Abstract
To gain further insight into the binding of the normal and variant human TSHβ subunits (TSHβ and TSHβv), we modeled the 2 monomeric proteins and studied their interaction with the TSH receptor ectodomain (TSHR-ECD) using molecular dynamics simulation Furthermore, analyzed their bioactivity in vitro using recombinant proteins to confirm that such binding was physiologically relevant. Examining the interaction of TSHβ and TSHβv with the TSHR-ECD model using molecular dynamic simulation revealed strong binding of these proteins to the receptor ECD. The specificity of TSHβ and TSHβv binding to the TSHR-ECD was examined by analyzing the hydrogen-bonding residues of these subunits to the FSH receptor ECD, indicating the inability of these molecules to bind to the FSH receptors. Furthermore, the modelling suggests that TSHβ and TSHβv proteins clasped the concave surface of the leucine rich region of the TSHR ECD in a similar way to the native TSH using dynamic hydrogen bonding. These mutually exclusive stable interactions between the subunits and ECD residues included some high-affinity contact sites corresponding to binding models of native TSH. Furthermore, we cloned TSHβ and TSHβv proteins using the entire coding ORF and purified the flag-tagged proteins. The expressed TSHβ subunit proteins retained bioactivity both in a coculture system as well as with immune-purified proteins. In summary, we showed that such interactions can result in a functional outcome and may exert physiological or pathophysiological effects in immune cells.
Keywords: molecular dynamics, hydrogen bonds, thyroid stimulating hormone, splice variant
TSH, which is primarily secreted from the anterior lobe of the pituitary gland, exists as noncovalently linked α and β subunits forming the heterodimeric (α/β) glycoprotein ligand for the TSH receptor (TSHR). Genetic organization of the native human TSHβ gene shows that it is localized to chromosome 1 and composed of 3 exons (exons 1-3), whereas the native mouse TSHβ gene is located in chromosome 3 and is composed of 5 exons (exons 1-5). The TSHβ gene can undergo differential splicing resulting in isoforms of TSHβ polypeptides encoded by exon 3 in humans and exon 5 in mice. The 9 amino acid N terminus region is known to function as the signal peptide (1) (Figure 1A and 1B).
Figure 1.
Gene organization of human and mouse TSHβ and TSHβv. (A) The human TSHβ subunit located on chromosome 1 consists of 3 exons, of which exon 1 is noncoding. The novel TSHβv is encoded only by exon 3 and a 21-base pair N terminus intronic segment shown here as signal peptide (SP). (B) Unlike the human TSHβ, the mouse TSHβ gene located on chromosome 3 consists of 5 exons, of which exons 1 through 3 are noncoding and exons 4 and 5 are the coding exons. The mouse novel TSHβv lacks exon 4 and is encoded by exon 5 only with a 21-base pair signal peptide. The corresponding protein sizes are indicated below each gene diagram.
The large extracellular domain of the TSHR (TSHR-ECD) is made up of the leucine-rich domain (LRD), which consists of α helices connected to 10 β-pleated sheets to form the concave floor of the LRD followed by a large hinge region (2). Models of heteromeric TSH, consisting of α and β subunits, show binding predominantly to the concave surface of the LRD and triggers activation of the TSHR resulting in thyroid cell growth and proliferation and synthesis and secretion of thyroid hormones. The TSHR-ECD, in addition to being the orthosteric site for TSH binding, is also the prime target for TSHR antibodies found in the autoimmune thyroid diseases (3, 4).
Previous studies from our laboratory (5, 6), and from others (7, 8), have clearly demonstrated that active TSH can exist as independent β subunits or its splice variant in mouse and humans. The human splice variant, TSHβv, is encoded by a partial open reading frame (ORF) consisting of exon 3 and a 27-nucleotide intronic sequence that forms its N terminus signal peptide in the truncated protein (1). Studies of this monomeric TSHβv protein have demonstrated its secretion by CD11+ lymphocytes and bone marrow-derived macrophages (ϕ) (6, 7) and has been shown to activate the TSHR. Furthermore, it has been shown that TSHβv is capable of inducing cAMP-mediated signaling in osteoblasts and osteoclasts (5). Studies by Klein et al. (8) have demonstrated that the mouse TSHβv is secreted in the thyroid by infiltrating lymphocytes in response to viral infection, whereas studies from our laboratory have shown that the variant protein was expressed in activated bone derived macrophages and could be regulated by T3 hormone (6).
These observations supported the concept that a bioactive TSHβ hormone can exist as a functional unit without coupling to an α subunit—a conclusion long considered artifactual when demonstrated after purification of bioactive β subunits from intact TSH by HPLC and thought to be due to trace contamination of the intact hormone. This concept found further support in our previous modeling data showing that the murine TSHβv is capable of binding to the TSHR-ECD with an affinity comparable to that of the native TSHβ (5). A recent study has also clearly outlined a molecular mechanism that could result in the formation of such TSHβ splice variants in immune cells (9), further strengthening the idea that an evolutionarily conserved heterodimer of TSH is not the only bioactive unit.
To gain further insight into the interactions of human TSHβ and TSHβv polypeptides with the TSHR-ECD, we carried out extensive molecular dynamic simulations of these human TSHβ proteins and mapped the bonding and probable contact sites of these monomeric polypeptides as well as confirming their bioactivity.
Materials and Methods
Molecular dynamics simulation of modeled TSHR-ECD and TSHβ proteins
The initial conformation of the TSHβ:TSHR-ECD complex was prepared using the published crystal structure of the FSH bound to the FSH receptor (FSHR) ECD (10) deposited in the Protein Data Bank (PDB ID: 4AY9 (11)). For our studies, the TSHR-ECD structure (PDB id: 3g04), which gives the coordinates of residues 30 through 257 (12), was superimposed on the FSH ECD; the hormone coordinates were obtained via homology modeling preformed with the program Modeller (13).
The coordinates of the initial model of the complex were sent to the Charmm-Gui server (14) that returned a complete set of input files ready to run the molecular dynamics (MD) simulation. The steps performed by the server included the generation of the topology file (based on the known topology of the protein residues), formation of the necessary disulfide bonds as specified by user input (based on the starting structure), specifying the shape and size of the simulation cell (determined by a user choice and specified solvent layer thickness), filling it with water, adding the requisite number of Na+ and Cl– ions to ensure both the neutrality of the system as well as an ionic strength of 0.15 M, and performing an initial energy minimization to eliminate steric clashes. The files provided by the server-included scripts to run the MD programs and the necessary parameter files. The systems thus prepared contained a total of 74 603 and 63 972 atoms for the TSHβ:TSHR-ECD and the TSHβv:TSHR-ECD complexes, respectively. The MD simulations used the NAMD (15) program and the Charmm-36 (16) force field and were carried out at constant temperature (300 K) and atmospheric pressure with periodic boundary condition implemented in a truncated octahedron box. The simulations kept the C-H bond length fixed allowing the use of 2-fs time step.
The initial structure provided by the Charmm-Gui server was further energy minimized to obtain a conformation that approximately corresponds to 0 K temperature. This conformation was then submitted to a schedule of warming MD runs at gradually increasing temperature until 300 K (room temperature) was reached. Running at 300 K and 1 atm required > 200 ns MD run to see the system stabilized (as evidenced by the average number of hydrogen bonds between the hormone and the receptor). Once the system appeared stabilized, an additional 400-ns simulation was run with both hormone variants.
The trajectories were visualized with the program VMD (17) to see if there were major changes in the complex. Most analyses were performed on the trajectories with the program Simulaid (18). Hydrogen bonds are defined by Simulaid as X···H-Y where X and Y are polar heavy atoms and the X···H-Y angle is above 90° and the X-Y distance is below threshold. Note that this definition ignores the actual charges; thus, it includes salt bridges because it is indeed the case for several of the hydrogen bonds thus defined. Hydrogen-bond lifetimes were calculated as the half-time of the bond autocorrelation function. Interface volume and surface was calculated with a Monte Carlo procedure as follows. In the first step, a set of uniformly distributed points was generated in a rectangle that enclosed the interface region. This was followed by a filtering procedure that eliminated points that were not in the interface. The ratio of the number of points left to the total number of points is an estimate of the ratio of the interface volume to the volume of the rectangle enclosing all the points. Points in the interface had to satisfy the following criteria: (1) be outside the van der Waals spheres of both members of the complex; (2) the angle between the lines drawn from the point to the nearest (heavy) atom in the 2 proteins exceeds 145°; (3) the circular variance (CV), shown to be a measure of “insideness” (19) with respect to the 2 proteins should each be > 0.6; and (4) the ratio of the CVs, calculated with the larger of the 2 CVs being the numerator, is < 3.5.
Cloning and expression of human TSHβ and TSHβv
To express the TSHβ and TSHβv proteins tagged with flag peptide at their C terminus, the entire coding sequence of the 2 proteins obtained from published work (1) was synthesized with the 5′ restriction enzyme site Xho I and a 3′ Bam HI sites. The double-digested cDNA was cloned into the mammalian expression vector pCDNA4/TO and cloned cDNA was sequence verified. These constructs were transfected into HEK293 cells and stable clones were obtained by selecting the cells with 500 µg/mL hygromycin for 2 weeks. The expressed protein was confirmed by intracellular staining of flag protein by flow cytometry and immunoblotting of total lysates with the anti-Flag antibody.
Protein purification.
Because the expressed protein was not secreted into the supernatant the proteins were purified from the lysates for TSHβ and TSHβv expressing clones. Briefly, total lysate was obtained from the cells using 1X RIPA buffer with the composition of 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, and 1 µg/mL leupeptin (Cell Signaling, MA Cat#9806) containing protease and phosphatase inhibitors. The lysate was clarified by centrifugation at 13 000g for 20 minutes at 4°C. Large amounts of lysate (~1-2 mg) was first precleared using IgG-bound Protein A in the ratio recommended by the manufacturer. Further, the clarified supernatant was mixed with protein A conjugated with anti-Flag in the proportions suggested by the manufacturer to maximize the binding capacity of the immuno-affinity column. The bound protein in the column was washed with 10 volumes of wash buffer and then eluted using low pH buffer (Glycine pH 2.0) and the eluate was neutralized immediately with 2M Tris pH 8.0 immediately. Fractions were pooled after OD measurement and dialyzed against cold PBS for 3 hours at 4°C.
Evaluation of the expressed protein.
The expressed protein was identified by immunoblot with anti-Flag and anti-TSH antibody and the sequence of protein fragments resolved on the gel was confirmed by mass spectrometry. For immunoblotting, the samples were reduced with 5X sample buffer containing 50 mM of DTT or no DTT and heated at 95°C for 5 minutes. The proteins separated on a 4% to 15% SDS-PAGE gel was transferred onto polyvinylidene fluoride membrane and proteins identified using anti-Flag antibody or anti-TSH. Bovine TSH was used as positive control. For mass spectrometry, the separated bands (17 KD/34 KD) were cut out from the resolved gel and sequenced. Peptides sequences were compared against known protein database and identified peptides represented as % coverage.
Bioactivity.
Bioactivity of the expressed proteins was examined by either (1) coculturing the cells that express the TSHβ and TSHβv proteins with the TSHR Glo cells (20) or (2) using the flag column purified and dialyzed protein on TSHRGlo cells. For the coculture, 1:1 ratio TSHRGlo and TSHβ/TSHβv positive cells at 25 × 103/well of a 96-well plate in complete DMEM medium were seeded together in 100 μL and incubated overnight at 37°C with 5% CO2. Untransfected HEK, TSHβ, or TSHβv expressing cells were used as controls. After 5 hours of incubation at 37°C, each well received 100 µL of Bright glow substrate. Luminesce was measured in a microplate reader after 3 minutes of lysis at room temperature. The purified protein was used at a final concentration 10 µg/well, which was added in a volume of 100 µL/well directly to TSHRGLO cells seeded at 50 × 103/well of 96-well plate overnight in complete medium.
Data analysis.
All statistical analysis of the data was performed using GraphPad Prism V6. One-way ANOVA was performed and multiple comparisons of data were calculated by Tukey correction as recommended by the software.
Results
Sequence alignments
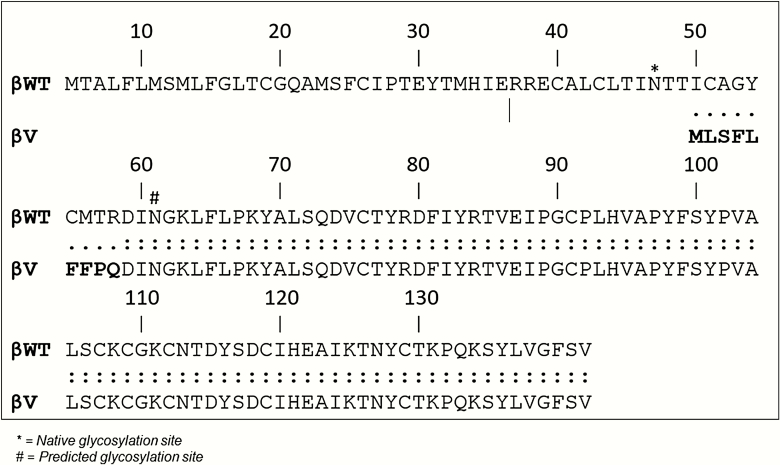
Before modeling studies, we carried out a sequence alignment of TSHβ and TSHβv to clearly indicate the amino acid overlap between the 2 polypeptides and to outline the conserved residues. The alignment also showed the portion of the truncated region in the TSHβv protein, which is missing and encoded by spliced exon 2 and the possible glycosylation sites on the TSHβ proteins. The 9 amino acid signal peptide at the N terminus (bolded) of the TSHβv protein derived from intron 2 (9) is also indicated (Figure 2).
Figure 2.
Protein alignment of TSH β and TSHβv. Alignment of the TSHβ and TSHβv protein sequences. The sequences are identical except in the 9 amino acid intronic sequence (in boldface type) that is seen in TSHβv. *Glycosylation sites on the protein.
Modeling the human TSHβ/TSHβv proteins binding to the TSHR-ECD
The number of hormone-TSHR-ECD hydrogen bonds formed during MD simulations > 200 ns is shown in Figure 3A and 3B. The illustration shows the history of residue pairs where at least 1 hydrogen bond existed between the residues resulting in pair bonding for more than 5% of the time. Each line in the timed plot represents a residue pair. When a line is broken, there were no hydrogen bonds between them at that time point. That each line begins soon after the start of the simulation indicates that both systems were well equilibrated.
Figure 3.
Hydrogen bonding profile of TSHβ and TSHβv. MD stimulation of TSHβ and TSHβv was carried out on a model of TSH receptor ectodomain for 400 ns. (A) Indicated is the profile of the hydrogen bonds that associate and dissociate during the interaction of TSHβ with the TSHR-ECD during simulation. (B) Profile of the hydrogen bonding for TSHβv with the TSHR-ECD during simulation. The y-axis represents the number of hydrogen bonds n(HB). TSHβv shows less hydrogen bonds formation in the latter half of the stimulation in contrast to TSHβ. ECD, ectodomain; TSHR, TSH receptor.
The major hydrogen-bonding residues for the TSHβ and TSHβv with the receptor ECD, based on their ability to stay in pairs for > 200 ns during the simulation, are summarized in Table 1 along with the % of time the residues were bonded together. From this analysis, we see that there were no overlapping residue pairs between the TSHβ and TSHβv. Residues with TSHβ binding were from residues distributed more to the N terminus of the ECD and 2 of the major subunit residues were common to both structures.
Table 1.
Major Hormone Receptor Hydrogen Bonds During MD Simulation
| TSH-β | |||
|---|---|---|---|
| TSHR ECD Residue | TSHβ Residue | % Bonded | Lifetime (ns) |
| Glu (E) 32 | Lys (K) 127 | 53.7 | >200 |
| Tyr (Y) 53 | Glu (E) 118a | 89.7 | >200 |
| Arg (R) 83 | Glu (E) 32 | 99.8 | >200 |
| Lys (K) 100 | Asp (D) 114 | 97.1 | >200 |
| Asp (D) 122 | Lys (D) 64 | 96.6 | >200 |
| Asp (D) 131 | Lys (D) 33 | 99.4 | >200 |
| Lys (K) 182 | VAL (V) 138a | 74.7 | >200 |
| TSH-βv | |||
| TSHR ECD Residue | TSHβ Residue | % Bonded | Lifetime (ns) |
| Arg (R) 9 | Glu (E) 118a | 98.7 | >200 |
| Glu (E) 32 | Arg (R) 80 | 97.2 | >200 |
| Arg (R) 51 | VAL (V) 138a | 89.2 | >200 |
Note: aResidues are common receptor residues.
Abbreviation: ns, nanosecond.
Figure 4A and 4B show the TSH:ECD complexes at the end of the MD run for TSHβ and TSHβv. The hydrogen-bonded residue pairs are visualized as lines connecting the Cα atoms of the corresponding residues. Observation of the animations of the trajectories showed that the conformation of the hormones changed significantly, especially the TSH-βv (Figure 4B). This suggested the need for a comparison of the root-mean-square fluctuations (RMSF). Figure 5 shows the RMSFs for the 2-subunit variants. The RMSFs were calculated after superimposing the ECD part of the complex onto the starting conformation and were then averaged over the atoms of each residue. Both had alternating stretches of low and high RMSF regions. As observed from the animation of the trajectory, the fluctuations were larger for the TSHβv. Also, TSHβ had significantly more low-RMSF stretches than TSHβv and the hormone residues forming stable hydrogen bonds were in a low RMSF region for both hormones (Figure 5).
Figure 4.
Representation of the last conformations of the 2 MD runs. The Cα atoms of the hydrogen-bonded residue pairs shown are connected with 2-colored lines coding for the residue type (red: acidic, blue: basic, green: polar). (A) TSHβ. (B) TSHβv. Residue GLU-32 is shown as van der Waals spheres in the 2 representations.
Figure 5.
Comparison of the RMSF. RMSFs were calculated after superimposing the ECD part of the complex onto the starting conformation and were then averaged over the atoms of each residue. (A) RMSF of the TSHβ residues during the simulations. (B) RMSF of TSHβv residues during the stimulation. We observe a higher RMSF for TSHβv, which indicate lower stability. ECD, ectodomain; RMSF, root-mean-square fluctuation.
The receptor-subunit interface
Observation of the animated trajectory and the last conformation of the MD simulation as indicated in Figure 6A and 6B also suggested that the hormone-receptor interface was not tight (i.e., there was a significant amount of space that could be filled by water between the subunit hormones and the receptor ECD). Therefore, we implemented a Monte Carlo procedure to estimate the volume of this space and applied it to 400 complexes selected from the trajectory using 107 random points. The calculation resulted in 175 ± 27 Å and 160 ± 25 Å for the ECD-TSH-β, and ECD-TSH-βv, respectively. Because the volume occupied by a water molecule is about 30 Å, this shows that the interface can accommodate several water molecules as seen on the animated trajectories.
Figure 6.
History of hydrogen-bonded residue pairs. The plots shown represent the hydrogen bonds between the TSHβ and TSHβv and the TSHR-ECD during the simulation. Each line in the plot represents a dynamic of the paired residue during 400 ns of simulation. The y-axis shows the residues for the receptor and hormone. (A) TSHβ, and (B) TSHβv. ECD, ectodomain.
Specificity of the modeling
To examine the specificity of the interaction of TSHβ proteins to the TSHR ECD, we first examined and mapped the residues on the TSHR ECD that were involved in hydrogen bonding with TSHβ based on criteria described earlier. Further, the TSHR ECD was aligned to the FSHR ECD (PDB id 4ay9) using the structural alignment option of the program Pymol (PyMOL Molecular Graphics System, version 2.0 Schrödinger, LLC). By this alignment, we found that of the 16 potential hydrogen bond-forming residues found in the TSHR-ECD, 15 were different in the FSHR ECD. This would suggest that the TSHβ protein is unlikely to bind to the FSHR ECD and thus can be considered as a strong indication for selectivity of the TSHβ and TSHβv to the TSHR-ECD.
Expression of recombinant human TSHβ and TSHβv protein
Having examined the binding of the human TSHβ and TSHβv to the TSHR-ECD in silico, we wanted to study the bioactivity of these 2 proteins in vitro. Figure 7A illustrates the constructs used for expression of human recombinant TSHβ and TSHβv in mammalian HEK 293 cells. After selecting stable clones of TSHβ and TSHβv, we checked the expression of the protein by intracellular staining in fixed cells by flow cytometry using an anti-Flag mAb (21). As indicated in Figure 7B and 7C, the % Flag positive cells for TSHβ was 90% and for TSHβv was 35% in these selected clones, indicating translated proteins. Anti-Flag antibody on untransfected HEK cells was used as a control. Anti-Flag immuno-affinity purified proteins prepared from these stable cells were analyzed under nonreducing conditions using the Protein Simple WES machine. By this analysis, we only observed protein bands with molecular weights of 34 KD for TSHβ and 24 KD of TSHβv, suggesting these purified proteins existed as dimeric forms (Figure 7D). On treating TSHβ protein with and without 200 mM of DTT and probing with anti-TSH (22), we observed that the dimeric protein corresponding to 34 KD reduced to the lower molecular protein (Figure 7E), thus confirming that these recombinant proteins existed as dimers under non-redox conditions. Mass spectrometric identification of the TSHβ of the purified expressed protein band resolved on a gel identified peptides corresponding to TSH.
Figure 7.
Expression and western blot analysis of recombinant TSHβ and TSHβv. (A) Schematic representation of the construct used for expression of the recombinant protein. The entire ORF of TSHβ or TSHβv was cloned into XhoI and BamHI sites of the mammalian vector and expressed in HEK293 cells. (B, C) Expression of the TSHβ and TSHβv recombinant proteins was confirmed in stable clones by flow cytometer by intracellular staining of the flag protein with an anti-flag monoclonal antibody (2 µg/106 cells). This was detected by anti-mouse, conjugated to phycoerythrin (anti-mouse PE). The graphs show FL1 that corresponds to the green channel on the x-axis and FL2 in the y-axis, which corresponds to the red channel (PE). The circled regions on the graph indicate the % positive stained cells for TSHβ and TSHβv in the representative clones. (D) Immunoblot carried out in the total lysates prepared from these cells and analyzed by anti-flag antibody using the protein simple WES system. The samples (1.2 mg/mL) were run under nonreduced conditions. TSHβ protein in lane 4 shows a 34-KD protein, whereas TSHβv (lane 6) shows a 24-KD protein, though the expected size of these proteins is 17 KD and 12 KD, respectively. The observed shift in size of these protein suggests dimers formation. Lane 1 shows the molecular weight markers and lane 2 positive WES running controls, which detects 44 KD ERK protein when probed with an anti-ERK antibody (45). Lanes 3 and 5 are controls for nonspecific binding of the antibodies; lane 3 is without lysate but primary antibody (anti-flag) and secondary (anti-mouse HRP) and lane 5 is without lysate but primary and secondary antibodies. (E) The high molecular weight TSHβ protein as indicated in panel D when reduced with 200 mM DTT was observed to be lower suggesting dimeric forms of the protein. ECD, ectodomain; HRP, horseradish peroxidase.
Bioactivity of the recombinant TSHβ proteins
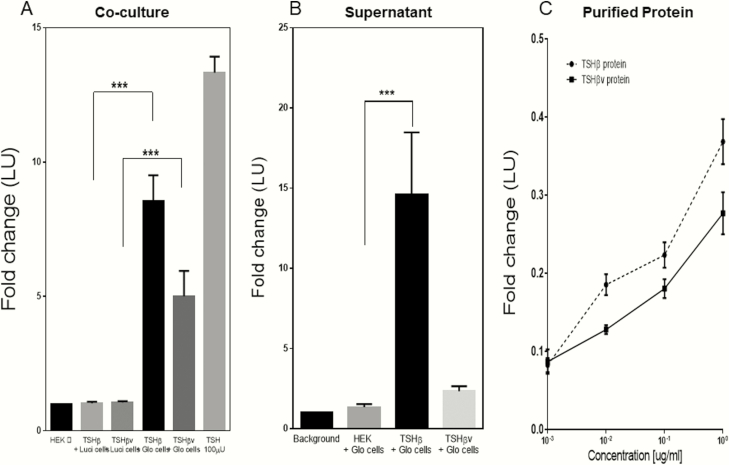
We initially examined bioactivity of the recombinant TSH β proteins by coculturing the protein expressing cells with TSHR-Glo cells (20). Figure 8A shows the cAMP responses observed as luciferase activity of the TSHR-Glo cells when cocultured with TSHβ and TSHβv cells. We observed nearly a 20-fold increase in luciferase activity with the TSHβ cocultured cells and 7-fold increase with TSHβv cocultured cells. Furthermore, on testing 5-fold concentrated and dialyzed supernatants on TSHR-Glo cells, we lost activity and only observed a 3-fold increase, whereas the concentrated supernatant from the TSHβv cells did not retain any activity (Figure 8B). However, immuno-affinity purified proteins from the lysates were then tested and a dose-dependent increase in cAMP responses was observed (Figure 8C). The inability of the purified proteins to show any response with control CHO-luci cells lacking the TSHR confirmed the specificity of the bioactivity of TSHβ and TSHβv observations.
Figure 8.
Bioactivity for the expressed proteins. We examined the bioactivity of the expressed proteins for cAMP response using the TSHRGlo cells. (A) HEK 293 cells expressing TSHβ or TSHβv were seeded together with TSHRGlo cells in equal ratios (20 000/well) in a 96-well plate and incubated for 48 hours at 37°C. At the end of 48 hours, the luciferase substrate BrightGlo was added to each well and luminescence emitted was read using a micro plate reader. (A) Graph shows responses of these cocultures cells. A ~9-fold increase in luciferase activity with TSHβ cells and ~4-fold increase with TSHβv cells respectively was observed when incubated with the TSHR Glo cells. TSHβ and TSHβv cells when cocultured with CHO luci (cells lacking TSHR) did not show any luciferase activity above the baseline cells (TSHRGlo/CHOluc cells). (B) Supernatant from TSHβ and TSHβv expressing cells concentrated 5× was added to TSHRGlo 40 000 cells per well seeded overnight in a 96-well plate. After 4 to 5 hours of stimulation by the supernatant of TSHRGlo cells, an equal amount of Bright Glo was added to each well and luminescence measured. (C) Dose-response of TSHβ and TSHβv lysate-purified protein measured using TSHRGlo cells as described previously is indicated in this graph after background subtraction.
Discussion
Recent studies have challenged the idea that pituitary hormones are “1-track” ligands; rather, they are capable of exerting their effects outside the target tissue. For example, in vitro and in vivo studies have shown the osteoprotective effect of pituitary TSH by activating osteoblasts and inhibiting osteoclasts (23-25) and also by its ability to enhance differentiation of preadipocytes (26). Further, the “forward feedback loop” action of the TSHβ splice variant (TSHβv) secreted by immune cells and macrophages in the bone marrow on osteoblasts are an added proof to the nontraditional functions of TSH subunits (23, 27). That human TSHβv secreted by bone macrophages is regulated by T3 (6) and the physiological effects it can have on the skeletal system or other tissues in the body led us to examine in detail the dynamics of binding of human TSHβ and TSHβv to the TSHR-ECD using computational modeling. This in silico binding analysis of the β subunits to the ECD was supported by further examination of the bioactivity of recombinant TSHβ subunits.
Native TSH is a member of the glycoprotein hormones and belongs to the cysteine-knot growth factor super family (28). It is secreted by the thyrotrope cells in the anterior pituitary as a heteromeric ~30-kDa glycoprotein consisting of a common α-subunit and a unique β-subunit, the latter being responsible for hormone specificity and binding to the orthosteric site of the TSHR. In terms of its carbohydrate content, the α subunit has 2 N-linked oligosaccharides, whereas the β subunit has only 1 in TSH and LH and 2 in chorionic gonadotropin and FSH (29). For the native TSH, N-linked glycosylation is necessary for proper folding, assembly, secretion, metabolic clearance, and biological activity (30).
The primary role of native TSH is to bind to the TSHR on the surface of thyrocytes and stimulate the production of thyroid hormones (T3 and T4) on the surface of thyroglobulin (31). Biochemical and homology modeling studies have clearly established the nature of the interactions of native TSH to the TSHR-ECD’s TSH “binding pocket” located in the horseshoe-shaped LRD floored by 9 β-pleated sheets held together by alpha helices (3, 32). However, the binding dynamics of the human TSHβ subunit or its splice variant (TSHβv) to the TSHR-ECD have not been studied. Previously, we reported that murine TSHβv has the ability to dock to the TSHR-ECD (5). Having derived the coordinates for TSHR-ECD from the FSH:FSHR-ECD from the PDB, we completed a 400-ns molecular dynamic simulation of the human TSHβ and TSHβv proteins interacting with the TSHR-ECD and followed the formation and dissociation of hydrogen binding to the receptor. The trajectories of these interactions were visualized with the program VMD (17) to see if there were major changes in the complex. The trajectories showed that the conformation of the hormones fluctuated significantly, especially the TSH-βv (Figure 4), suggesting increased flexibility of the residues within the truncated TSHβ protein. Examining the RMSF between the subunits after superimposing the ECD, we again observed larger fluctuations in the trajectories of TSHβv. The significantly lower RMSF and the larger number of TSHβ:ECD contact residues observed (Table1) suggested the formation of more stable hydrogen bonds with this complex, which would result in its stronger affinity and thus better activity, as observed in our subsequent experimental studies.
Although the interaction of heteromeric TSH to the receptor ECD is not restricted to the LRD (33), we limited our simulation to the LRD because (1) there is no experimentally available structure of the linker region (not even a reasonable homology model), (2) nor is there reliable information on the relative positions of the ECD and the TMD of the TSHR, and (3) even though we have some success in generating models for protein loops, the length of the linker region loop is way too long for obtaining reliable results. Although there are different types of interactions between the TSH and the TSHR (32, 34, 35), we focused our analysis on hydrogen bonding because it was seen to be the dominant interaction. That 15 of the 16 residues that are involved in hydrogen bond formation with residues in the TSHR-ECD were different to the FSHR-ECD gave a good indication of the specificity of TSHβ and TSHβv binding.
In addition to this extrathyroidal effect of pituitary TSH, our own studies and those of others (5, 6, 8, 9) have indicated that TSH activity can exist as a bioactive molecule in the absence of the α subunit. This unique ability of TSH to exist solely as a β subunit, or a splice variant of β, was first demonstrated in immune cells that homed to the thyroid on viral infection in a mouse model (8). This splice variant of TSHβ (TSHβv), found in bone marrow cells and peripheral blood leukocytes (1, 5, 6, 8) is essentially a truncated bioactive form of TSHβ.
Native TSH, consisting of the α and β subunits, are transcribed from 2 genes located in different chromosomes, and assembled and folded in the endoplasmic reticulum before being secreted as an active hormone (36). Though these 2 subunits are independent of each other, they are each further stabilized by disulfide bonds within the α and β chains (37, 38). Thus, it remains an enigma that a hormone that has coevolved as a heteromeric unit can also exist in immune cells as a bioactive molecule as a β subunit (1, 5, 39), although some earlier studies on pituitary monolayers in vitro have shown that free subunits of α and β can be released from these cells in addition to the mature heterodimeric native TSH (40). Furthermore, biochemically stripped β subunit purified from native TSH subunits has been shown to be a bioactive molecule (41), although it is still unclear if such preparations were devoid of any trace, unreduced TSH, as a contaminant. It is known that native bioactive TSH can be effectively produced as recombinant protein in mammalian cells (42); however, to our knowledge, there are no data as to production of bioactive recombinant human TSHβ or TSHβv although immunoprecipitation of human serum has shown the presence of TSHβv (43). Recently, there has been some insight on the mechanistic aspect of the formation of TSHβv isoform in immune cells (9), but it was not known if the proteins could bind to the TSHR ECD and trigger the formation of cAMP via activation of Gα s.
Thus, to demonstrate bioactive nature of TSHβ or TSHβv, we produced recombinant protein in HEK 293. Flow cytometric analysis of fixed cells with antibody to the fusion protein carried out on several stable clones (Figure 7B and 7C) showed that protein is produced in these cells and TSHβ protein was effectively secreted into supernatant although the splice variant was not secreted out. Coculturing of these cells with TSHRGlo cells (20) showed nearly 8-fold increase of cAMP response with TSHβ expressing cells and ~4-fold increase with TSHβv. However, testing 5× concentrated supernatant from these positive clones we observed a ~13-fold increase of cAMP and response with TSHβ, whereas no significant increase with TSHβv, suggesting inability of truncated protein to get secreted from the cells or instability of protein because immunopurified protein from the cell lysate retained its bioactivity though devoid of the α alpha subunit. On immunoblot analysis of the lysate from these cells under nonreduced conditions, we observed only a 34 KD and 24 KD forms of TSHβ and TSHβv, respectively, rather than a 17 KD and 12 KD protein as the expected protein sizes. Treating these samples with 200 mM DTT, we observed a reduction in size of this dimeric protein, suggesting that these proteins do exist as dimeric units in this recombinant preparation. Although we have not explored further if these dimeric units can result in different signaling profile or block the binding of native TSH to its cognate receptor, it has been shown that mutations in the β subunit of native TSH can certainly lead to altered signaling profiles (44).
Thus, mapping the binding of these TSHβ and TSHβv to the receptor ectodomain and using recombinant protein to explore the biological effects of these proteins gave us better insight into the role of TSHβ isoforms in different tissue sites.
Acknowledgments
Financial Support: This work was supported in part by National Institute of Health grant number DK069713 and the Veterans Administration Merit Award number BX000800 (to T.D.), the Segal Family Fund, and in part through the computational resources and staff expertise provided by the Department of Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Glossary
Abbreviations
- CV
circular variance
- ECD
ectodomain
- FSHR
FSH receptor
- LRD
leucine-rich domain
- ORF
open reading frame
- MD
molecular dynamics
- RMSF
root-mean-square fluctuation
- TSHR
TSH receptor
Additional Information
Disclosure Summary: T.F.D. is member of the Board of Kronus Inc, Starr, Idaho; M.Z. is inventor on patents on FSH, bone, and body fat regulation; these patents are owned by Icahn School of Medicine at Mount Sinai, with M.Z. being the recipient of royalties should they arise per institutional policies; M.Z. also consults for several financial platforms, including Gerson Lehrman Group and Guidepoint, on drugs for osteoporosis and genetic bone diseases. M.M., R.B., M.R.A., and R.L have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.
References
- 1. Klein JR. Biological impact of the TSHβ splice variant in health and disease. Front Immunol. 2014;5:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115(8):1972-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19(6):673-716. [DOI] [PubMed] [Google Scholar]
- 4. Sanders J, Miguel RN, Furmaniak J, Smith BR. TSH receptor monoclonal antibodies with agonist, antagonist, and inverse agonist activities. Methods Enzymol. 2010;485:393-420. [DOI] [PubMed] [Google Scholar]
- 5. Baliram R, Chow A, Huber AK, et al. Thyroid and bone: macrophage-derived TSH-β splice variant increases murine osteoblastogenesis. Endocrinology. 2013;154(12): 4919-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baliram R, Latif R, Morshed SA, Zaidi M, Davies TF. T3 regulates a human macrophage-derived TSH-β splice variant: implications for human bone biology. Endocrinology. 2016;157(9):3658-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schaefer JS, Klein JR. A novel thyroid stimulating hormone beta-subunit isoform in human pituitary, peripheral blood leukocytes, and thyroid. Gen Comp Endocrinol. 2009;162(3):241-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vincent BH, Montufar-Solis D, Teng BB, Amendt BA, Schaefer J, Klein JR. Bone marrow cells produce a novel TSHbeta splice variant that is upregulated in the thyroid following systemic virus infection. Genes Immun. 2009;10(1):18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein JR. Novel splicing of immune system thyroid stimulating hormone β-subunit-genetic regulation and biological importance. Front Endocrinol (Lausanne). 2019;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang X, Liu H, Chen X, et al. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc Natl Acad Sci U S A. 2012;109(31):12491-12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berman H, Henrick K, Nakamura H. Announcing the worldwide protein data bank. Nat Struct Biol. 2003;10(12):980. [DOI] [PubMed] [Google Scholar]
- 12. Sanders J, Chirgadze DY, Sanders P, et al. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17(5):395-410. [DOI] [PubMed] [Google Scholar]
- 13. Webb B, Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics. 2014;47:5.6.1-5.632. [DOI] [PubMed] [Google Scholar]
- 14. Jo S, Kim T, Iyer VG, Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem. 2008;29(11):1859-1865. [DOI] [PubMed] [Google Scholar]
- 15. Phillips JC, Braun R, Wang W, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26(16):1781-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Best RB, Zhu X, Shim J, et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J Chem Theory Comput. 2012;8(9):3257-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Humphrey W, Dalke A, Schulten K. VMD—visual molecular dynamics. J Molec Graph. 1996;14:33-38. [DOI] [PubMed] [Google Scholar]
- 18. Mezei M. Simulaid: a simulation facilitator and analysis program. J Comput Chem. 2010;31(14):2658-2668. [DOI] [PubMed] [Google Scholar]
- 19. Mezei M. A new method for mapping macromolecular topography. J Mol Graph Model. 2003;21(5):463-472. [DOI] [PubMed] [Google Scholar]
- 20. Latif R, Lau Z, Cheung P, Felsenfeld DP, Davies TF. The “TSH receptor glo assay”—a high-throughput detection system for thyroid stimulation. Front Endocrinol (Lausanne). 2016;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. RRID:AB_439685. http://antibodyregistry.org/AB_439685. [Google Scholar]
- 22. RRID:AB_661822. http://antibodyregistry.org/AB_661822. [Google Scholar]
- 23. Abe E, Marians RC, Yu W, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151-162. [DOI] [PubMed] [Google Scholar]
- 24. Sun L, Vukicevic S, Baliram R, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2008;105(11):4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zaidi M, Sun L, Davies TF, Abe E. Low TSH triggers bone loss: fact or fiction? Thyroid. 2006;16(11):1075-1076. [DOI] [PubMed] [Google Scholar]
- 26. Lu S, Guan Q, Liu Y, et al. Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis. 2012;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baliram R, Latif R, Zaidi M, Davies TF. Expanding the role of thyroid-stimulating hormone in skeletal physiology. Front Endocrinol (Lausanne). 2017;8:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDonald NQ, Hendrickson WA. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993;73(3):421-424. [DOI] [PubMed] [Google Scholar]
- 29. Grossmann M, Wong R, Teh NG, et al. Expression of biologically active human thyrotropin (hTSH) in a baculovirus system: effect of insect cell glycosylation on hTSH activity in vitro and in vivo. Endocrinology. 1997;138(1):92-100. [DOI] [PubMed] [Google Scholar]
- 30. Baenziger JU, Green ED. Pituitary glycoprotein hormone oligosaccharides: structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochim Biophys Acta. 1988;947(2):287-306. [DOI] [PubMed] [Google Scholar]
- 31. Szkudlinski MW, Grossmann M, Weintraub BD. Structure-function studies of human TSH: new advances in design of glycoprotein hormone analogs. Trends Endocrinol Metab. 1996;7(8):277-286. [DOI] [PubMed] [Google Scholar]
- 32. Núñez Miguel R, Sanders J, Chirgadze DY, Blundell TL, Furmaniak J, Rees Smith B. FSH and TSH binding to their respective receptors: similarities, differences and implication for glycoprotein hormone specificity. J Mol Endocrinol. 2008;41(3):145-164. [DOI] [PubMed] [Google Scholar]
- 33. Mueller S, Kleinau G, Jaeschke H, Paschke R, Krause G. Extended hormone binding site of the human thyroid stimulating hormone receptor: distinctive acidic residues in the hinge region are involved in bovine thyroid stimulating hormone binding and receptor activation. J Biol Chem. 2008;283(26):18048-18055. [DOI] [PubMed] [Google Scholar]
- 34. Kleinau G, Mueller S, Jaeschke H, et al. Defining structural and functional dimensions of the extracellular thyrotropin receptor region. J Biol Chem. 2011;286(25):22622-22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kleinau G, Worth CL, Kreuchwig A, et al. Structural-functional features of the thyrotropin receptor: a class A G-protein-coupled receptor at work. Front Endocrinol (Lausanne). 2017;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weintraub BD, Wondisford FE, Farr EA, et al. Pre-translational and post-translational regulation of TSH synthesis in normal and neoplastic thyrotrophs. Horm Res. 1989;32(1-3):22-24. [DOI] [PubMed] [Google Scholar]
- 37. Fairlie WD, Stanton PG, Hearn MT. The disulphide bond structure of thyroid-stimulating hormone beta-subunit. Biochem J. 1996;314 (Pt 2):449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82(2):473-502. [DOI] [PubMed] [Google Scholar]
- 39. Peele ME, Carr FE, Baker JR Jr, Wartofsky L, Burman KD. TSH beta subunit gene expression in human lymphocytes. Am J Med Sci. 1993;305(1):1-7. [DOI] [PubMed] [Google Scholar]
- 40. Chin WW, Maloof F, Martorana MA, Pierce JG, Ridgway EC. Production and release of thyrotropin and its subunits by monolayer cultures containing bovine anterior pituitary cells. Endocrinology. 1981;108(2):387-394. [DOI] [PubMed] [Google Scholar]
- 41. Williams JF, Davies TF, Catt KJ, Pierce JG. Receptor-binding activity of highly purified bovine luteinizing hormone and thyrotropin, and their subunits. Endocrinology. 1980;106(5):1353-1359. [DOI] [PubMed] [Google Scholar]
- 42. Cole ES, Lee K, Lauziere K, et al. Recombinant human thyroid stimulating hormone: development of a biotechnology product for detection of metastatic lesions of thyroid carcinoma. Biotechnology (N Y). 1993;11(9):1014-1024. [DOI] [PubMed] [Google Scholar]
- 43. Liu C, Li L, Ying F, Xu C, Zang X, Gao Z. A newly identified TSHβ splice variant is involved in the pathology of Hashimoto’s thyroiditis. Mol Biol Rep. 2012;39(12):10019-10030. [DOI] [PubMed] [Google Scholar]
- 44. Kalveram L, Kleinau G, Szymanska K, Scheerer P, Rivero-Muller A, Gruters-Kieslich A, Biebermann H. The pathogenic TSH beta-subunit variant C105Vfs114X causes a modified signaling profile at TSHR. Int J Mol Sci. 2019;20(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. RRID:AB_2315650. http://antibodyregistry.org/AB_2315650. [Google Scholar]