Abstract
Genotyping of the genus Paracoccidioides showed its diversity and geographical distribution. Four species constituting the Paracoccidioides brasiliensis complex and Paracoccidioides lutzii are etiological agents of paracoccidioidomycosis (PCM). However, there are no studies comparing the clinical and epidemiological aspects between PCM caused by the P. brasiliensis complex and by P. lutzii. Demographic and clinical data from 81 patients with PCM—confirmed by mycological and/or histopathological examination—from Mato Grosso do Sul state (Brazil) were studied. All patients underwent serology by immunodiffusion with antigens obtained from the P. brasiliensis complex (ExoPb and gp43) and Cell Free Antigens obtained from P.lutzii (CFAPl).The cases were classified regarding their serological profile into three groups: G1: PCM patients seropositive to ExoPb and/or gp43 and seronegative to CFAPl (n = 51), assumed to have PCM caused by P. brasiliensis complex; G2: PCM patients seronegative to gp43 and seropositive to CFAPl (n = 16), with PCM caused by P. lutzii; and G3: PCM patients seropositive to ExoPb or gp43 and seropositive to CFAPl (n = 14), with undetermined serological profile, was excluded from the analyses. The Fisher's exact test or the Mann-Whitney U test, and cluster analysis according to Ward’s method and Euclidean distance were used to analyze the results. Patients with serological profile suggestive of P. lutzii lived predominantly in municipalities in the Central and Southern regions of the state, while those with serological profile indicative of the P. brasiliensis complex were distributed throughout the state. No differences were found between the two groups regarding gender, age, schooling, rural work, clinical form, severity, organs involved, intensity of pulmonary involvement, degree of anemia, erythrocyte sedimentation rate values, and therapeutic response. PCM patients with serological profile suggestive of P. lutzii and PCM patients with serological profile indicative of P. brasiliensis complex showed the same clinical and radiological presentations.
Author summary
Paracoccidioidomycosis (PCM) is a disease caused by fungi living in the soil, which are inhaled mainly by rural workers from Latin America. These fungi are classified into the Paracoccidioides brasiliensis complex and Paracoccidioides lutzii. PCM may involve any organ and its treatment frequently results in fibrotic scars and functional limitations, which not rarely lead to early retirement. The mycological examination performed by routine laboratory tests cannot identify these species. However, the serological tests can suggest what species the etiological agent is, long as an antigen from both species is used. In order to determine whether there are differences in epidemiological aspects, clinical manifestations, severity, treatment response, and intensity of lung lesions according to the species involved, we compared 51 patients with serological profile suggestive of PCM caused by P. brasiliensis complex with 16 others caused by P. lutzii. Our results showed no differences between these species related to the parameters evaluated. Nevertheless, other clinical variables should be evaluated to detect differences regarding species. This study reinforces the importance of using antigens from both species for serological tests in PCM diagnosis, determination of severity, treatment follow-up and control of cure.
Introduction
Paracoccidioidomycosis (PCM) is a systemic disease caused by thermo-dimorphic fungi Paracoccidioides, and it is the most significant endemic mycosis of Latin America- especially in Brazil, Venezuela, Colombia, and Argentina- because of its high incidence [1]. PCM patients present an antigen-specific immunosuppression, which can be recovered after appropriate treatment [2, 3]
Several species of the genus Paracoccidioides involved in PCM etiology have been identified, such as P. brasiliensis, P. americana, P. restrepiensis, and P. venezuelensis. Collectively, these constitute the P. brasiliensis species complex. P. lutzii, which does not belong to this species complex, has also been identified as an etiological agent of PCM [4, 5].
Accurate identification of these agents depends on molecular evaluation of clinical specimens. However, this is infeasible because of the slow fungal growth, low frequency of Paracoccidioides spp isolation in culture, and because the molecular identification of fungi is not performed to routine laboratories. Therefore, it has been proposed to use specific P. lutzii antigens to perform serological tests in PCM so as to differentiate between its two main causative species [6].
Although only a few studies have been conducted to determine the exact geographical distribution of P. lutzii in Brazil, it is known to predominate in the Midwest Region of Brazil, [7, 8]. PCM caused by P. lutzii is known to occur in Mato Grosso do Sul [6]. This state is in the Midwest region of Brazil and is the neighboring state of Mato Grosso, where many P. lutzii cases have been reported previously [9], despite the absence of information on the geographical distribution in the State.
Since the identification of P. lutzii, it has been questioned whether it causes a different clinical presentation of PCM compared to P. brasiliensis infection.
This study aimed to compare the demographical, clinical and radiological aspects of PCM cases serologically identified as caused by the P. brasiliensis complex with cases caused by P. lutzii in Mato Grosso do Sul state.
Methods
Study design, setting and patients
The present study was conducted from March 2008 to March 2012 with PCM patients monitored by the Reference Service for Infectious and Parasitic Diseases at the University Hospital of Federal University of Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul state, Brazil.
The inclusion criteria for this study were as follows: a confirmed laboratory diagnosis of PCM characterized by the identification of the typical Paracoccidioides spp. yeast forms in clinical samples by mycological or histopathological examinations from patients presenting symptoms consistent with active infection. Clinical, sociodemographic, and laboratory variables were prospectively recorded on a standardized form. Such variables were compared between two different group of patients, i. e., a group with PCM presumably caused by the P. brasiliensis complex and another group with PCM supposedly caused by P. lutzii.
Thirty-four patients in this study participated in a previous study about serology of PCM due to P. lutzii [6] and 60 patients participated in a study about prevalence of the acute/subacute PCM in Mato Grosso do Sul [10].
Definition of study groups
Serology was performed in all patients included at the Immunology Laboratory of the Federal University of São Paulo (UNESP), São Paulo state, Brazil. Three antigenic preparations—(1) P. brasiliensis exoantigen (ExoPb); (2) purified 43 kDa glycoprotein (gp43); (3) Cell Free Antigen from P. lutzii isolate (CFAPl)—were used for the detection of specific antibodies via double immunodiffusion (DID) in agar gel.
The ExoPb and gp43 antigens obtained from B339 isolates and the CFAPI antigen obtained from EPM 208 isolates were identified through polymerase chain reaction (PCR) as P. brasiliensis and P. lutzii, respectively. The P. brasiliensis complex was differentiated from P lutzii using the primers HSPMMT1 and PLMMT1 as molecular markers [4].
DID was conducted according to the modified Ouchterlony´s method [11]. For every sample, tests were performed with each one of the antigens: ExoPb, gp43, and CFAPl.
Depending on the antigen identification by serum antibodies, patients were distributed into 3 groups: group1: PCM patients seropositive to ExoPb and/or gp43 and seronegative to CFAPl, with 51 patients; group 2: PCM patients seronegative to gp43 and seropositive to CFAPl, with 16 patients; and group 3: PCM patients seropositive to ExoPb or gp43 and seropositive to CFAPl, with 14 patients.
Group 1 patients were serologically compatible with the P. brasiliensis complex and group 2 with P. lutzii etiology. Finally, PCM patients in group 3 have not a determined etiology and they were excluded from the comparative analysis.
The flow diagram (Fig 1) summarizes the number of subjects enrolled and included in the study.
Fig 1. Flow diagram of PCM cases and their serological results.

DID: Double agar gel immunodiffusion test; ExoPb: P. brasiliensis exoantigen; gp43: purified 43 kDa glycoprotein; CFAPl: Cell free antigen from P. lutzii.
Sample size
Sample size calculation was performed for difference between proportions, considering an error type α of 0.05, 80% test power, and 35% differences, according to Jekel et al. (2005) [12]. Every arm of this study should have at least 16 patients.
Prevalence rate
Prevalence rates were calculated by dividing the number of cases treated at the study site, which is a reference for PCM, by the number of inhabitants in the state in 2010. We employed such year as the cases were diagnosed between 2008 and 2012, and the number of inhabitants was estimated on the records of the Brazilian Institute of Geographic and Statistic (IBGE), 2010 survey [13]
Demographic and clinical data
Demographic and clinical data were collected prospectively using a standardized protocol.
PCM patients were classified into acute/subacute or chronic forms. The acute/subacute form was subdivided into moderate and severe forms, and the chronic form into mild, moderate, and severe forms [1, 14]. The criteria used in the classification of severity were weight loss, dissemination of the disease, intensity of pulmonary involvement, serum levels of specific antibodies, and degree of lymph node enlargement [1, 14].
Antifungal drugs were administered according to the Brazilian guidelines [15]. The follow-up regimen included reevaluation after 1 month of treatment, and every 2 months thereafter until clinical cure was reached. Clinical cure was defined as the disappearance of the previously detected symptomatology and regression of the erythrocyte sedimentation rate or C-reactive protein to normal values [1]. Patients were then evaluated every 2–3 months until serological cure was reached.
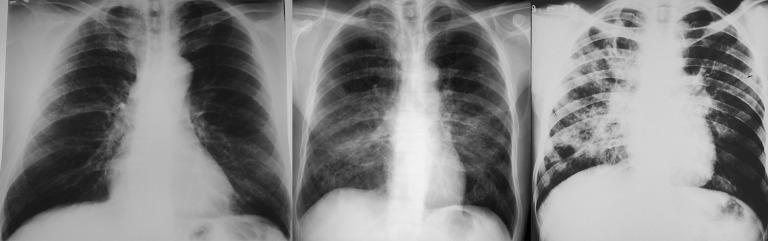
The severity of pulmonary involvement was assessed based on standard posteroanterior and lateral views of chest roentgenograms obtained at patient admission. The same radiologist analyzed all the radiographs and classified the severity of pulmonary involvement into mild, moderate or severe: a) mild–only one or two focal pulmonary lesions, or alveolar lesions even if involving more than one third of the total pulmonary parenchyma; b) moderate–three or more focal lesions were present, or interstitial or mixed lesions involving less than one third of the total lung parenchyma; c) severe–interstitial or mixed lesions involving more than one third of the total lung parenchyma. Fig 2 illustrates the radiography of mild, moderate and severe cases (Fig 2) [16].
Fig 2. Chest roentgenograms of three cases.
From left to right: mild, moderate, and severe.
Statistical analysis
The Fisher's exact test was used to compare categorical variables, and the Mann-Whitney U test to compare continuous variables. To study the similarities in prevalence rate between different municipalities, cluster analysis was performed according to Ward’s method and Euclidean distance. Significance was set up at p≤0.05. The software used was SPSS version 21.0 (IBM Corporation, Chicago, IL, USA).
Ethical considerations
The Ethics Committee of the Federal University of Mato Grosso do Sul approved the present study with ID number 354898. All patients signed a statement of written informed consent for data collection. In the cases of minors, their parents or legal guardians did it on their behalf.
Results
All 81 patients included have confirmed PCM. In 57 (70.4%) of them, the confirmation was by histopathological examination; in 13 (16.0%) of them, it was by direct mycological examination; and in the other 11 patients (13.6%), it was by both examinations.
Out of the 81 PCM patients studied, 51 (62.9%) had PCM serologically compatible with the P. brasiliensis complex etiology (group 1) and16 (19.8%) had it compatible with P. lutzii etiology (group 2). In the remaining 14 (17.3%), the species was serologically undetermined (group 3).
Except for ethnicity, no significant differences in the demographic variables were found between group1 and group 2, as shown in Table 1.
Table 1. Demographic findings of 81 paracoccidioidomycosis patients according to their serological profile.
| Variable | Group 1 n = 51 Nº (%) or median [range] |
Group 2 n = 16 Nº (%) or median [range] |
Group 3 n = 14 Nº (%) or median [range] |
Group 1 vs Group2 p |
|---|---|---|---|---|
| Gender | ||||
| Male | 48 (94.1) | 15 (93.8) | 14 (100) | 1.00# |
| Female | 3 (5.9) | 1 (6.2) | 0 | |
| Ethnicity | 0.05# | |||
| White | 28 (54.9) | 3 (18.8) | 4 (28.6) | 0.03& |
| Black | 3 (5.9) | 1 (6.2) | 0 | 0.57& |
| Mixed | 16 (31.4) | 10 (62.5) | 7 (50,0) | 0.83& |
| Indigenous | 0 | 0 | 1 (7.1) | |
| Unknown* | 4 (7.8) | 2 (12.5) | 2 (14.3) | |
| Age (years) | 47.4 [4–76] | 49.5 [35–66] | 51 [32–69] | 0.89† |
| Education (years) | 0.71# | |||
| ≤ 4 | 29 (56.9) | 9 (56.2) | 13 (92.9) | |
| > 4 | 8 (15.7) | 4(25.0) | 1 (7.1) | |
| Unknown* | 14 (27.4) | 3 (18.8) | 0 | |
| Rural workers | 0.67# | |||
| Yes | 41 (80.4) | 15 (93.8) | 14 (100) | |
| No | 6 (11.8) | 1 (6.2) | 0 | |
| Unknown* | 4 (7.8) | 0 (0.0) | 0 |
Group1: PCM patients seropositive to ExoPb and/or gp43 and seronegative to CFAPl. Group 2: PCM patients seronegative to gp43 and seropositive to CFAPl. Group 3: PCM patients seropositive to ExoPb or gp43 and seropositive to CFAPl.
* It was not included in the statistical analysis.
#: Fisher´s Exact test.
&: comparison of two proportions test.
†: Mann-Whitney U test.
The distribution of cases according to the municipality of origin is shown in Fig 3 (Fig 3). It is observed that most patients live in the center of the State. This distribution, according to the prevalence rate and similarities, is presented in Table 2 and Fig 4. Three cases of the group 3 were not from Mato Grosso do Sul, being one from São Paulo state, another from Mato Grosso and the other one from Paraguay.
Fig 3. Distribution of 78 cases of paracoccidioidomycosis from the Mato Grosso do Sul state.
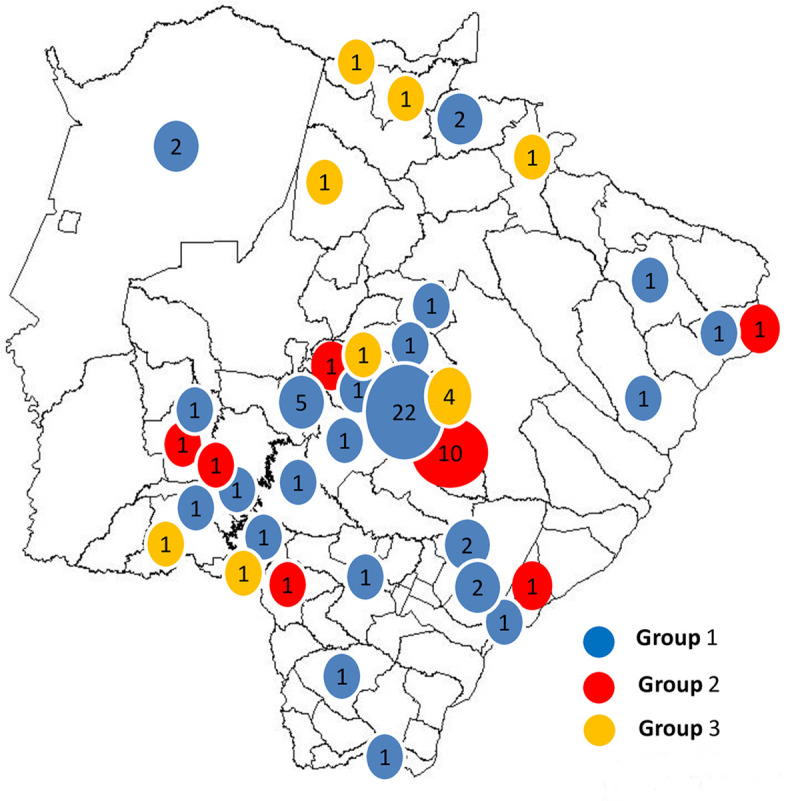
Table 2. PCM cases and prevalence rate (PR) per 100,000 inhabitants according to their municipalities and serological profiles.
| State/Municipalities | Population (year 2010) | Group 1 n (PR) |
Group 2 n (PR) |
Group 3 n (PR) |
|---|---|---|---|---|
| Mato Grosso do Sul | 2,449,024 | 51 (2.1) | 16 (0.7) | 11 (0.4) |
| Campo Grande | 786,797 | 22 (2.8) | 10 (1.3) | 4 (0.5) |
| Dois Irmãos do Buriti | 10,363 | 5 (48.2) | 0 (0.0) | 0 (0.0) |
| Alcinópolis | 4,569 | 2 (43.8) | 0 (0.0) | 0 (0.0) |
| Angélica | 9,185 | 2 (21.8) | 0 (0.0) | 0 (0.0) |
| Corumbá | 103,703 | 2 (1.9) | 0 (0.0) | 0 (0.0) |
| Ivinhema | 22,341 | 2 (9.0) | 0 (0.0) | 0 (0.0) |
| Amambaí | 34,730 | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| Anastácio | 23,835 | 1 (4.2) | 0 (0.0) | 0 (0.0) |
| Aparecida do Taboado | 22,320 | 1 (4.5) | 1 (4.5) | 0 (0.0) |
| Bandeirantes | 6,609 | 1 (15.1) | 0 (0.0) | 0 (0.0) |
| Dourados | 196,035 | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Guia Lopes da Laguna | 10,366 | 1 (9.6) | 1 (9.6) | 0 (0.0) |
| Inocência | 7,669 | 1 (13.0) | 0 (0.0) | 0 (0.0) |
| Japorã | 7,731 | 1 (12.9) | 0 (0.0) | 0 (0.0) |
| Jaraguari | 6,341 | 1 (15.8) | 0 (0.0) | 0 (0.0) |
| Jardim | 24,346 | 1 (4.1) | 0 (0.0) | 0 (0.0) |
| Maracaju | 37,405 | 1 (2.7) | 0 (0.0) | 0 (0.0) |
| Ponta Porã | 77,872 | 1 (1.3) | 1 (1.3) | 1 (1.3) |
| Sidrolândia | 42,132 | 1 (2.4) | 0 (0.0) | 0 (0.0) |
| Taquarussu | 3,518 | 1 (28.4) | 0 (0.0) | 0 (0.0) |
| Terenos | 17,146 | 1 (5.8) | 1 (5.8) | 1 (5.8) |
| Três Lagoas | 101,791 | 1 (1.0) | 0 (0.0) | 0 (0.0) |
| Batayporã | 10,936 | 0 (0.0) | 1 (9.1) | 0 (0.0) |
| Bonito | 19,587 | 0 (0.0) | 1 (5.1) | 0 (0.0) |
| Bela Vista | 23,181 | 0 (0.0) | 0 (0.0) | 1 (4.3) |
| Costa Rica | 19,695 | 0 (0.0) | 0 (0.0) | 1 (5.1) |
| Pedro Gomes | 7,967 | 0 (0.0) | 0 (0.0) | 1 (12.6) |
| Rio Verde de Mato Grosso | 18,890 | 0 (0.0) | 0 (0.0) | 1 (5.3) |
| Sonora | 14,833 | 0 (0.0) | 0 (0.0) | 1 (6.7) |
Group1: PCM patients seropositive to ExoPb and/or gp43 and seronegative to CFAPl. Group 2: PCM patients seronegative to gp43 and seropositive to CFAPl. Group 3: PCM patients seropositive to ExoPb or gp43 and seropositive to CFAPl.
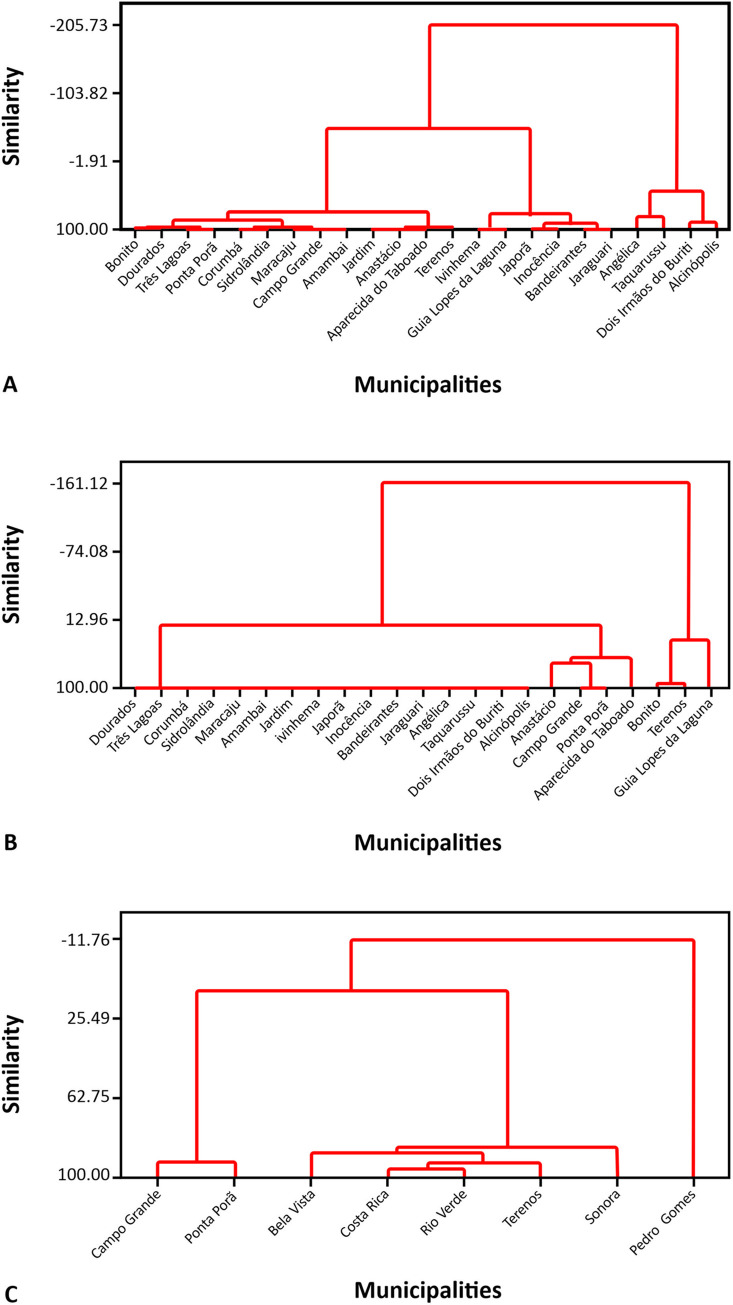
Fig 4. Dendrograms related to the prevalence rates of paracoccidioidomycosis (PCM) per 100,000 inhabitants, January 2008—December 2012.
A. Group 1, PCM patients seropositive to gp43 and/or ExoPb and seronegative to CFAPl (51 cases). B. Group 2, PCM patients seronegative to gp43 and ExoPb and seropositive to CFAPl (16 cases). C. Group 3, PCM patients seropositive to gp43 or ExoPb and seropositive to CFAPl (11 cases). The reference year for the population was 2010.
The highest rates of the disease serologically compatible with the P. brasiliensis complex etiology were found in Dois Irmãos do Buriti (48.2/100,000 inhabitants) and Alcinópolis (43.8/100,000 inhabitants), while the highest rates involving P. lutzii were observed in Guia Lopes da Laguna (9.6/100,000 inhabitants) and Batayporã (9.1/100,000 inhabitants).
An evaluation of group 1 PCM patients serologically compatible with the P. brasiliensis complex etiology reveals the formation of three geographic groups of municipalities, according to the similarity of prevalence: the first one, made up of Bonito, Dourados, Três Lagoas, Ponta Porã, Corumbá, Sidrolândia, Maracaju, Campo Grande, Amambai, Jardim, Anastácio, Aparecida do Taboado, and Terenos; the second one, constituted by Ivinhema, Guia Lopes da Laguna, Japorã, Inocência, Bandeirantes and Jaraguari; and the third one, composed of Angélica, Taquarussu, Dois Irmão do Buriti and Alcinópolis (Fig 4A).
Fig 4B (Fig 4B) reveals the formation of two groups of municipalities according to the prevalence rates of PCM patients, serologically compatible with P. lutzii etiology: the first one, constituted by Dourados, Três Lagoas, Corumbá, Sidrolândia, Maracaju, Amambaí, Jardim, Ivinhema, Japorã, Inocência, Bandeirantes, Jaraguari, Angélica, Taquarussu, Dois Irmãos do Buriti, Alcinópolis, Anastácio, Campo Grande, Ponta Porã, and Aparecida do Taboado; and the second, formed by Bonito, Terenos, and Guia Lopes da Laguna.
In sequence, an evaluation of group 3 with PCM patients by serologically undetermined species, reveals the formation of two geographic groups according of prevalence rate: the first one, made up by Campo Grande, Ponta Porã, Bela Vista, Costa Rica, Rio Verde, Terenos and Sonora; and the second one, constituted by Pedro Gomes (Fig 4C).
Group1 and 2 did not differ regarding clinical form, organs involved, intensity of pulmonary involvement, treatment, and progress after treatment (Table 3).
Table 3. Clinical aspects of 81 paracoccidioidomycosis patients according to their serological profiles.
| Variables | Group 1N° (%) or median [range] | Group 2N° (%) or median [range] | Group 3 N° (%) or median [range] |
Group 1 vs Group2 p |
|---|---|---|---|---|
| Clinical form/severity (n = 81) | 1.00 | |||
| Acute/subacute | 5 (9.8) | 1 (6.3) | - (-) | 1.00 |
| Moderate | 2 (40.0) | 1 (100.0) | (-) | |
| Severe | 3 (60.0) | - (-) | ||
| Chronic | 46 (90.2) | 15 (93.8) | 14 (100.0) | 0.41 |
| Mild | 13 (28.3) | 2 (13.3) | 1 | |
| Moderate | 22 (47.8) | 10 (66.7) | 8 | |
| Severe | 10 (21.7)) | 3 (20.0) | 5 | |
| Unclassified* |
|
|
- (-) | |
| Lesion site (n = 81) | ||||
| Lungs | 45 (88.2) | 14 (87.5) | 14 (100.0) | 1.0 |
| Oral cavity and/or oropharynx | 26 (51.0) | 12 (75.0) | 9 | 0.15 |
| Larynx | 11 (21.6) | 2 (12.5) | 5 | 0.72 |
| Lymph node | 11 (21.6) | 6 (37.5) | 7 | 0.21 |
| Central nervous system | 4 (7.8) | 1 (6.3) | 2 | 1.00 |
| Adrenal glands | 7 (13.7) | 3 (18.8) | 1 | 0.69 |
| Intensity of lung lesions, on chest X-ray- (n = 41) | NA | 0.90 | ||
| Mild | 16 (53.3) | 5 (45.4) | ||
| Moderate | 9 (30.0) | 4 (36.4) | ||
| Severe | 5 (16.7) | 2 (18.2) | ||
| Albumin (g/dL) (n = 50) | 3.8 [2.0–5.1] | 3.3 [1.3–3.9] | 3.5 [1.4–4.5] | 0.14 |
| Hemoglobin (g/dL) (n = 72) | 13.8 [8.5–16.6] | 13.1 [9.5–19.0] | 13.9 [9.7–16.4] | 0.67 |
| ESR (mm/hour) (n = 56) | 23 [1.0–117.0] | 61.5 [2.0–90.0] | 50.0 [3.0–115.0] | 0.17 |
| Antifungal compound (n = 77) | ||||
| Cotrimoxazole | 40 (80.0) | 15 (93.7) | 12 (85.7) | 0.43 |
| Itraconazole | 8 (16.0) | 1 (6.3) | 2 (14.3) | |
| Amphotericin B* | 1 (2.0) | - (-) | - | |
| Fluconazole* | 1 (2.0) | - (-) | - | |
| Completed treatment (n = 80) | 0.26 | |||
| Yes | 22 (44.0) | 10 (62.5) | 8 (57.1) | |
| No | 28 (56.0) | 6 (37.5) | 6 (42.9) | |
| Death (n = 81) | 1.00 | |||
| Yes | 1 (2.0) | - (-) | - (-) | |
| No | 50 (98.0) | 16 (100) | 14 (100.0) | |
| Relapse (n = 41) | 0.65 | |||
| Yes | 4 (18.2) | 3 (30.0) | 1 (7.1) | |
| No | 18 (81.8) | 7 (70.0) | 13 (82.9) | |
| Cure with pulmonary sequelae (n = 41) | 0.61 | |||
| Yes | 19 (86.4) | 8 (80.0) | 7 (87.5) | |
| No | 3 (13.6) | 2 (20.0) | 1 (7.1) |
* It was not included in the statistical analysis
Group1: PCM patients seropositive to ExoPb and/or gp43 and seronegative to CFAPl. Group 2: PCM patients seronegative to gp43 and seropositive to CFAPl. Group 3: PCM patients seropositive to ExoPb or gp43 and seropositive to CFAPl. * It was not included in the statistical analysis: Fisher´s Exact test to compare categorical variables. Mann-Whitney U test to compare numerical variables. NA: not available
Discussion
The recent identification of two different PCM-causing agents, the P. brasiliensis complex and P. lutzii [4, 5] raises the issue of whether there are clinical and epidemiological differences in the disease caused by them. While this study was ongoing, additional PCM-causing species were identified: P. americana, P. restrepiensis, and P. venezuelensis. Together with P. brasiliensis, these fungal species now form the Paracoccidioides brasiliensis complex [5].
The reference standard for Paracoccidioides species identification is molecular methods of the clinical isolates from culture. As Paracoccidioides spp growing in culture media is difficult and molecular methods are not available in routine clinical laboratory, we used serology as the strategy to presume the species causing PCM, as proposed by Gegembauer et al (2014) [6].
Our patients were classified regarding their results of immunodiffusion: group 1 with serological pattern suggestive of the P. brasiliensis complex; group 2 with serological profile suggestive of P. lutzii; and group 3 with undetermined serological profile. As the purpose of our study is to investigate differences between PCM caused by P. lutzii and PCM caused by the P. brasiliensis complex, the group 3 was excluded from the comparative analyses.
The main biochemical characteristic that differentiates P. lutzii from the P. brasiliensis complex is the absence of gp43 expression [17, 18]. Sera from patients with PCM caused by P. lutzii identified by molecular method do not react against gp43 and other antigens obtained from P. brasiliensis complex isolates, such as exo-antigen B339, which is traditionally used in the PCM immunodiagnosis. In addition, these sera have 100% reactivity against CFA obtained from P. lutzii isolates [18], and 100% sensitivity (IC 80.5–100) and 100% specificity (IC 94–100) of CFAPl have been reported [6]. Although other studies show CFAPl is not specific antigen for P. lutzii [17, 19], patients seropositive to CFAPl and seronegative to gp43 have PCM presumably caused by P. lutzii [6]. In contrast, patients seronegative to CFAPl and seropositive to gp43 have PCM presumably caused by the P. brasiliensis complex [6].
About one-fifth of the PCM cases presented a serological response profile suggestive of PCM caused by P. lutzii which occurs mainly in the Brazilian Midwest Region, having been isolated from human cases in Goiás and Mato Grosso [4, 7, 20, 21]. Mato Grosso do Sul is bordered on the north by Mato Grosso and on the northeast by Goiás, but the distribution of PCM by P. lutzii was concentrated in the south-central region of Mato Grosso do Sul. The low population density in northern Mato Grosso do Sul, where only a few cases of PCM have been reported, can justify the lack of P. lutzii cases in this area, as observed in the present study. Moreover, it should be emphasized that the place of residence of the patients may not reflect the place of infection, since most cases presented the chronic form, in which the incubation period can often extend into decades; therefore, the infection could have been acquired in another municipality or state.
Before this study, it was already speculated that at least some of the patients from Mato Grosso do Sul were infected by P. lutzii. The positivity of DID reactions performed in this study, with antigens extracted from the isolate B339 of the P. brasiliensis complex (74.5%) [22] was found to be lower than that observed in the southeast region of Brazil. This result suggested that there could be another species of the genus Paracoccidioides involved, similar to findings reported in a study undertaken in Mato Grosso state [21].
Two results found in the present study suggest PCM autochthony caused by P. lutzii in Mato Grosso do Sul state. First, the occurrence of an acute/subacute form in a patient serologically compatible with P. lutzii etiology, with a shorter incubation period (from a few weeks to months). Even though this patient was born in Cáceres (Mato Grosso state), where P. lutzii is quite common; it is unknown how long he lived in Campo Grande, capital of Mato Grosso do Sul state. Second, four patients with PCM serologically compatible with P. lutzii etiology were born and remained in the MS state, and reported never having left the state. Other Brazilian states have reported humans and animals infected by P. lutzii, as such as São Paulo [23] in the Southeast region; and Paraná [24] and Rio Grande do Sul [25], in South region.
The evaluation of the prevalence rates and the geographic distribution in Mato Grosso do Sul state of the three groups of patients showed no peculiarity in neither of them.
The population of Mato Grosso do Sul in 2010 was 47.6% white. In the present study, a lower percentage of white people was found within P. lutzii cases when compared to the P. brasiliensis complex cases. More than a racial susceptibility to the Paracoccidioides species, this finding may be related to the geographical area where the infection occurred. The agricultural areas of MS were occupied by different migratory movements. The population of the South of the country, predominantly white, has been an important colonizer in the state in recent years. Consequently, it is possible that the individual was infected with Paracoccidioides in his/her childhood in his/her hometown, progressing to disease after decades, due to endogenous reactivation. Out of the 20 PCM patients born in the southern region of the country, 18 (90.0%) were serologically compatible with the P. brasiliensis complex etiology.
Although the geographical distribution of P. lutzii cases is not yet defined, the methodology proposed by Gegembauer et al [6] for the classification of the species involved in PCM will certainly help to accomplish this task.
The clinical manifestations of PCM are determined by host-parasite interactions. The variable clinical presentations of PCM are usually attributed to the host's immunological status [1, 13]. Nonetheless, some intriguing regional differences related to the clinical manifestations of PCM are known, such as the absence of the acute/subacute form in the state of Rio Grande do Sul [26], in contrast to the interior São Paulo state, where cases of the acute/subacute disease forms reach 25% [27]. A number of patients with acute/subacute forms have also been reported in Goiás state [28]. Thus, it is difficult to accepted that these differences are related only to host differences and not also to environmental conditions, and consequently, to fungal species.
To the best of our knowledge, there are no publications comparing the clinical manifestations presented by PCM patients caused by agents of the P. brasiliensis complex with those from cases caused by P. lutzii. A recent publication compared findings from PCM patients caused by P. brasiliensis with those by P. americana, indicating no differences between them [29].
The present study showed no differences in the variables studied, including clinical form and organs affected, neither found any association between clinical severity and the species involved. Hahn et al [30] have recently described the clinical characteristics of 34 patients with PCM due to P lutzii, who presented clinical features like those previously described in cases of P. brasiliensis complex. However, no comparative evaluation was not undertaken by these authors. A report of a case of fatal fungemia caused by P. lutzii in a patient who did not exhibit any immunosuppressive co-morbidity led to the hypothesis that this species is more pathogenic than the P. brasiliensis complex [31], yet, a possible primary immunodeficiency was not investigated, as it was confirmed by Schimke et al. [32].
Therefore, although there were differences in virulence mechanism [33]; protein expression and antigenicity of P. lutzii [17, 34]; and, patterns of host-parasite interaction and pathology [35], these findings seem to have no impact on clinical manifestation, as they have on serology. Serology in PCM is particularly useful not only for diagnosis, but also severity assessment and control of cure during the treatment follow-up [1].
This study has some limitations, such as its relatively small sample size, which was enough to a safe statistical analysis, though. Another limitation is the non-molecular identification of the etiological agent. However, the serological strategy used in order to define PCM by P. lutzii could falsely identify a P. brasiliensis complex isolate as P. lutzii, since there are rare reports of molecularly confirmed PCM by P. brasiliensis complex seropositive for CFAPl [19], or seronegative for gp43 [36]. and much rarer is the occurrence of both exceptions at the same time.
In conclusion, our study suggests that there are no differences in demographic aspects, pulmonary clinical and radiological manifestations, therapeutic response and progress of PCM caused by either P. lutzii or the P. brasiliensis complex. The significance of this knowledge is the maintenance of the epidemiological, clinical, radiological and therapeutic approaches, persisting the well-known differences of sensitivity of the serologic tests. Future studies, a multicentric or systematic review with meta-analysis, with a greater sample of the Paracoccidioides species are necessary to confirm these findings and to evaluate the importance of the routine identification of the Paracoccidioides species in clinical management and public health. We would like to emphasize the need to include CFA from P. lutzii in routine serological tests in endemic areas for both Paracoccidioides species, improving the diagnosis and allowing a better monitoring of the treatment control.
Supporting information
(DOC)
(XLSX)
Acknowledgments
The authors thank the study participants and the team of health professionals from the Infectious and Parasitic Diseases Unit (UDIP) at Maria Aparecida Pedrossian University Hospital of the Federal University of Mato Grosso do Sul for their support in this project.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [to Z.P.C]; Fundação de Apoio ao Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul [to R.P.M]; and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 (to A.M.M). B.M.M recieved research fellowship from Conselho Nacional de Conselho Nacional de Desenvolvimento Científico e Tecnológico. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mendes RP, Cavalcante RS, Marques SA et al. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol J, 2017; 11: 224–82. 10.2174/1874285801711010224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benard G, Hong MA, Del Negro GMB, Batista L, Shikanai-Yasuda MA, Duarte AJS. Antigen-specific immunosuppression in paracoccidioidomycosis. Am J Trop Med Hyg 1996; 54: 7–12. 10.4269/ajtmh.1996.54.7 [DOI] [PubMed] [Google Scholar]
- 3.Benard G, Romano CC, Cacere CR, Juvenale M, Mendes-Giannini MJS, Duarte AJS. Imbalance of IL-2, IFN-γ and IL-10 secretion in the immunosupression associated with human paracoccidioidomycosis. Cytokine 2001; 13: 248–52. 10.1006/cyto.2000.0824 [DOI] [PubMed] [Google Scholar]
- 4.Teixeira MM, Theodoro RC, de Carvalho MJ, Hahn RC, et al. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol, 2009; 52(2): 273–83. 10.1016/j.ympev.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Turissini DA, Gomez OM, Teixeira MM, McEwen JG, Matute DR. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet Biol, 2017; 106: 9–25. 10.1016/j.fgb.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gegembauer G, Araujo LM, Pereira EF et al. Serology of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl Trop Dis. 2014; 8(7): e2986 10.1371/journal.pntd.0002986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theodoro RC, Teixeira MeM, Felipe MS et al. Genus Paracoccidioides: Species recognition and biogeographic aspects. PLoS One, 2012; 7(5): e37694 10.1371/journal.pone.0037694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn RC, Macedo AM, Fontes CJ, Batista RD, Santos NL, Hamdan JS. Randomly amplified polymorphic DNA as a valuable tool for epidemiological studies of Paracoccidioides brasiliensis. J Clin Microbiol, 2003; 41(7): 2849–54. 10.1128/jcm.41.7.2849-2854.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Queiroz Júnior LeP, de Camargo ZP, Tadano T et al. Serological and antigenic profiles of clinical isolates of Paracoccidioides spp. from Central Western Brazil. Mycoses, 2014; 57(8): 466–72. 10.1111/myc.12183 [DOI] [PubMed] [Google Scholar]
- 10.Fabris LR, Andrade UV, Santos AF et al. Decreasing prevalence of the acute/subacute clinical form of paracoccidioidomycosis in Mato Grosso do Sul, Brazil. Rev Inst Med Trop Sao Paulo, 2014; 56(2): 121–5. 10.1590/S0036-46652014000200006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camargo ZP, Taborda CP, Rodrigues EG, Travassos LR. The use of cell-free antigens of Paracoccidioides brasiliensis in serological tests. J Med Vet Mycol. 1991; 29(1): 31–8. [PubMed] [Google Scholar]
- 12.Jekel JF, Katz DL, Elmore JG. Tamanho da amostra, randomização e teoria da probabilidade. In: ___. Epidemiologia, estatística e Medicina Preventiva. Artmed 2ed., Porto Alegre, Brasil; 2005: 205–219.]
- 13.IBGE. Censo Demográfico 2010. https://censo2010.ibge.gov.br/resultados.html; 2011.
- 14.Mendes RP. The Gamut of Clinical Manifestations In: Franco M, Lacaz CS, Restrepo-Moreno A, Del-Negro G, editors. Paracoccidioidomycosis. Boca Raton: CRC press; 1994. [Google Scholar]
- 15.Shikanai-Yasuda MA, Telles-Filho FQ, Mendes RP et al. [Guidelines in paracoccidioidomycosis]. Rev Soc Bras Med Trop, 2006; 39(3): 297–310. 10.1590/s0037-86822006000300017 [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro SM, Nunes TF, Cavalcante RS, Paniago AMM, Mendes RP. A scoping study of pulmonary paracoccidioidomycosis: severity classification on radiographic and tomographic evaluation. J Venom Anim Toxins incl Trop Dis (accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitão NP, Vallejo MC, Conceição PM, Camargo ZP, Hahn R, Puccia R. Paracoccidioides lutzii Plp43 is an active glucanase with partial antigenic identity with P. brasiliensis gp43. PLoS Negl Trop Dis. 2014;8(8):e3111 10.1371/journal.pntd.0003111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenhard-Vidal A, Assolini JP, Chiyoda FAS, Ono MA, Sano A, Itano EN. Polyclonal antibodies to Paracoccidioides brasiliensis are able to recognise antigens from different strains from Paracoccidioides species complex, including Paracoccidioides lutzii LDR2. Mycoses. 2018;61(11):826–32. 10.1111/myc.12819 [DOI] [PubMed] [Google Scholar]
- 19.Buccheri R, Morais VDS, Kamikawa CM, Vidal MSM, Naves G, Del Negro GMB, et al. Case Report: Misleading Serological Diagnosis of Paracoccidioidomycosis in a Young Patient with the Acute Form Disease:. Am J Trop Med Hyg. 2018;98(4):1082–5 10.4269/ajtmh.17-0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richini-Pereira VB, Bosco SeM, Theodoro RC, Macoris SA, Bagagli E. Molecular approaches for eco-epidemiological studies of Paracoccidioides brasiliensis. Mem Inst Oswaldo Cruz. 2009, 104(4): 636–43. 10.1590/s0074-02762009000400018 [DOI] [PubMed] [Google Scholar]
- 21.Batista J, de Camargo ZP, Fernandes GF, Vicentini AP, Fontes CJ, Hahn RC. Is the geographical origin of a Paracoccidioides brasiliensis isolate important for antigen production for regional diagnosis of paracoccidioidomycosis? Mycoses, 2010; 53(2): 176–80. 10.1111/j.1439-0507.2008.01687.x [DOI] [PubMed] [Google Scholar]
- 22.Paniago AM, Aguiar JI, Aguiar ES et al. [Paracoccidioidomycosis: a clinical and epidemiological study of 422 cases observed in Mato Grosso do Sul]. Rev Soc Bras Med Trop, 2003; 36(4): 455–9. 10.1590/s0037-86822003000400004 [DOI] [PubMed] [Google Scholar]
- 23.Hrycyk MF, Garcia Garces H, Bosco SMG, de Oliveira SL, Marques SA, Bagagli E. Ecology of Paracoccidioides brasiliensis, P. lutzii and related species: infection in armadillos, soil occurrence and mycological aspects. Med Mycol. 2018;56(8):950–62 10.1093/mmy/myx142 [DOI] [PubMed] [Google Scholar]
- 24.Takayama A, Itano EN, Sano A, Ono MA, Kamei K. An atypical Paracoccidioides brasiliensis clinical isolate based on multiple gene analysis. Med Mycol. 2010;48(1):64–72. 10.3109/13693780902718065 [DOI] [PubMed] [Google Scholar]
- 25.Mendes JF, Klafke GB, Albano APN, Cabana Â, Teles AJ, de Camargo ZP, et al. Paracoccidioidomycosis infection in domestic and wild mammals by Paracoccidioides lutzii. Mycoses. 2017;60(6):402–6. 10.1111/myc.12608 [DOI] [PubMed] [Google Scholar]
- 26.Colares SM, Marcantônio S, Zambonato S, Severo LC. [Acute/subacute disseminated paracoccidioidomycoses. First case in Rio Grande do Sul]. Rev Soc Bras Med Trop, 1998; 31(6): 563–7. 10.1590/s0037-86821998000600010 [DOI] [PubMed] [Google Scholar]
- 27.Bellissimo-Rodrigues F, Machado AA, Martinez R. Paracoccidioidomycosis Epidemiological Features of a 1,000-Cases Series from a Hyperendemic Area on the Southeast of Brazil. Am J Trop Med Hyg, 2011; 85(3): 546–50. 10.4269/ajtmh.2011.11-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrade ALSS. Paracoccidioidomicose linfático-abdominal: contribuição ao seu estudo. Rev Pat Trop, 1983. p. 165–256. [Google Scholar]
- 29.Macedo PM, Teixeira MM, Barker BM, Zancopé-Oliveira RM, Almeida-Paes R, Francesconi do Valle AC. Clinical features and genetic background of the sympatric species Paracoccidioides brasiliensis and Paracoccidioides americana. PLoS Negl Trop Dis. 2019;13(4):e0007309 10.1371/journal.pntd.0007309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn RC, Rodrigues AM, Della Terra PP, Nery AF et al. Clinical and epidemiological features of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl Trop Dis, 2019; 13(6): e0007437 10.1371/journal.pntd.0007437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn RC, Rodrigues AM, Fontes CJ, et al. Fatal fungemia due to Paracoccidioides lutzii. Am J Trop Med Hyg, 2014; 91(2): 394–8. 10.4269/ajtmh.13-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schimke LF, Hibbard J, Martinez-Barricarte R et al. Paracoccidioidomycosis associated with a heterozygous STAT4 mutation and impaired IFN-γ immunity. J Infect Dis, 2017; 216(12): 1623–34. 10.1093/infdis/jix522 [DOI] [PubMed] [Google Scholar]
- 33.Scorzoni L, de Paula e Silva AC, Singulani JeL, Leite FS, de Oliveira HC, da Silva RA, et al. Comparison of virulence between Paracoccidioides brasiliensis and Paracoccidioides lutzii using Galleria mellonella as a host model. Virulence. 2015;6(8):766–76. 10.1080/21505594.2015.1085277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado GC, Moris DV, Arantes TD, Silva LR, Theodoro RC, Mendes RP, et al. Cryptic species of Paracoccidioides brasiliensis: impact on paracoccidioidomycosis immunodiagnosis. Mem Inst Oswaldo Cruz. 2013;108(5):637–43 10.1590/0074-0276108052013016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siqueira IM, Fraga CL, Amaral AC, Souza AC, Jerônimo MS, Correa JR, et al. Distinct patterns of yeast cell morphology and host responses induced by representative strains of Paracoccidioides brasiliensis (Pb18) and Paracoccidioides lutzii (Pb01). Med Mycol. 2016;54(2):177–88. 10.1093/mmy/myv072 [DOI] [PubMed] [Google Scholar]
- 36.del Negro GM, Benard G, de Assis CM, Vidal MS, Garcia NM, Otani C, et al. Lack of reactivity of paracoccidioidomycosis sera in the double immunodiffusion test with the gp43 antigen: report of two cases. J Med Vet Mycol. 1995;33(2):113–6. 10.1080/02681219580000241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.