Abstract
OBJECTIVE
To assess whether ageing processes influence angiogenesis in renal cell carcinoma (RCC) we carried out a pilot study of vascular properties in a series of archival primary kidney tumours in patients of different ages.
PATIENTS AND METHODS
A cohort of patients with RCC was identified restrospectively, with an age range of 35–84 years.
Paraffin-embedded, formalin-fixed sections of surgical tumour specimens were stained for endothelial (CD31, von Willebrand factor [vWF]), pericyte (alpha smooth muscle actin [SMA]) and leucocytic (CD45) markers, as well as for proliferative (Ki67) and angiogenic activity (tumour endothelial markers [TEMs], delta-like 4 [Dll4], Dll1, endothelial nitric oxide synthase [eNOS]).
Vascular properties were compared between patients above and below 65 years of age.
RESULTS
Microvascular density (MVD) within capillary hot spots was generally higher in patients with non-metastatic clear-cell RCC (ccRCC; n = 21) than in those with metastatic RCC (mRCC; n = 9).
Patients with ccRCC who were more than 65 years old showed significantly higher MVD than their younger (<65 years) counterparts. There were dividing (Ki67-positive) endothelial and mural cells in both small (<20 µm) capillary and large (>20 µm), pre-capillary vessels, suggesting the involvement of both angiogenic and remodelling/arteriogenic processes.
Tumour endothelial markers (TEM1, TEM7, TEM8), Notch ligands (Dll1, Dll4), and other molecular characteristics (eNOS) were analysed. Age-related differences were observed in the frequency of pre-capillary vessels expressing Dll1, which was significantly higher in tumours of younger patients (<65 years), while eNOS was more prevalent among capillaries associated with ccRCC in older patients (>65 years).
CONCLUSIONS
The results of the present study suggest that age influences the structural and molecular properties of the tumour vasculature in ccRCC.
We postulate that vascular ageing could also be relevant in the context of anti-angiogenic therapy.
Keywords: RCC, angiogenesis, arteriogenesis, ageing, endothelial nitric oxide synthase
INTRODUCTION
Neovascularization is critical for the progressive growth of solid tumours, but the mechanisms responsible for driving this process are still poorly understood, multifactorial and variable, especially between different tumour types [1]. The practical relevance of these processes is underscored by the growing interest in, and recent approval, of several novel anti-cancer therapeutics designed to target the tumour vasculature [1–3]. In this regard, primary kidney tumours, especially clear-cell RCC (ccRCC) in its metastatic presentation (mcRCC), constitute a particularly compelling case due to their unique pathogenesis. ccRCC is frequently driven by loss of the von Hippel–Lindau (VHL) tumour suppressor gene and this mutation results in constitutive activation of the hypoxia response pathway [4]. This, in turn, drives a uniquely high expression of vascular endothelial growth factor (VEGF) [5]. Events of this nature are believed to result in both the high vascularity and therapeutic responsiveness of these kidney tumours to agents targeting VEGF-driven angiogenesis [6,7], of which four (sunitinib, sorafenib, bevacizumab, pazopanib) are currently approved for clinical use in mcRCC [6,8].
Moderate levels of VEGF expression are normally present in renal parenchyma and are required for normal kidney homeostasis in the absence of angiogenesis [9]. However, the exuberant up-regulation of this growth factor in ccRCC qualitatively changes the responses of resident endothelial cells and provokes fulminant blood vessel growth [10], along with several molecular changes [11]. In this regard, Notch and its ligands, especially delta-like 1 and 4 (Dll1 and Dll4), are of particular interest as important regulators of pre-capillary ‘large’ [12] and capillary ‘small’ blood vessel growth [13], respectively. The growing blood vessel network is also equipped with feedback mechanisms to control blood volume distribution, through processes of vessel remodelling and regulation of vascular tone. The latter is executed, at least in part, by endothelial nitric oxide synthase (eNOS), which acts as the sensor of the intravascular shear stress [14], and a mechanism of the reactive vasodilatation through local production of nitric oxide (NO) [15,16]. While these mechanisms have been detected in various cancer settings and linked to VEGF-driven angiogenesis [17], their role in ccRCC remains relatively unexplored [18,19].
In several epithelial cancers, high microvascular density (MVD) can predict increased disease aggressiveness [20]. In ccRCC, however, this linkage appears to be more complex, controversial and largely unexplained. Clearly, methodological factors, such as different blood vessel detection and quantification protocols, heterogeneity of tissue samples (e.g. tumour stage, grade, size or degree of hypoxia [21]), as well as random variablility in clinical and pathological characteristics of the respective patient populations could have contributed to the aforementioned inconsistencies.
Alternatively, however, the variable results of MVD analyses could also be a reflection of more biologically meaningful properties of the ccRCC patient populations [22,23]. In this regard, one largely overlooked aspect of the inter-individual diversity amongst patients with ccRCC is their age. Indeed, the age of patients included in various ccRCC studies may span nearly six decades [24] and such a long period of time could be consequential for the status of the kidney and tumour microcirculation [25]. Age-related effects could be inferred from studies on the role of ageing in the function of the vascular system as a whole [26] and the changing functionality of the VEGF pathway [27]. Ageing is also known to modulate tumour angiogenesis in experimental settings [28–30] and could affect both tumour responses and side-effects of anti-angiogenic therapies [31]. These effects either are a direct consequence of ageing and exhaustion within the endothelial and bone marrow compartments [26,28,31] or are related to age-dependent vascular co-morbidities such as atherosclerosis, thrombosis and inflammatory conditions [31]. Analyses of these processes in ccRCC are presently lacking.
In this pilot study aimed at exploring some of the aforementioned questions, we report the results of vascular analysis performed in a cohort of 39 patients with kidney tumours, of whom 21 were diagnosed with ccRCC and were in the age range 36–79 years. We show that age-related differences exist between ccRCC tumours removed from younger (<65 years) and from older patients (>65 years). For instance, higher MVD counts, lower prevalence of Dll1-positive pre-capillary vessels, and higher numbers of eNOS-positive microvessels were found in older patients. We postulate that age-specific vascular features (both architectural and molecular) could be of translational value in malignant kidney tumours, especially with respect to the further development of anti-angiogenic agents for this disease.
PATIENTS AND METHODS
The clinical, pathological and other relevant characteristics of the patient cohort included in the present study are summarized in Table 1. Overall, 44 samples were analysed in detail, including 21 cases of ccRCC, nine cases of metastatic RCC (mRCC), five cases of non-ccRCC, four cases of oncocytoma and five specimens of normal kidney. Among the patients with ccRCC, 11 were older than 65 years, and 10 were younger (individual specimens were occasionally found inadequate for specific analyses, as indicated). All tumours included in the present study were diagnosed, and patients were cared for at the Department of Urology, Sunnybrook Hospital, University of Toronto (L.K). Preliminary analysis was conducted using an independent small sample of six ccRCC tumours obtained from the Provincial Specialist Hospital, Research and Development Center in Wroclaw, Poland (K.P-R.). Normal human kidney slides were purchased from Biochain (catalogue no. T2234142; Harvard, CA, USA) and Abcam (catalogue no. ab4347, Cambridge, MA, USA).
TABLE 1.
Clinical and pathological characteristics
| Age (median), years | N (male/female) | Tumour size (median), cm | Grade (median) | |
|---|---|---|---|---|
| Normal human kidney (control) | 50–60 (NA) | 5 (4/1) | None | N/A |
| Oncocytoma | 63–81 (78.5) | 4 (2/2) | 2.0–7.5 (3.75) | N/A |
| ccRCC | 36–79 (60.5) | 21 (9/12) | 1.3–13 (5.5) | 1–4 (2.5) |
| Non-ccRCC* | 51–70 (62) | 5 (3/2) | 2.1–7 (5.0) | 1–3 (2) |
| mRCC | 35–84 (63) | 9 (5/4) | 2.5–12 (5.0) | 2–4 (3) |
Non-ccRCC cohort consists of patients with papillary RCC (two), chromophobe eosinophilia RCC (one), leiomyosarcoma (one) and haemangiopericytoma (one).
ANTIBODIES
Mouse anti-PECAM (NCL-CD31-1A10) was purchased from Novocastra (UK). Rabbit polyclonal anti-tumour endothelial marker-1 (anti-TEM1) (no. 67275) was purchased from Abcam. Mouse anti-TEM7 and anti-TEM8 were generated in the laboratory of B.S.C. (National Cancer Institute, Frederick, MD, USA). Monoclonal mouse anti-human CD45 and anti-human von Willebrand Factor (vWF, A0082) antibodies were purchased from Dako (Missisauga, ON, Canada). Ki67 and anti-smooth muscle actin (SMA, A-2547) antibodies were purchased from Sigma (St Louis, MO, USA). Rabbit polyclonal anti-eNOS antibody (PA1-37624) was from Affinity Bio Reagents (Golden, CO, USA). Rabbit anti-mouse polyclonal antibody against Dll1 (LS-B72) was from Lifespan Biosciences Inc. (Seattle, WA USA). Rabbit polyclonal antibody to Dll4 was purchased from Abcam (catalogue no. ab7280). Antibodies recognizing angiopoietin-1/2 and phospho-vascular endothelial growth factor receptor-2 (phospo-VEGFR-2) were from R&D Systems (Minneapolis, MN, USA).
IMMUNOHISTOCHEMICAL ANALYSIS
Paraffin-embedded, formalin-fixed tissue samples were sectioned at 5 µm. All tissues were de-waxed in xylene and rehydrated through a graded series of ethanol, as described previously [32]. Microwave antigen retrieval was performed using Vector Antigen Unmasking Solution (H-3300, Vector Laboratories, Burlingame, CA, USA) at pH 6.0. Primary antibodies were incubated with the samples overnight at 4 C. For bright-field visualization, slides processed using the Vector AEC kit were mounted using Vectamount (H-5000) media (Vector Laboratories). For immunofluorescent staining, the appropriate secondary antibodies conjugated with AlexaFlour 488, 594 and 350 fluorophores (Molecular Probes, CA, USA) were used accordingly. Slides were mounted using Vectashield with DAPI (H-1500, Vector Laboratories), and viewed and analysed using a Zeiss Axiophot microscope.
MVD AND PRE-CAPILLARY VESSEL DENSITY (PVD) ANALYSES
All samples were analysed by a blinded observer using pre-coded samples. Upon staining of tissues with pan-endothelial markers (CD31, vWF), MVD was evaluated by counting capillary-sized vessels (<20 µm in diameter). The density of larger (>20 µm) vessels was designated as PVD, irrespective of their arterial or venous identity. For MVD counts, tumour sections stained for CD31 were first surveyed under low magnification to locate microvascular ‘hot spots’, defined as areas of the highest capillary density within a given slide, as previously described [20]. All microvessels within each of the three independent hot spot areas per specimen were counted under ×400 magnification and their mean MDV was expressed as vessel density per high power field (hpf) [20]. Similar procedures were applied to determine PVD using vWF immunofluorescence to highlight endothelial cells of larger vessels. Again, three areas containing clusters of the largest pre-capillary vessels were selected for each tissue specimen under low power (×50). The number of blood vessels in each such region (pre-capillary hot spot) was subsequently counted under ×400 magnification and expressed as the vessel number per hpf, or ‘hot spot PVD’. We also independently evaluated tumours for global PVD, using CD31-stained sections. In this case, the entire section area was divided into nine equivalent regions (3 × 3 fields). Photographs of identical fields defined by the intersecting vertices were taken at ×100 magnification and the numbers of pre-capillary vessels in these regions were calculated and expressed as the mean global PVD. The sizes (longest width) of all pre-capillary vessels in these regions were measured and their numbers in each specimen plotted as indicated.
CELL PROLIFERATION OF AND NECROSIS
Triple staining for vWF (vWF/factor VIII related antigen), alpha-SMA (α-SMA) and Ki67 was used to determine the mitogenic activity of the respective vascular (endothelial and mural) cells. Tumour cell proliferation rate was also assessed by Ki67 staining. Positivity of various cells for Ki67 was evaluated at ×400 magnification and the number of cells with positive nuclei were counted in 10 independent fields per specimen and the mean determined. Tumour necrosis was evaluated by examining at least two different haematoxylin and eosin-stained slides from each specimen, followed by morphometric measurements of the respective viable and necrotic regions. Necrotic area was calculated as the percentage of the entire tumour section area occupied by necrotic masses.
EXPRESSION OF FUNCTIONAL VASCULAR MARKERS
The functional state of tumour microvessels was assessed by immunofluorescent staining for eNOS, Dll1 and other markers, as indicated. For eNOS immunostaining, slides were exposed to the respective antibody and co-stained for CD31. The number of capillary and pre-capillary blood vessels positive for eNOS was assessed in 10 random fields per section under ×400 and ×200 magnifications, respectively. The mean number of eNOS-positive vessels per hpf was used for comparisons between patients. For Dll1 analysis, the entire slide was viewed under ×400 and ×200 magnifications. All vessels that stained positive for Dll1 were measured and classified as either capillaries or pre-capillaries (as described earlier). The respective vessel categories were enumerated and expressed as the number of Dll1-positive vessels per slide. Slides were also stained for CD45 and vWF to evaluate the extent and perivascular location of the infiltrating leucocytic cells.
STATISTICAL ANALYSES
Statistical analysis was conducted by t-test between two groups using SPSS version 12 for Windows.
RESULTS
HETEROGENEOUS VASCULAR PATTERNS IN PRIMARY RENAL TUMOURS
Immunostaining for pan-endothelial markers, including CD31 and vWF, shows a rich network of blood vessel capillaries in both normal and neoplastic kidneys (Fig. 1A,B and data not shown). However, while the cortical vasculature of the normal kidney is well organized, with defined glomeruli and larger supplying vessels, the tumour-associated microcirculation is profoundly disorganized with capillary, pre-capillary and larger vascular structures present throughout the parenchyma at variable densities, often clustering into microvascular hot spots (Fig. 1B). The MVD within these latter regions is often viewed as informative as to the intensity of the angiogenic process and the related disease aggressiveness [20]. Therefore, we assessed hot spot MVD in a diverse cohort of archival tissue specimens, including normal kidney, benign kidney tumours (oncocytoma) and aggressive RCC, of either ccRCC or non-ccRCC morphology. The ccRCC samples also included material from patients who eventually progressed to develop mRCC (Table 1).
FIG. 1.

Microvascular density in primary kidney tumours. A, B, Representative patterns of capillary blood vessels shown by staining for the pan-endothelial marker CD31. Well-organized glomerulal structures in cortical sections of the normal kidney A, contrast with chaotic blood vessels in RCC B,. C. Hot spot MVD of benign (oncocytoma) and malignant (RCC) kidney tumours. Notably, MVD in oncocytoma resembles that of normal kidney cortex (NK, dotted line), while an extremely wide MVD distribution is observed in RCC. D, Separation of the RCC cohort into RCC sub-sets shows higher MVD in ccRCC, than in non-ccRCC (nccRCC) and metastatic (mRCC) tumours. The latter difference is statistically significant (P < 0.05), even though the values for individual patients continue to exhibit wide heterogeneity. Median values are denoted by thick horizontal lines.
Vascular densities shown by CD31 staining in these respective tissues were vastly different. While MVD in oncocytoma resembled that of the normal renal cortex, some RCC lesions were highly vascular, but as a whole tumour vasculature exhibited considerable numerical variation between individual patients (Fig. 1C). Even when tumours were stratified into non-ccRCC, ccRCC and mRCC sub-sets, the hot spot capillary vessel densities varied markedly within each of these patient groups (Fig. 1D). Interestingly, tumour sections representative of mRCC exhibited significantly lower MVD values then those of patients with ccRCC, who did not progress to mRCC after surgery. Although counter-intuitive, this finding is consistent with a previous report [33].
AGE-RELATED CHANGES IN THE VASCULARITY OF ccRCC
Even upon restriction of our MVD analysis to ccRCC, the largest sub-set within our cohort, we observed a marked patient-to-patient variability. We sought to understand the nature of this diversity by plotting the data as a function of several different parameters, including tumour linear length, tumour grade, mitogenic activity, necrotic region, and other features, none of which correlated with the tumour MVD counts (data not shown).
Since tumour angiogenesis was previously shown to be modulated by vascular ageing [26,28,31], we also plotted MVD values as a continuous function of age, or upon stratification into groups of patients: either <65 or >65 years old (Fig. 2A,B). Interestingly, this stratification showed notable differences, in that tumours resected from patients >65 years exhibited significantly higher MVD than those from their younger counterparts (≤65). These changes were not linked to noticeable differences in mitotic indices of cancer cells or to the extent of tumour necrosis (data not shown).
FIG. 2.
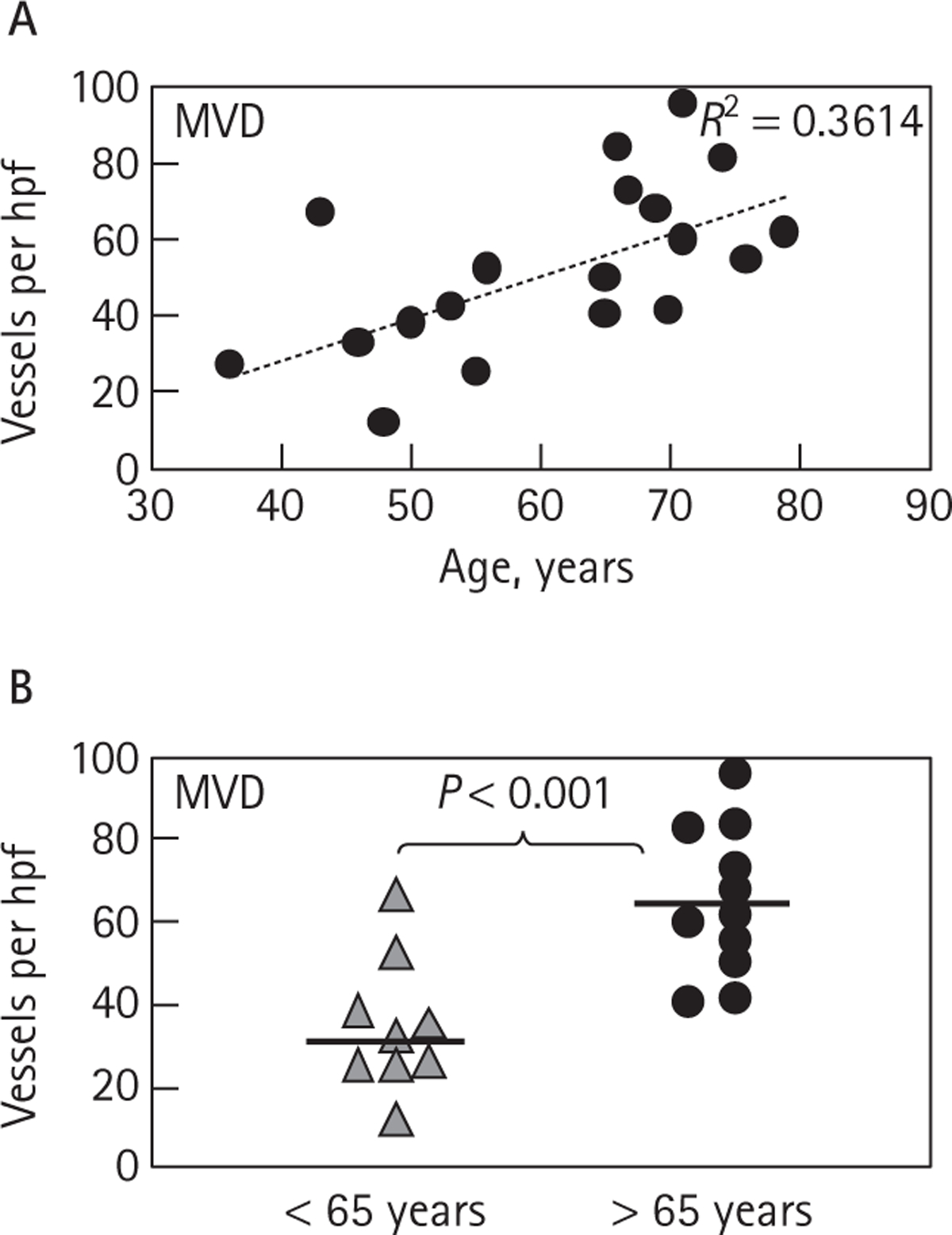
Age-related distribution of MVD in ccRCC. A, Gradual increase in MVD values with the age of patients with ccRCC. B, MVD of patients with ccRCC older than 65 years is markedly higher than in younger patients (P < 0.001). Median values are denoted by thick horizontal lines.
Since blood vessel growth and remodelling implicitly involves mitogenesis of endothelial and mural cells [31], samples of ccRCC were subjected to triple immunofluorescent staining for markers of proliferation (Ki67), endothelium (vWF) and mural cells (α-SMA). As might be expected, tumour blood vessels in both younger and older patients contained dividing endothelial cells (Ki67/vWF-positive) and pericytes (Ki67/α-SMA-positive), but their corresponding vascular cells in the normal kidney remained completely Ki67-negative (data not shown). Overall, the low numbers of endothelial nuclei unequivocally positive for Ki67 in standard histological sections did not permit an accurate quantification and comparison of this mitogenic activity between different patient sub-sets.
Notably, features of endothelial and smooth muscle cell proliferation were apparent in both capillary and larger, pre-capillary tumour blood vessels (Fig. 3), an observation that suggests a co-existence of angiogenic and an arteriogenic-like vascular growth [34]. To assess whether the related (‘neo’)arteriogenesis [34] exhibited age-dependent properties, we assessed the PVD in ccRCC tumours of patients >65 years and those <65 years. PVD counts were collected either within the pre-selected clusters of large vessels (‘PVD hot spots’) or in random fields across the entire cross-section of each tumour specimen (‘PVD global count’; Fig. 4A). While there was a trend towards higher density of intermediately sized vessels (20–50 μm in diameter) in older patients, this difference (unlike MVD) did not reach statistical significance, and the densities of larger vessels (50–500 μm) were quite similar in both age groups (Fig. 4B,C). Overall, this analysis suggests that certain characteristics of tumour-related vascular structures, especially the hot spots of capillary microvessels, correlate with age of patients with ccRCC.
FIG. 3.

Evidence of vascular cell proliferation in capillary and pre-capillary vessels in ccRCC. Triple immunofluorescence for endothelial (vWF/blue), mural (SMA/green) and proliferation markers (Ki67/red and red arrows) shows evidence of proliferation of pericytic/smoth muscle A, B, D, and endothelial cells C. Proliferating vascular cells were found throughout ccRCC-associated vascular structures, including capillary A, B, medium-sized D and larger, pre-capillary vessels (C, red dotted line shows the vessel diameter of approximately 100 μm).
FIG. 4.
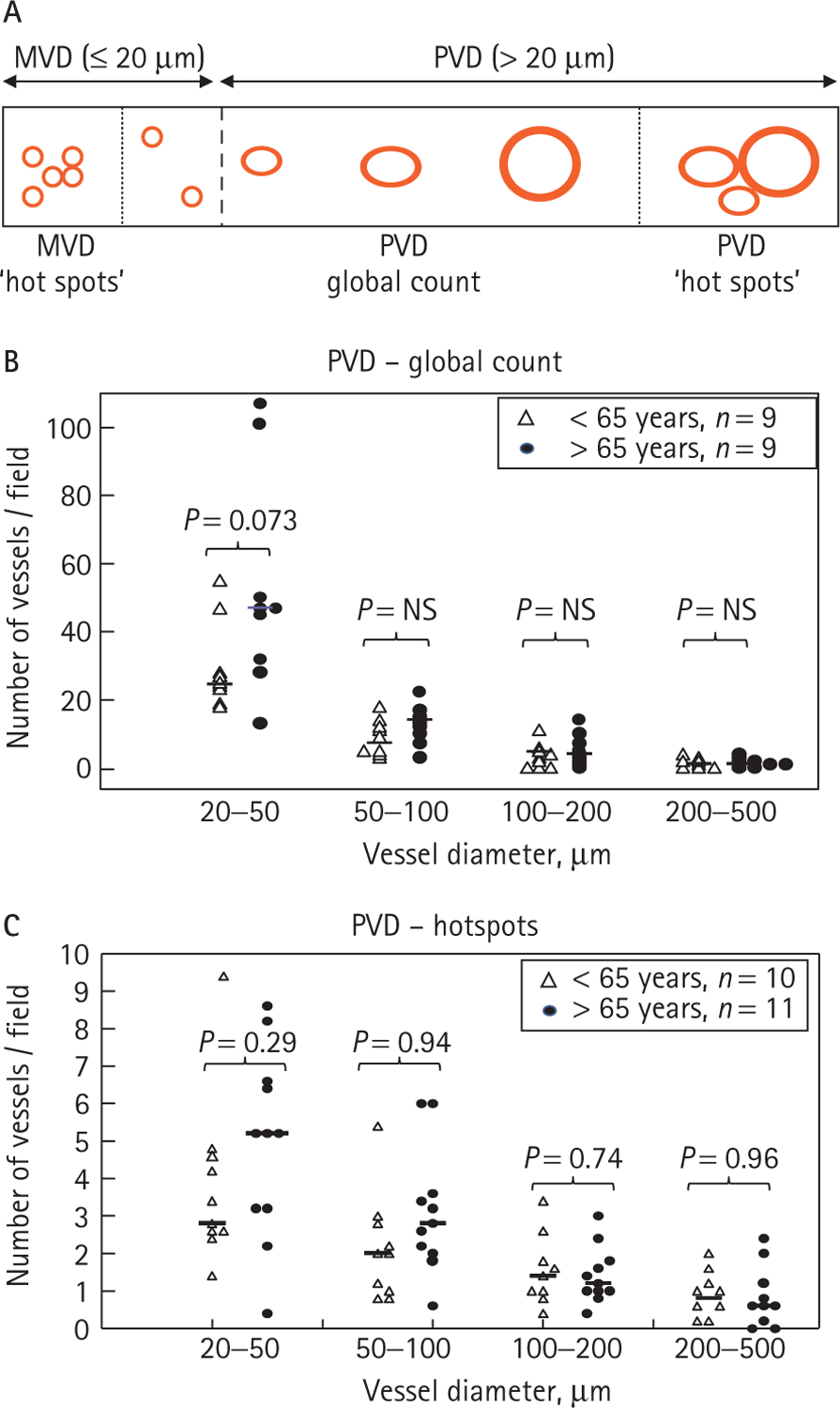
Analysis of age-related changes in ccRCC-associated pre-capillary blood vessels. A, Illustration depicting the criteria used to evaluate capillary (MVD) and pre-capillary (PVD) vascular densities in the present study. Counts were either performed within the hot spots (clusters) of capillary (<20 μm) or larger (20–500 μm) blood vessels, or the respective vascular structures were enumerated in random fields across the entire tissue specimen (global count). B, Global count of pre-capillary blood vessels (PVD) in ccRCC tumours of patients younger (triangle) or older (filled circle) than 65 years. A trend towards greater density of intermediate-sized (20–50 μm) vessels in older patients was observed, consistent with the results of the MVD count (Fig. 2B). However, with increasing vessel sizes, their densities were lower and more indistinguishable between both groups of patients. C, Vascular density count (PVD) in hot spots of pre-capillary vessels shows a trend similar to that of a global count B. Median values are denoted by short horizontal lines.
AGE-RELATED MOLECULAR FEATURES OF ccRCC-RELATED BLOOD VESSELS
We reasoned that the aforementioned structural differences (MVD) between tumour blood vessels in younger and older ccRCC patients must be related to some underlying molecular processes and characteristics [31]. In this regard, several molecular hallmarks of tumour-related blood vessels have been described in recent years, including TEMs [31,35], up-regulation/activation of VEGF receptors, and changes in other angiogenesis-regulating molecules [2], such as elements of the angiopoietin system, Notch ligands and several other entities [36]. Some of these molecules have already been evaluated as a function of vascular ageing (e.g. TEM1/glycosialin, VEGFR-2/flk-1), but on a very limited scale and only in experimental studies [31].
To extend this analysis to human ccRCC, we surveyed several angiogenesis-related molecules by immunofluorescence. This included staining for several human TEMs (TEM1, TEM7 and TEM8), Dll1, Dll4 and eNOS, along with the presence of myelocytic (CD45-positive) cells in the perivascular tumour regions (Fig. 5 and data not shown). Staining patterns for TEM1, TEM7, TEM8, Dll4 and CD45 were similar in the ccRCC specimens from both younger and older patients (data not shown). In the case of TEM1 and TEM8, the staining was found in both endothelial and in some perivascular cells, while the TEM7 signal was primarily detected in a sub-set of endothelial cells (Fig. 5A–C). This pattern could reflect the emerging and differential association of some of these molecules (TEM1) with endothelial, mural [37] and endothelial progenitor cell populations [38]. ccRCC tissues stained poorly for the Notch ligand, Dll4 (Fig. 5E), even though the same reagents yielded a robust and endothelial-specific signal of human glioblastoma specimens (data not shown).
FIG. 5.
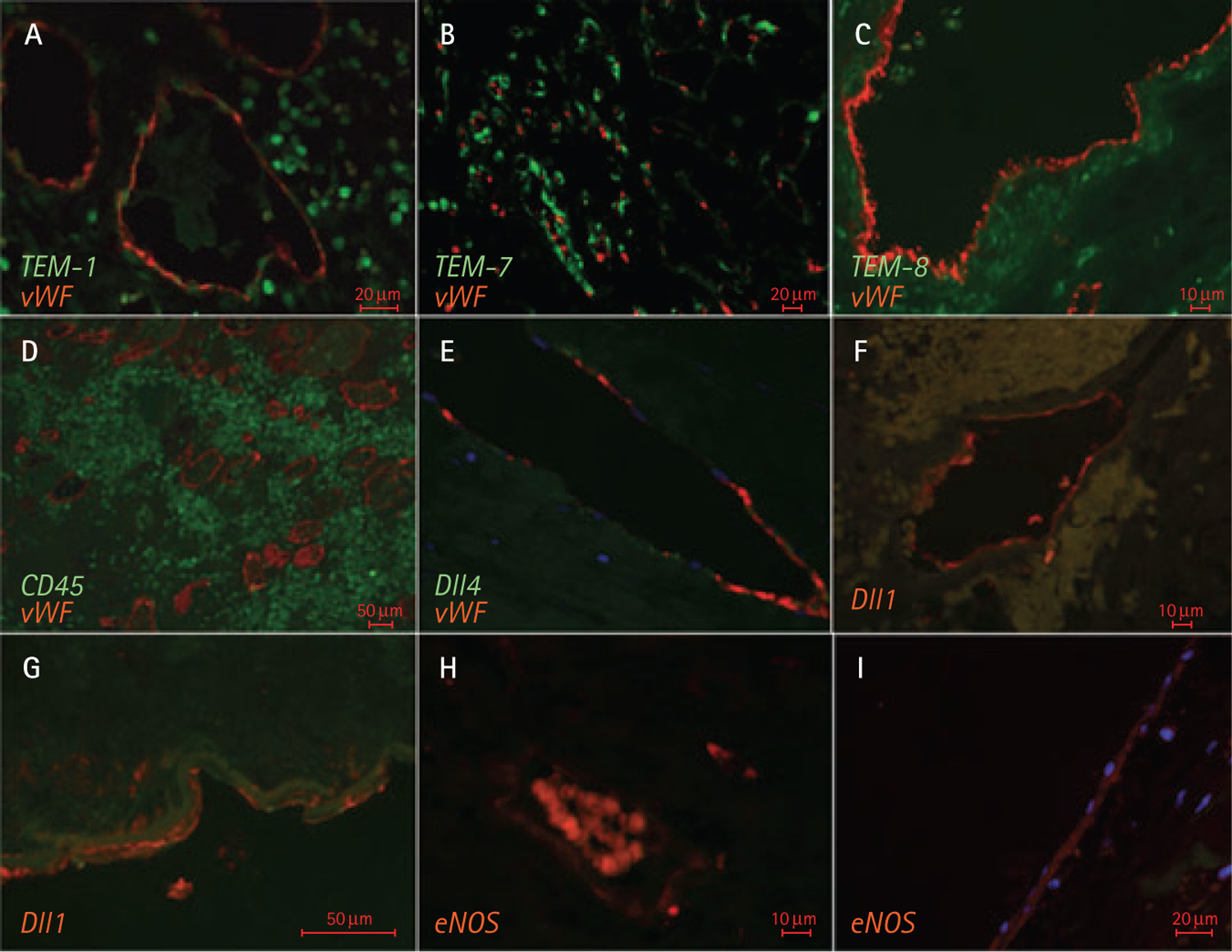
Immunofluorescent staining for vascular markers associated with ccRCC. A, Expression of TEM1 (green) by endothelial and extravascular cells infiltrating ccRCC. Endothelial cells are highlighted by co-staining for vWF (red). B, TEM7 staining (green) co-localized with vessels but was predominantly non-overlapping with vWF (red) in ccRCC tumours. C, Staining of ccRCC for TEM8 (green) was predominantly perivascular; vWF staining is shown in red. D, Massive perivascular infiltration of the tumour mass with CD45-positive (green) leucocytic cells. Blood vessels are highlighted by staining for vWF (red). E, Endothelial pattern of ccRCC staining for Dll4 (green) and vWF (red) – large pre-capillary vessel. F, G, Staining of pre-capillary ccRCC-associated vessels for Dll1. H, I, Specific immunoreactivity of endothelial cells in small- and large-calibre blood vessels with the anti-eNOS antibody. In H, the unspecific intravascular signal is associated with autofluorescence of red blood cells.
Interestingly, age-related differences were noted in the case of another Notch ligand, Dll1, which has been implicated in both angiogenesis and arteriogenesis [12]. Thus, the number of Dll1-positive capillaries did not differ significantly between tumours in younger and older ccRCC patients. However, similar analysis of pre-capillary vessels (>20 μm) showed that the Dll1 antibody was able to decorate a greater fraction of tumour vessels in patients who were younger than 65 years than in their older counterparts (>65 years; Fig. 6A,B). In both instances, Dll1 was present mainly on the surface of endothelial cells (Fig. 5).
FIG. 6.

Age-related expression of Dll1 in the vasculature of ccRCC tumours. A, Similar frequencies of Dll1-expressing capillary blood vessels in patients younger and older than 65 years. B, Lower number of Dll1-positive pre-capillary vessels (>20 μm) per hpf in patients with ccRCC > 65 years old than in their younger counterparts (P < 0.05). Median values are denoted by thick horizontal lines.
Age-related differences were also noted in the case of eNOS expression (Figs 5,7). This marker of increased vascular shear stress [14] was upregulated more frequently in ccRCC-associated capillaries of older patients (P < 0.02) than in those who were younger than 65 years. A similar trend was observed in the case of larger vessels, but in this case it did not reach statistical significance. Taken as a whole, these observations show both structural and molecular, age-related differences in the vasculature of ccRCC.
FIG. 7.
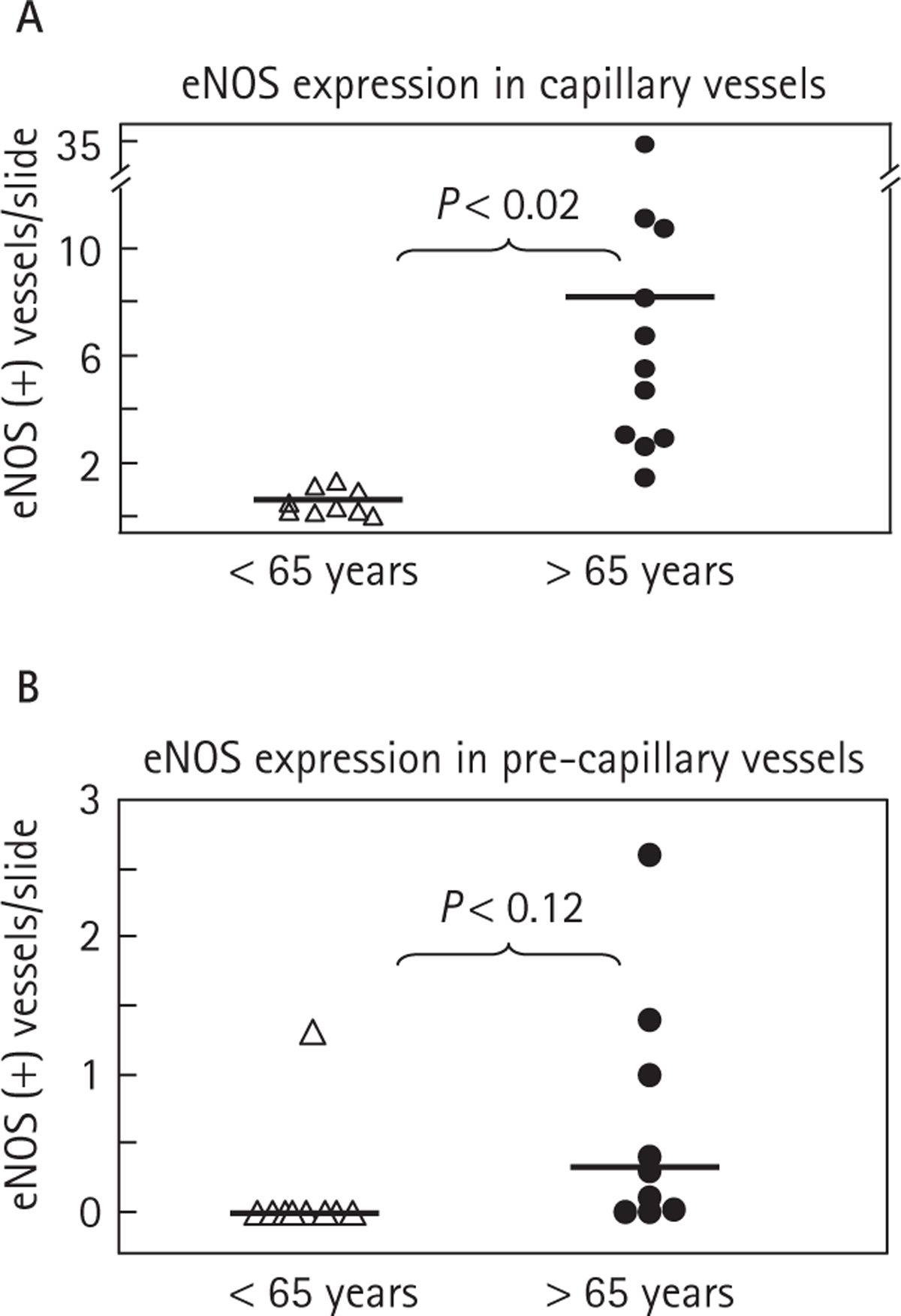
Age-related expression of eNOS in the vasculature of ccRCC tumours. A, Markedly higher frequency of eNOS-expressing capillary blood vessels in patients older than 65 years than in those younger than 65 (P < 0.02). B, Frequencies of eNOS-positive pre-capillary vessels (>20 μm) in patients with ccRCC younger and older than 65 years. Interestingly, a virtual absence of eNOS in most younger patients contrasts with the positivity for this shear stress-related marker in approximately 50% of older patients. However, this trend has not reached statistical significance. Median values are denoted by thick horizontal lines.
DISCUSSION
The exploratory analysis of ccRCC in the present study suggests that kidney tumours can exhibit age-related alterations in their vascular characteristics. It is striking that such differences were noticeable even in a very small cohort of patients, and larger studies are warranted to investigate this pattern more fully. Thus, we observed a higher capillary density (MVD) and higher frequency of microvessels positive for eNOS in patients older than 65 years than in tumours that originated in younger patients. Conversely, vascular Dll1 expression was more prevalent in the latter group, but only in larger, pre-capillary tumour vessels. The specific reasons for this pattern are presently unknown. Nonetheless, Dll1 has been implicated in postnatal arteriogenesis [12], a process that is regulated by shear stress, of which the elevated expression of eNOS is a frequently found indicator [14]. It is conceivable that with age the coupling between the formation of larger ‘feeding’ vessels (‘neo-arteriogenesis’) [34] and their corresponding tumour capillaries (angiogenesis) is altered and leads to a greater shear stress and remodelling within the microcirculation.
We observed an increased mitogenic activity in both endothelial and mural cell compartments of blood vessels within ccRCC tumours. Because this occurred in both capillary and pre-capillary vessels, one can surmise that ccRCC progression triggers both angiogenic (capillary) and arteriogenic (pre-capillary) vascular growth, as we suggested in a previous report [34]. While much attention is devoted to tumour angiogenesis, the concomitant circumferential/arteriogenic remodelling of larger tumour vessels (neo-arteriogenesis) is relatively unstudied and poorly understood. In the context of tissue ischaemia, formation of larger vessels (collaterals) is attributed to retrograde shear stress, endothelial activation and influx of monocytic cells [15]. Other contributing changes include the expression of inflammatory chemokines, adhesion molecules [11], Dll1 [12] and nitric oxide signalling [39]. Thus, unlike angiogenesis, the growth of larger vessels could be independent of hypoxia [40]. Instead, these vessels could be influenced by bone marrow cells which, in turn, could be affected by an age-dependent exhaustion of bone marrow [31,41,42]. It remains to be established whether these processes are relevant in the context of ccRCC.
Irrespectively of mechanistic considerations, the linkage between vascularity and progression of kidney cancers has long been perplexing. Thus, while some studies found a positive correlation between high MVD and poor patient survival [21,43,44], others concluded the opposite to be true [21,24,33,45,46], or questioned the prognostic significance of MVD in ccRCC altogether [47,48]. These discrepancies motivated several methodological refinements to capture the heterogeneity of the tumour-related vasculature with a greater precision, for instance, by pointing to the preponderance of larger vessels in more aggressive lesions [33] or by analysis of more subtle features of the vascular architecture [49], including its fractal properties [46]. Interestingly, some of this work also showed the existence of both cellular and molecular heterogeneity within the blood vessel networks of ccRCC tumours, including the presence of distinct CD34-positive and CD34-negative subsets of tumour capillaries [50]. Variable amounts of circulating endothelial progenitor cells [51] and differences in the expression of various angiogenesis-related genes other than VEGF have also been reported [52]. It remains to be seen whether these processes are affected by vascular ageing.
It is of considerable interest whether the age-related vascular properties of ccRCC, and especially mRCC, have therapeutic relevance [6]. Nearly 50% of all patients with RCC are more than 65 years old, although the disease can emerge as early as the third decade of life [8]. While age is recognized as a factor relevant for the cardiovascular safety of anti-angiogenic agents [8], its influence on therapeutic efficacy and mechanisms of biological response has received much less attention. Nonetheless, it is intriguing that in one preliminary study, which addressed age-related responses to therapy, sorafenib appeared most efficacious in patients with RCC who were older then of 65 years [7].
In conclusion, the present study suggests that ageing could influence structural, molecular and possibly functional characteristics of kidney tumour blood vessels. Indeed, processes related to vascular ageing could potentially affect multiple characteristics of the disease, including tumour growth and metastasis [53], aggressiveness [54] and the effects of various therapeutics [55], including those with anti-angiogenic and non-angiogenic mechanisms of action. The present, exploratory analysis, which is based on a small patient cohort, represents an essential first step towards understanding how ageing affects vascular architecture and molecular profiles in RCC. Follow-up studies are required to determine if these vascular variables can help to evaluate tumour vascular responses to anti-angiogenic agents. Age-related alterations in the vasculature could also help explain some of the inconsistencies observed between independent patient cohorts regarding the prognostic value of vascular density counts.
What’s known on the subject? and What does the study add?
Little is known about the impact of vascular ageing on the angiogenic features of clear cell renal cell carcinoma, a disease in which antiangiogenic therapy currently has a well established role. It is also rather surprising that this question has not been raised in a disease context where patients’ age may differ by several decades. We provide the first glimpse in to the related vasclar changes, including morphology and some of the molecular features.
ACKNOWLEDGEMENTS
We would like to thank Dr Xun Zhang for help with statistical analysis and Dr Halka Klement for technical help with the triple immunostaining of blood vessels. We are grateful to our colleagues for their advice and inspiration, and to our families, especially to Anna and Danuta Rak for their inexhaustible patience and support.
Source of Funding: this work was supported by grants from the Cancer Research Society and partially by funds from the Canadian Cancer Society, both to J.R., who is also a recipient of the Jack Cole Chair in Pediatric Oncology. Infrastructure support was provided by Fonds de la Recherche en Sante Quebec.
Abbreviations:
- ccRCC
clear-cell RCC
- Dll
delta-like
- eNOS
endothelial nitric oxide synthase
- hpf
high power field
- mRCC
metastatic RCC
- MVD
microvascular density
- PVD
pre-capillary vessel density
- SMA
smooth muscle actin
- TEM
tumour endothelial marker
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Folkman J, Kalluri R. Chapt 11: Tumor angiogenesis In Kufe DW, Pollock RE, Weichselbaum RR et al. eds. Cancer Medicine, 6th edn. Hamilton, London: BC Decker Inc., 2003: 161–94 [Google Scholar]
- 2.Kerbel RS. Tumor angiogenesis. N Engl J Med 2008; 358 (19): 2039–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3 (5): 391–400 [DOI] [PubMed] [Google Scholar]
- 4.Brown LF, Berse B, Jackman RW et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol 1993; 143: 1255–62 [PMC free article] [PubMed] [Google Scholar]
- 5.Kaelin WG Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 2008; 8 (11): 865–73 [DOI] [PubMed] [Google Scholar]
- 6.Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol 2009; 27 (19): 3225–34 [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356 (2): 125–34 [DOI] [PubMed] [Google Scholar]
- 8.Dutcher JP, Tannir N, Bellmunt J, Escudier B. Experience with sorafenib and the elderly patient. Med Oncol 2009. [Epub ahead of print] [DOI] [PubMed]
- 9.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF – a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol 2007; 106 (2): 32–7 [DOI] [PubMed] [Google Scholar]
- 10.Schoenfeld J, Lessan K, Johnson NA et al. Bioinformatic analysis of primary endothelial cell gene array data illustrated by the analysis of transcriptome changes in endothelial cells exposed to VEGF-A and PlGF. Angiogenesis 2004; 7 (2): 143–56 [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000; 6 (4): 389–95 [DOI] [PubMed] [Google Scholar]
- 12.Limbourg A, Ploom M, Elligsen D et al. Notch ligand Delta-like 1 is essential for postnatal arteriogenesis. Circ Res 2007; 100 (3): 363–71 [DOI] [PubMed] [Google Scholar]
- 13.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer 2007; 7 (5): 327–31 [DOI] [PubMed] [Google Scholar]
- 14.Jones EA, le Noble F, Eichmann A. What determines blood vessel structure? Genetic prespecification vs. hemodynamics. Physiology 2006; 21: 388–95 [DOI] [PubMed] [Google Scholar]
- 15.Scholz D, Cai WJ, Schaper W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis 2001; 4 (4): 247–57 [DOI] [PubMed] [Google Scholar]
- 16.Searles CD. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am J Physiol Cell Physiol 2006; 291 (5): C803–16 [DOI] [PubMed] [Google Scholar]
- 17.Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res 2007; 67 (4): 1407–10 [DOI] [PubMed] [Google Scholar]
- 18.Sun S, Du R, Gao J et al. Expression and clinical significance of Notch receptors in human renal cell carcinoma. Pathology 2009; 41 (4): 335–41 [DOI] [PubMed] [Google Scholar]
- 19.Patel NS, Li JL, Generali D, Poulsom R, Cranston DW, Harris AL. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res 2005; 65 (19): 8690–7 [DOI] [PubMed] [Google Scholar]
- 20.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8 [DOI] [PubMed] [Google Scholar]
- 21.Imao T, Egawa M, Takashima H, Koshida K, Namiki M. Inverse correlation of microvessel density with metastasis and prognosis in renal cell carcinoma. Int J Urol 2004; 11 (11): 948–53 [DOI] [PubMed] [Google Scholar]
- 22.Rohan RM, Fernandez A, Udagawa T, Yuan J, D’Amato RJ. Genetic heterogeneity of angiogenesis in mice. FASEB J 2000; 14 (7): 871–6 [DOI] [PubMed] [Google Scholar]
- 23.Lyden D, Young AZ, Zagzag D et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 1999; 401 (6754): 670–7 [DOI] [PubMed] [Google Scholar]
- 24.Yildiz E, Ayan S, Goze F, Gokce G, Gultekin EY. Relation of microvessel density with microvascular invasion, metastasis and prognosis in renal cell carcinoma. BJU Int 2008; 101 (6): 758–64 [DOI] [PubMed] [Google Scholar]
- 25.Long DA, Mu W, Price KL, Johnson RJ. Blood vessels and the aging kidney. Nephron Exp Nephrol 2005; 101 (3): e95–9 [DOI] [PubMed] [Google Scholar]
- 26.Reed MJ, Edelberg JM. Impaired angiogenesis in the aged. Sci Aging Knowledge Environ 2004; 2004 (7): e7. [DOI] [PubMed] [Google Scholar]
- 27.Rivard A, Berthou-Soulie L, Principe N et al. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem 2000; 275 (38): 29643–7 [DOI] [PubMed] [Google Scholar]
- 28.Pili R, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A. Altered angiogenesis underlying age-dependent changes in tumor growth. J Natl Cancer Inst 1994; 86 (17): 1303–14 [DOI] [PubMed] [Google Scholar]
- 29.Franco S, Segura I, Riese HH, Blasco MA. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res 2002; 62 (2): 552–9 [PubMed] [Google Scholar]
- 30.Shimada T, Takeshita Y, Murohara T et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation 2004; 110 (9): 1148–55 [DOI] [PubMed] [Google Scholar]
- 31.Klement H, Croix B St, Milsom C et al. Atherosclerosis and vascular aging as modifiers of tumor progression, angiogenesis, and responsiveness to therapy. Am J Pathol 2007; 171 (4): 1342–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Nedawi K, Meehan B, Micallef J et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008; 10 (5): 619–24 [DOI] [PubMed] [Google Scholar]
- 33.Delahunt B, Bethwaite PB, Thornton A. Prognostic significance of microscopic vascularity for clear cell renal cell carcinoma. Br J Urol 1997; 80 (3): 401–4 [DOI] [PubMed] [Google Scholar]
- 34.Yu JL, Rak JW. Host microenvironment in breast cancer development: inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res 2003; 5 (2): 83–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Croix B St., Rago C, Velculescu V et al. Genes expressed in human tumor endothelium. Science 2000; 289 (5482): 1197–202 [DOI] [PubMed] [Google Scholar]
- 36.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer 2005; 5 (6): 436–46 [DOI] [PubMed] [Google Scholar]
- 37.Christian S, Winkler R, Helfrich I et al. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol 2008; 172 (2): 486–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagley RG, Rouleau C, Martin T St et al. Human endothelial precursor cells express tumor endothelial marker 1/endosialin/CD248. Mol Cancer Ther 2008; 7 (8): 2536–46 [DOI] [PubMed] [Google Scholar]
- 39.Prior BM, Lloyd PG, Ren J et al. Arteriogenesis: role of nitric oxide. Endothelium 2003; 10 (4–5): 207–16 [DOI] [PubMed] [Google Scholar]
- 40.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin 2008; 40 (8): 681–92 [PubMed] [Google Scholar]
- 41.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003; 108 (4): 457–63 [DOI] [PubMed] [Google Scholar]
- 42.Xu Q The impact of progenitor cells in atherosclerosis. Nat Clin Pract Cardiovasc Med 2006; 3 (2): 94–101 [DOI] [PubMed] [Google Scholar]
- 43.Yoshino S, Kato M, Okada K. Evaluation of the prognostic significance of microvessel count and tumor size in renal cell carcinoma. Int J Urol 1998; 5 (2): 119–23 [DOI] [PubMed] [Google Scholar]
- 44.Nativ O, Sabo E, Reiss A, Wald M, Madjar S, Moskovitz B. Clinical significance of tumor angiogenesis in patients with localized renal cell carcinoma. Urology 1998; 51 (5): 693–6 [DOI] [PubMed] [Google Scholar]
- 45.Edeline J, Rioux-Leclercq N. Renal cell carcinoma and prognostic factors. Ann Pathol 2008; 28 (5): 374–80 [DOI] [PubMed] [Google Scholar]
- 46.Sabo E, Boltenko A, Sova Y, Stein A, Kleinhaus S, Resnick MB. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin Cancer Res 2001; 7 (3): 533–7 [PubMed] [Google Scholar]
- 47.MacLennan GT, Bostwick DG. Microvessel density in renal cell carcinoma: lack of prognostic significance. Urology 1995; 46 (1): 27–30 [DOI] [PubMed] [Google Scholar]
- 48.Gelb AB, Sudilovsky D, Wu CD, Weiss LM, Medeiros LJ. Appraisal of intratumoral microvessel density, MIB-1 score, DNA content, and p53 protein expression as prognostic indicators in patients with locally confined renal cell carcinoma. Cancer 1997; 80 (9): 1768–75 [DOI] [PubMed] [Google Scholar]
- 49.Okon K, Kawa R. Microvascular network in renal carcinomas. Quantitative and tissue microarray immunohistochemical study. Pol J Pathol 2008; 59 (2): 107–15 [PubMed] [Google Scholar]
- 50.Yao X, Qian CN, Zhang ZF et al. Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clin Cancer Res 2007; 13 (1): 161–9 [DOI] [PubMed] [Google Scholar]
- 51.Vroling L, van der Veldt AA, de Haas RR et al. Increased numbers of small circulating endothelial cells in renal cell cancer patients treated with sunitinib. Angiogenesis 2009; 12 (1): 69–79 [DOI] [PubMed] [Google Scholar]
- 52.Yilmazer D, Han U, Onal B. A comparison of the vascular density of VEGF expression with microvascular density determined with CD34 and CD31 staining and conventional prognostic markers in renal cell carcinoma. Int Urol Nephrol 2007; 39 (3): 691–8 [DOI] [PubMed] [Google Scholar]
- 53.Ershler WB, Moore AL, Shore H, Gamelli RL. Transfer of age-associated restrained tumor growth in mice by old-to-young bone marrow transplantation. Cancer Res 1984; 44 (12 Pt 1): 5677–80 [PubMed] [Google Scholar]
- 54.Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer 2005; 5 (8): 655–62 [DOI] [PubMed] [Google Scholar]
- 55.Sprenger CC, Plymate SR, Reed MJ.


