Abstract
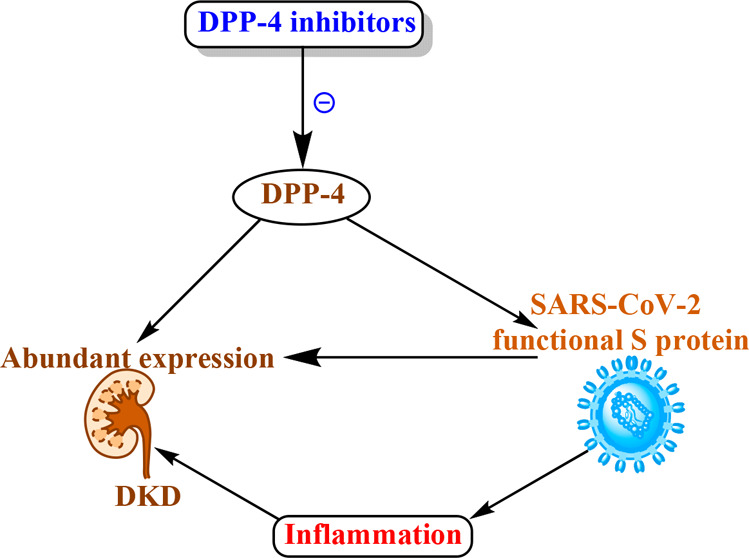
Dipeptidyl peptidase-4 (DPP-4) is expressed ubiquitously in many tissues, including kidney, respiratory tract, and immune cells. Human DPP-4 has been identified as a functional receptor for the spike glycoprotein of the Middle East respiratory syndrome coronavirus. A large interface has been predicted in the docking of DPP-4/SARS-CoV-2 spike protein. Globally, 40% of diabetic patients develop diabetic kidney disease (DKD), a leading cause of end-stage renal disease. DPP-4 inhibitors possess anti-inflammatory properties which suggest their potential implication in DKD and SARS-CoV-2 immunopathogenesis.
Keywords: dipeptidyl peptidase-4, DPP-4 inhibitor, diabetic kidney disease, COVID-19, SARS-CoV-2, inflammation
Since the evolution of COVID-19 due to SARS-CoV-2 in late December 2019 from the city of Wuhan, China, it has now spread to maximum parts of the world and has become a global pandemic and urgently need medication for the treatment. Reports have been suggested that the higher risk of mortality and complications in patients with diabetes is similar to that in two earlier coronavirus outbreaks, i.e., severe acute respiratory syndrome corona virus (SARS-CoV) in Asia in 2002 and Middle East respiratory syndrome corona virus (MERS-CoV) in Saudi Arabia in 2012.1 During this recent COVID-19 global pandemic, a report from Italy showed that more than two-thirds of patient deaths were due to SARS-CoV-2 infection with diabetes comorbidity.1 It has also been noticed that the increased death rate of diabetic patients with COVID-19 infection might be due to possible pathophysiological mechanisms, such as direct pancreatic cell damage, immune response impairment, chronic inflammation, and increased coagulation activities.2 Hence, diabetes contributed principally to the MERS-CoV disease severity and mortality.3 Diabetes leads to diabetic kidney disease (DKD), which is reported to be the major complications observed in both type 1 and 2 diabetic patients.4 Therefore, the clinical management of DKD patients it has become an urgent topic of discussion for when these patients are infected with SARS-COV-2. Therefore, the clinical use of DPP-4 inhibition could be a potential approach to handle this emerging global situation. Patients with renal disease or cardiovascular risks have shown a worse prognosis during the COVID-19 pandemic.5 Hence, it seems reasonable to take extra precautions to protect renal and cardiovascular patients who bear the maximum chances to be affected by the SARS-CoV-2. In kidney, DPP-4 is expressed abundantly at the brush border S1–S3 segment of the proximal tubule. Also, the expression of DPP-4 in vascular endothelium and involvement of vascular inflammation in DKD is well-identified.6 Furthermore, DPP-4 co-immunoprecipitates and redistributes with Na+–H+ exchanger (NHE3) to the microvilli-enriched fractions. In the proximal tubule, the binding of angiotensin II (Ang-II) to the AT1 receptor stimulates NHE3 activities through multiple signaling pathways.6 DPP-4 also plays a central role in glucose and insulin metabolism. DPP-4 degrades incretins including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide, ultimately leading to reduced insulin secretion and abnormal visceral adipose tissue metabolism.7 DPP-4 regulates postprandial glucose via degradation of GLP-1.7 DPP-4 expression is higher in visceral adipose tissue and directly correlates with adipocyte inflammation and insulin resistance.7 The expression of DPP-4 has been found to be upregulated in the glomeruli of DKD patients.8 DPP-4 is also known as the T-cell activation antigen CD26, is found in immune cells including T and B lymphocytes, and plays an essential role in immune system regulation by activating T cells and upregulating CD86 expression and the NF-κB pathway.1 The involvement of the immune system in DKD pathogenesis has been reported.9 The contribution of renal inflammation in advanced DKD has been well-reported.10 Furthermore, DPP-4 increases inflammation in type 2 diabetes patients via both catalytic and noncatalytic mechanisms; hence, the enzymatic activity of DPP-4 is cleavage and affects the function of several cytokines, chemokines, and growth factors.11 Recent evidence suggested that SARS-CoV-2 binds to DPP4/CD26 when entering the respiratory tract.12 Therefore, it seems that the interaction between SARS-CoV-2 S protein and human DPP4/CD26 is responsible for the hijacking of cells and virulent activities.12 It has also been shown that DPP-4 acts as a CoV coreceptor, having a mechanism similar to the host cell entry of SARS-CoV-2.13 It has also been described that inflammation plays an essential role in SARS-CoV-2 infection.14 Evidence suggested that MERS-CoV can bind to the receptor-binding domain (RBD) and interact with T cells and nuclear factor, including NF-κB, which is a key agent for the progression of inflammation. A study using antibodies targeting DPP-4 showed the inhibition of human coronavirus–Erasmus Medical Center (hCoV-EMC) infection of human bronchial epithelial cells and Huh-7 cells.1 It seems that DPP-4 can accelerate SARS-CoV-2 entry into the respiratory tissues and spread to other tissues including kidney and may contribute in the cytokine storm and immunopathogenesis.15
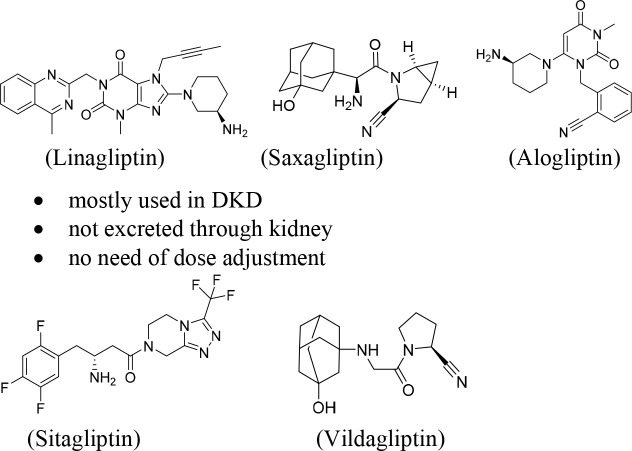
DPP-4 inhibitors, such as linagliptin, alogliptin, saxagliptin, sitagliptin, and vildagliptin (gliptin class of drugs), represent a promising class of drugs which have been used in the treatment of type 2 diabetes patients6 (Figure 1). DPP-4 inhibitors have shown some promising results in the treatment of DKD patients by acting as antioxidants as well as activating endogenous antioxidant defense systems, exhibiting anti-inflammatory properties, maintaining GLP-1 signaling, and increasing insulin secretion.6 Clinical use of a combination of angiotensin receptor inhibitors (ARBs) and DPP-4 inhibitors has become an emerging therapeutic approach for the treatment of DKD patients to maintain blood glucose levels.6 In a phase III clinical trial, treatment with linagliptin in adult patients showed decrease in albuminuria by 28% without affecting glycemia or blood pressure.6 Type 2 diabetes patients with renal dysfunction require dose adjustment of any DPP-4 inhibitor except for linagliptin because it is not eliminated through the kidney. Therefore, linagliptin is mostly the first choice of drug for the treatment of type 2 diabetes patients with renal failure. DPP-4 inhibitors also shown to have renoprotective effects beyond glycaemic control.7 In addition, they have shown important beneficial renal effects mainly by reducing the risk of incident of both microalbuminuria and macroalbuminuria, exert several favorable effects on metabolism and cardiovascular function, and therefore have shown better effects on comorbidities associated with type 2 diabetes patients. Besides, GLP-1 (an incretin hormone) receptor agonists have reported to exhibit potential anti-inflammatory and antiadipogenic activities, and hence, reduce insulin resistance.7 Thus, the beneficial effects of both DPP-4 inhibition and GLP-1 activation, through GLP-1-dependent signaling pathways, the reduction of inflammatory cascade, and the reduction of peripheral insulin resistance, have been well-described.7 Therefore, clinical applications of DPP-4 inhibitors can restore GLP-1 signaling and insulin secretion in type 2 diabetes patients which would be beneficial for the DKD patients.
Figure 1.
Five approved DPP-4 inhibitors for the clinical use in the treatment of DKD.
Furthermore, preclinical evidence suggest that administration of linagliptin showed decrease of kidney fibrosis in experimental DKD mice models.6 In long-term treatment of in db/db type 2 diabetes mice showed reduction of albuminuria and renal injury. Linagliptin was also shown to normalize of the podocalyxin levels with no effects on glucose metabolism and blood pressure during long-term treatment.8 Linagliptin was also reported to show decreasing albuminuria and kidney hypertrophy and increasing mRNA and protein levels for the antioxidative enzymes including catalase and manganese superoxide dismutase (MnSOD) in a DKD model with DBA/2J diabetic mice.6 Therefore, DPP-4 inhibition seems to have renoprotective abilities through stimulation of the antioxidant defense systems and inhibition of fibrosis. Sitagliptin, an another DPP-4 inhibitor, attenuated DKD and inhibited transforming growth factor (TGF-β1, a factor responsible for fibrosis development) expression in diabetic mice model.6 DPP-4 inhibitors possibly interfere the virulence activities of SARS-CoV-2 and may antagonize the multiorgan acute and chronic damage through several additive mechanisms such as enhancing of GLP-1 and anti-inflammatory activities, stimulating direct pulmonary anti-inflammatory activities, downregulating the macrophage activities, and reducing the overproduction of cytokines.15
With these available evidence, DPP-4 might represent a potential target to reduce the pathological progression of the DKD with SARS-CoV-2 infection. However, DPP-4 inhibitors need clinical investigation urgently for the evaluation of their efficacy. DPP-4 inhibitors may act as viral entry inhibitors, may inhibit the viral replication process, and may be capable of reducing the cytokine storm and inflammation.
The author declares no competing financial interest.
References
- Iacobellis G. (2020) COVID-19 and diabetes: Can DPP4 inhibition play a role?. Diabetes Res. Clin. Pract. 162, 108125. 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A.; Bhowmik B.; do Vale Moreira N. C. (2020) COVID-19 and diabetes: Knowledge in progress. Diabetes Res. Clin. Pract. 162, 108142. 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A.; Hui D. S.; Perlman S. (2015) Middle East respiratory syndrome. Lancet 386 (9997), 995–1007. 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera-Chimal J.; Jaisser F. (2020) Pathophysiologic mechanisms in diabetic kidney disease: A focus on current and future therapeutic targets. Diabetes, Obes. Metab. 22, 16–31. 10.1111/dom.13969. [DOI] [PubMed] [Google Scholar]
- Zhou F.; Yu T.; Du R.; Fan G.; Liu Y.; Liu Z.; Xiang J.; Wang Y.; Song B.; Gu X.; Guan L.; Wei Y.; Li H.; Wu X.; Xu J.; Tu S.; Zhang Y.; Chen H.; Cao B. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (10229), 1054–1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikov M.; Pavlović N.; Stanimirov B.; Đanić M.; Goločorbin-Kon S.; Stankov K.; Al-Salami H. (2020) DPP-4 Inhibitors: Renoprotective Potential and Pharmacokinetics in Type 2 Diabetes Mellitus Patients with Renal Impairment. Eur. J. Drug Metab. Pharmacokinet. 45 (1), 1–14. 10.1007/s13318-019-00570-y. [DOI] [PubMed] [Google Scholar]
- Muskiet M. H. A.; Tonneijck L.; Smits M. M.; van Baar M. J. B.; Kramer M. H. H.; Hoorn E. J.; Joles J. A.; van Raalte D. H. (2017) GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 13 (10), 605–628. 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- Sharkovska Y.; Reichetzeder C.; Alter M.; Tsuprykov O.; Bachmann S.; Secher T.; Klein T.; Hocher B. (2014) Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase-4 inhibition in a mouse model of type-2 diabetic nephropathy. J. Hypertens. 32 (11), 2211–23. 10.1097/HJH.0000000000000328. [DOI] [PubMed] [Google Scholar]
- Wilson P. C.; Wu H.; Kirita Y.; Uchimura K.; Ledru N.; Rennke H. G.; Welling P. A.; Waikar S. S.; Humphreys B. D. (2019) The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl. Acad. Sci. U. S. A. 116 (39), 19619–19625. 10.1073/pnas.1908706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada J.; Makino H. (2016) Innate immunity in diabetes and diabetic nephropathy. Nat. Rev. Nephrol. 12 (1), 13–26. 10.1038/nrneph.2015.175. [DOI] [PubMed] [Google Scholar]
- Doupis J.; Veves A. (2008) DPP4 inhibitors: a new approach in diabetes treatment. Adv. Ther. 25 (7), 627–43. 10.1007/s12325-008-0076-1. [DOI] [PubMed] [Google Scholar]
- Vankadari N.; Wilce J. A. (2020) Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerging Microbes Infect. 9 (1), 601–604. 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V. S.; Mou H.; Smits S. L.; Dekkers D. H.; Müller M. A.; Dijkman R.; Muth D.; Demmers J. A.; Zaki A.; Fouchier R. A.; Thiel V.; Drosten C.; Rottier P. J.; Osterhaus A. D.; Bosch B. J.; Haagmans B. L. (2013) Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495 (7440), 251–4. 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. (2020) COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 20 (5), 269–270. 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solerte S. B.; Di Sabatino A.; Galli M.; Fiorina P. (2020) Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol. 57 (7), 779–783. 10.1007/s00592-020-01539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]