HIV/AIDS epidemic in Estonia. AIDS Care. 2013;25:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutter D, van Oudheusden AP, Hilvers AG, et al. A new test-strip for demonstrating erythrocytes and haemoglobin in urine (author's transl) [in German]. Dtsch Med Wochenschr. 1974;99:2332–2335. [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020. doi: 10.7326/M20-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kummer N, Wille SM, Poll A, et al. Quantification of EtG in hair, EtG and EtS in urine and PEth species in capillary dried blood spots to assess the alcohol consumption in driver's licence regranting cases. Drug Alcohol Depend. 2016;165:191–197. [DOI] [PubMed] [Google Scholar]
- 34.Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction. 2008;103:1039–1047. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham WE, Markson LE, Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. HCSUS Consortium. HIV Cost and Services Utilization. J Acquir Immune Defic Syndr. 2000;25:115–123. [DOI] [PubMed] [Google Scholar]
- 36.Wilson IB, Fowler FJ, Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2014;18:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiau S, Krause KD, Valera P, et al. The burden of COVID-19 in people living with HIV: a syndemic perspective. AIDS Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kay ES, Musgrove K. From HIV to coronavirus: AIDS service organizations adaptative responses to COVID-19, Birmingham, Alabama. AIDS Behav. 2020. doi: 10.1007/s10461-020-02879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdiserri RO, Holtgrave DR. Responding to pandemics: what we've learned from HIV/AIDS. AIDS Behav. 2020. doi: 10.1007/s10461-020-02859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kretchy IA, Asiedu-Danso M, Kretchy JP. Medication management and adherence during the COVID-19 pandemic: perspectives and experiences from low-and middle-income countries. Res Soc Adm Pharm. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marasca C, Ruggiero A, Fontanella G, et al. Telemedicine and support groups in order to improve the adherence to treatment and health related quality of life in patients affected by inflammatory skin conditions during COVID-19 emergency. Clin Exp Dermatol. 2020. doi: 10.1016/j.sapharm.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein MB. EDITORIAL: COVID-19 and anxiety and depression in 2020. Depress Anxiety. 2020;37:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Team CC-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]