Abstract
The National Toxicology Program has recently introduced the practice of examining longitudinal histological sections of the female rodent uterus to improve the identification of non-neoplastic lesions, pre-neoplastic lesions, and uterine tumors. This practice has created a need for reference material that includes normal histology, spontaneous lesions, and inducible lesions in longitudinal as well as transverse sections of the body of the uterus, uterine horns, cervix and vagina. Using three archived NTP reproductive and developmental toxicity studies, the authors reviewed longitudinal and transverse sections of uteri from female Harlan Sprague Dawley (Hsd:SD) rats for cystic endometrial hyperplasia (CEH). The purposes of this review were to 1) evaluate if existing criteria for CEH in transverse uterine sections could be applied to longitudinal sections to develop diagnostic features of CEH in longitudinal uterine sections of rat uterus; and 2) create an atlas of the normal estrous cycle phases in longitudinal sections of young and mature adult Hsd:SD rat uteri. The information provided in this original article should help facilitate the examination of longitudinal sections of the uterus in future commercial and governmental rodent studies.
Keywords: uterus, longitudinal, estrous cycle, cystic endometrial hyperplasia, rat pathology
Introduction
The National Toxicology Program (NTP) has recently introduced the practice of examining longitudinal histological sections of the female rodent reproductive tract, including the body of the uterus, uterine horns, cervix, and vagina in the NTP 2-year bioassay.1 Although longitudinal histological sections have been used for NTP reproductive assessment by continuous breeding (RACB) and modified one generation (MOG) studies, this practice was first implemented in the NTP 2-year bioassay during the oral gavage study of Tetrabromobisphenol A (TBBPA) to improve the identification of nonneoplastic lesions, pre-neoplastic lesions, and uterine tumors, particularly uterine tumors that may not have been identified grossly.2,3 Although the recent NTP Historical Control database on the Harlan Sprague Dawley (Hsd:SD) rat is based on longitudinal sectioning of the uterus,1,4 the industry standard is to process and review transverse sections of each uterine horn.5,6 In fact, most of the references available for the normal histology, spontaneous lesions and inducible lesions in the female rat and mouse reproductive tract predominantly include transverse sections of uterus.7–13 As the NTP requires transverse and sagittal (i.e. longitudinal) sections of uterus to be processed and evaluated in its reproductive and developmental toxicity studies,14 there is a need for reference material that includes normal histology, spontaneous lesions, and inducible lesions in transverse and longitudinal histological sections of the body of the uterus, uterine horns, cervix and vagina.7–13
Cystic endometrial hyperplasia (CEH) is considered the most common, age-related change in the uterus of rats and mice.15 CEH is thought to result from prolonged, estrogen stimulation and is not believed to be a preneoplastic lesion.7,9,13,16 CEH is occasionally observed in adult rats, but is a very common lesion in old mice.9 Criteria for CEH are currently available for sections of uterus in the NTP Nonneoplastic Lesion Atlas (NTP NNLA) and the INHAND document: Nonproliferative and Proliferative Lesions of the Rat and Mouse Female Reproductive System.9,10 Terminology for CEH in the NTP NNLA is uterus, endometrium – hyperplasia, cystic and in the INHAND document it is hyperplasia, glandular, cystic – uterus, both for which cystic endometrial hyperplasia (CEH) is the synonym. The criteria and accompanying photomicrographs for this lesion is of the transverse uterus.9,10 Reference material for the histology of the normal estrous cycle of the rodent and descriptions of specific uterine lesions are not readily available for longitudinal histological sections of the rodent uterus. 7–13,15
The purpose of this manuscript was to review the diagnoses of CEH in longitudinal and transverse histological sections of uteri from female Hsd:SD rats from archived NTP reproductive and developmental toxicity studies. Existing diagnostic criteria for CEH in transverse sections would be applied to CEH in longitudinal sections of rat uterus to develop the diagnostic features of this lesion in the longitudinal rat uterus. This purpose was extended to include the development of an atlas of the normal phases of the estrous cycle in longitudinal sections of uterus from young and mature adult female Hsd:SD rats, which was needed to distinguish normal physiologic changes in the uterus associated with the estrous cycle from CEH.
Materials and Methods
Longitudinal and transverse histological sections of uteri from female Harlan Sprague Dawley rats (Hsd:SD) were reviewed from three NTP reproductive and developmental toxicity studies including one reproductive assessment by continuous breeding (RACB) study and two modified one generation (MOG) studies. Female Hsd:SD rats chosen for this review included those with original diagnoses in longitudinal and transverse uterine sections of uterus, endometrium, hyperplasia, cystic (i.e., CEH) and uterus, endometrium, cyst, as cysts were often diagnosed concurrently with CEH in these studies. The NTP’s protocol for obtaining longitudinal and transverse sections of the rodent uterus is summarized in Supplemental Figure S1.
Sixteen females with CEH from the RACB study (Study A), two females with CEH from the first MOG study (Study B), eleven females with endometrial cysts (only) from the RACB study, and six females with endometrial cysts (only) from the second MOG study (Study C) were chosen for review. During this review, a “control” was defined as a female Hsd:SD rat without a diagnosis of CEH or endometrial cyst. For each Hsd:SD rat diagnosed with CEH or endometrial cyst, a “control” female without the diagnosis of CEH or endometrial cyst was examined from the corresponding study, generation, and/or treatment group for a total of 83 animals reviewed. Ages of animals reviewed ranged from postnatal day (PND) 83 to 276. Treated animals were compared to both a treated “control” and an untreated “control;” whereas, untreated animals were only compared to an untreated “control.” Table 1 includes the female Hsd:SD rats reviewed with or without an initial diagnosis of CEH from Study A and Study B. Table 2 includes the female Hsd:SD rats reviewed with or without an initial diagnosis of cyst (only) from Study A and Study C. Females that were found dead or euthanized moribund were not included in this review.
Table 1.
Study Design for Review of Cystic Endometrial Hyperplasia (CEH) in Longitudinal and Transverse Sections of Uterus in Female Harlan Sprague Dawley (Hsd:SD) Rats Previously Diagnosed with or without CEH
| Original Diagnosis: CEH | Original Diagnosis: No CEH | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Generation | PPM1 | Animal Number | CEH Grade | Age (Days)2 | Treated and Control Females for Comparison | |||
| Control Animal Number3 | Age (Days) | Treated Animal Number3 | Age (Days) | ||||||
| A | F0 | 0 | 22 | Mild | 275 | 46 | 276 | ||
| 0 | 36 | Mild | 276 | 8 | 275 | ||||
| 5000 | 150 | Minimal | 275 | 40 | 276 | 142 | 275 | ||
| 5000 | 160 | Mild | 276 | 2 | 275 | 184 | 276 | ||
| 5000 | 162 | Mild | 276 | 26 | 275 | 158 | 275 | ||
| 5000 | 172 | Minimal | 276 | 30 | 276 | 176 | 276 | ||
| F1cP4 | 0 | 1100 | Minimal | 256 | 1112 | 250 | |||
| 0 | 1102 | Minimal | 248 | 1082 | 250 | ||||
| 0 | 1168 | Minimal | 247 | 1042 | 251 | ||||
| 0 | 1178 | Minimal | 246 | 1096 | 248 | ||||
| 0 | 1180 | Minimal | 250 | 1056 | 252 | ||||
| 0 | 1198 | Mild | 241 | 1154 | 254 | ||||
| F1cNP5 | 0 | 1008 | Minimal | 91 | 1006 | 91 | |||
| 0 | 1038 | Minimal | 91 | 1158 | 93 | ||||
| 0 | 1064 | Mild | 91 | 1048 | 91 | ||||
| 0 | 1146 | Minimal | 91 | 1050 | 91 | ||||
| B | F1 | 0 | 1084 | Minimal | 127 | 1060 | 128 | ||
| 0 | 1158 | Minimal | 132 | 1092 | 128 | ||||
PPM = parts per million
Age of animal at termination
Treated and control animals chosen from same treatment group and generation for comparison purposes
F1cP = F1c Parental Females
F1cNP = F1c Nonparental Females
Table 2.
Study Design for Review of Cystic Endometrial Hyperplasia (CEH) in Longitudinal and Transverse Sections of Uterus in Female Harlan Sprague Dawley (Hsd:SD) Rats Previously Diagnosed with or without Endometrial Cyst
| Original Diagnosis: Cyst | Original Diagnosis: No Cyst or CEH | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Generation | PPM1 | Animal Number | CEH Grade | Age (Days)2 | Females for Comparison | |||
| Control Animal Number3 | Age (Days) | Treated Animal Number3 | Age (Days) | ||||||
| A | F0 | 2500 | 98 | Not Diagnosed | 275 | 32 | 276 | 108 | 275 |
| 5000 | 156 | Not Diagnosed | 275 | 38 | 276 | 144 | 275 | ||
| F1cP3 | 750 | 1358 | Not Diagnosed | 250 | 1090 | 245 | 1526 | 250 | |
| 750 | 1514 | Not Diagnosed | 248 | 1156 | 250 | 1396 | 254 | ||
| 2500 | 1602 | Not Diagnosed | 255 | 1022 | 254 | 1604 | 254 | ||
| 2500 | 1634 | Not Diagnosed | 250 | 1132 | 248 | 1644 | 250 | ||
| 2500 | 1718 | Not Diagnosed | 251 | 1130 | 250 | 1664 | 255 | ||
| F1cNP4 | 0 | 1138 | Not Diagnosed | 91 | 1136 | 91 | |||
| 0 | 1172 | Not Diagnosed | 93 | 1026 | 91 | ||||
| 0 | 1194 | Not Diagnosed | 91 | 1028 | 91 | ||||
| 2500 | 1628 | Not Diagnosed | 91 | 1020 | 91 | 1608 | 91 | ||
| C | F1-Fert | 0 | 1026 | Not Diagnosed | 131 | 1090 | 130 | ||
| 0 | 1074 | Not Diagnosed | 129 | 1084 | 126 | ||||
| 0 | 1170 | Not Diagnosed | 133 | 1076 | 125 | ||||
| F1-Fert | 1000 | 1478 | Not Diagnosed | 127 | 1014 | 133 | |||
| 6000 | 1942 | Not Diagnosed | 129 | 1056 | 128 | 2036 | 124 | ||
| F1-Tox | 0 | 1080 | Not Diagnosed | 83 | 1094 | 83 | |||
PPM = parts per million
Age of animal at termination
Treated and control animals chosen from same treatment group and generation for comparison purposes
F1cP = F1c Parental Females
F1cNP = F1c Nonparental Females
Females with original diagnoses of CEH or cyst (only) were compared to approximately age-matched females demonstrating the corresponding phase of the normal estrous cycle. This was done to confirm the diagnosis of CEH and to compare and contrast histologic changes of CEH from normal physiologic changes observed in the uterus during the estrous cycle. To make the comparisons, we developed a reference atlas of the normal estrous cycle phases observed in longitudinal sections of uterus. Female rats were divided into two PND age groups: young adult (~PND 91) and mature adult (~PND 250). The estrous cycle phases were staged in longitudinal and transverse sections of uteri from young adult and mature adult female Hsd:SD rats using criteria from The Female Rat Reproductive Cycle: A Practical Histological Guide to Staging.11 Photomicrographs were acquired from young adult and mature adult Hsd:SD rats demonstrating normal proestrus, estrus, metestrus, and diestrus in longitudinal and transverse sections of uterus for the atlas (Figures 1a–8e) using preferred estrous cycle phase terms proposed in the INHAND document: Nonproliferative and Proliferative Lesions of the Rat and Mouse Female Reproductive System.9 Longitudinal sections of vagina and uterine cervix were included in the atlas for estrous cycle staging purposes. Ovaries were also evaluated to confirm estrous cycle phases, but photomicrographs were not included. Estrous cycle-specific changes in the ovary are well-documented and can be reviewed in Westwood FR11 and the INHAND document.9
Figure 1.






Young adult female Hsd:SD rat, PND 91, Study A, 0 ppm. All sections stained with hematoxylin and eosin. Longitudinal uterus, normal proestrus (a & b). The uterine lumen (double arrow) and endometrial glands (arrow) exhibit dilation appropriate for proestrus (a). Endometrial glands are minimally to mildly dilated and lined by normal cuboidal to columnar epithelium (arrows); a mitotic figure is present in endometrial lining cell (arrowhead) (b). Transverse uterus, normal proestrus (c & d). Uterine lumen (double arrow) and endometrial glands (arrow) exhibit dilation appropriate for this phase (c). Endometrial glands are dilated and lined by low cuboidal epithelium (arrow); the endometrial epithelium is low columnar (arrowhead) (d). Vagina, normal proestrus (e & f). The vaginal epithelium consists of four layers (double arrow) (e). The four layers include the stratum germinativum (SGerm), stratum granulosum (SG), stratum corneum (SC) and stratum mucification (SM) (f).
Figure 8.
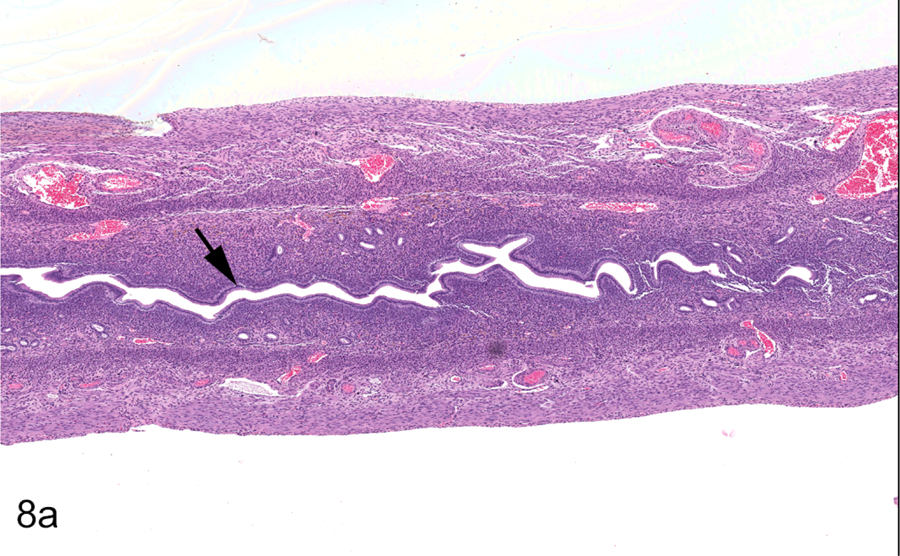





Mature adult female Hsd:SD rat, PND 254, Study A, 0 ppm. Longitudinal uterus, normal diestrus (a & b). All sections stained with hematoxylin and eosin. Uterus is small and inactive with a slit-like lumen (arrow) (a). Uterine lumen (arrowhead) and endometrial glands (arrow) are lined by cuboidal to low columnar epithelium (b). Transverse uterus, normal diestrus (c & d). Uterus is small and inactive with a slit-like lumen (arrow) (c). Uterine lumen (arrowhead) and endometrial glands (arrow) are lined by cuboidal to low columnar epithelium (d). Vagina, normal diestrus (e & f). Vaginal epithelium is low and consists of the stratum germinativum (arrow) (e). Stratum germinativum (SGerm) includes the stratum basale (arrowhead) and stratum spinosum (arrow) (f).
A list of diagnostic features for CEH in longitudinal sections of Hsd:SD rat uteri (see Results) was developed in part from this review and criteria in the INHAND document and the NTP NNLA.9,10 These diagnostic features were used to help diagnose CEH in longitudinal and transverse sections of rat uteri from the archived NTP Studies A, B, and C. Animals with diagnoses of CEH were compared to a range of normal physiological changes observed in longitudinal and transverse sections of rat uterus during the different phases of the estrous cycle. The new rat estrous cycle atlas for longitudinal uterine sections was utilized for this comparison (Figures 1a–8e). The few females in persistent estrus or repetitive pseudopregnancy were not excluded from the review. After initial evaluation, longitudinal and transverse sections of uterus were read without knowledge of original diagnosis or treatment group to refine the list of diagnostic features of CEH in longitudinal sections of uterus and to obtain consistency of grading for CEH across all studies using the grading criteria in Table 3. For CEH, “abnormality” was defined as an increase in the number of glands, increases in stroma and/or cystic glandular dilation.
Table 3.
Grading Criteria
| Uterine tissue | Grade |
|---|---|
| No discernible abnormality | Normal |
| 1–25% abnormal | Minimal |
| 26–50% abnormal | Mild |
| 51–75% abnormal | Moderate |
| >75% abnormal | Marked |
Results
Longitudinal and transverse histological sections of uterus from young adult and mature adult female rats with or without original diagnoses of CEH or cyst (only) from Studies A, B, and C were evaluated against age and estrous phase-matched normal uteri from Study A used for the new atlas (Figures 1a–8e). In this atlas, the authors observed similar histological changes for the estrous cycle in the longitudinal sections of young and mature adult Hsd:SD rat uterus as that previously described by Westwood FR11 and the INHAND document.9 We briefly summarized the salient histologic features for each estrous cycle phase in the longitudinal uterus and vagina and compared the transverse and longitudinal sections of uterus. For proestrus (Figures 1a–f, young rat; and 2a–f, mature rat), the uterine lumen and endometrial glands exhibited minimal to mild dilation appropriate for the phase. In the longitudinal and transverse sections of both young and mature adult Hsd:SD rats, blood vessels were generally prominent in the oblique muscle layer of the myometrium at this phase and the endometrial stroma occasionally showed minimal to mild edema with increases in inflammatory cell infiltrates. The endometrial epithelium was cuboidal to tall columnar and mitoses were increased during this phase. In sections of vagina, three distinct epithelial layers were typically observed (i.e. stratum germinativum, stratum granulosum, and stratum mucification) with the stratum corneum present in the later stages of proestrus. For estrus (Figures 3a–f, young rat; and 4a–f, mature rat), notable apoptosis of epithelial cells lining the endometrial lumen and glands was present in both young adult and mature adult rat uteri. In the longitudinal and traverse sections, the uterine lumen was less dilated than observed in proestrus and had returned to its normal shape and volume in most sections reviewed. There was decreased mitotic activity in the mucosal and glandular epithelia; however, increased inflammatory cell infiltrates were present in the endometrial stroma. In general, prominent vessels in the oblique muscle layer were not as obvious as observed during proestrus in both longitudinal and transverse sections in young and mature Hsd:SD rats. Vaginal changes observed in both young adult and mature adult uteri were characterized by a keratinized stratum corneum that progressively increased to form a layer of cornified epithelial cells some of which had sloughed into the vaginal lumen. For metestrus (Figures 5a–f, young rat; and 6a–f, mature rat), there was a return of mitotic activity in both young adult and mature adult rats. The endometrial epithelium was reduced in size to low columnar, and mitotic activity and apoptosis were both present. In general, blood vessels in the oblique muscle layer appeared more prominent in both transverse and longitudinal sections during this phase in both young adult and mature adult rats. In the vagina, there was dehiscence of the stratum corneum, loss of the stratum granulosum, increased numbers of inflammatory cell infiltrates traversing the vaginal epithelium, with increased desquamated cells, keratin debris, and/or inflammatory cells in the vaginal lumen. For diestrus (Figures 7a–f, young rat; and 8a–f, mature rat), the longitudinal and transverse sections of uterus were small with slit-like lumens and lined by cuboidal to low columnar epithelium. Mitoses and apoptotic cells in the endometrial lining and glands were rare. In general, blood vessels in the oblique muscle layer appeared more prominent in both transverse and longitudinal sections during this phase in both young adult and mature adult rats. The vaginal epithelium was thin and consisted of 3–5 layers of cells in thickness. There were decreased inflammatory cells in the vaginal mucosa and lumen and no keratin debris was present. No differences in the estrous cycle phases were observed in longitudinal versus transverse sections of uteri from the young adult and mature adult rats. Likewise, no differences in the estrous cycle phases were observed between the two age groups.
Figure 2.



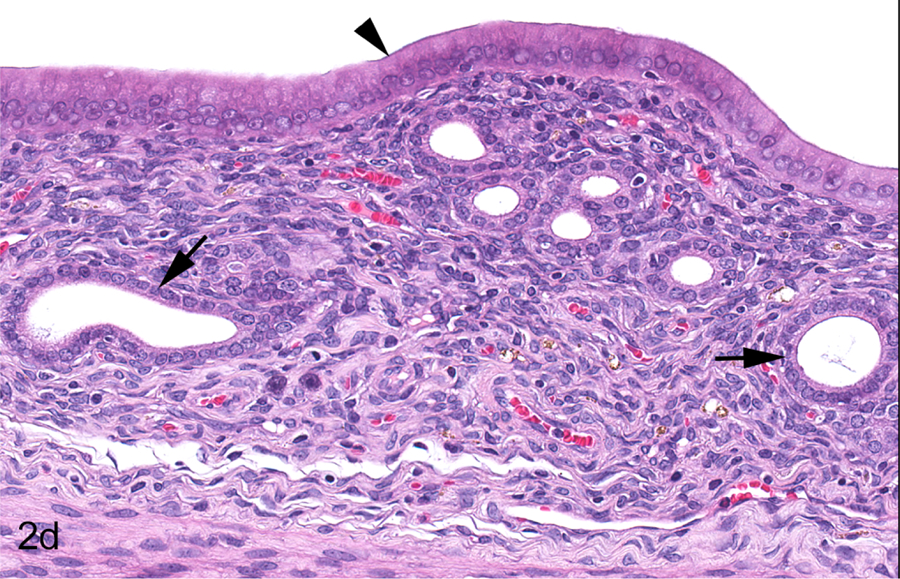


Mature adult female Hsd:SD rat, PND 248, Study A, 0 ppm. All sections stained with hematoxylin and eosin. Longitudinal uterus, normal proestrus (a & b). The uterine lumen (double arrow) and endometrial glands (arrow) exhibit dilation appropriate for proestrus (a). Endometrial cells are columnar lining the lumen (arrowhead) and cuboidal in the glands (arrows); glands exhibit dilation appropriate for this phase (b). Transverse uterus, normal proestrus (c & d). The uterine lumen (double arrow) and endometrial glands (arrow) exhibit dilation appropriate for this phase (c). Endometrial cells are columnar lining the lumen (arrowhead) and cuboidal in the glands (arrows); glands exhibit dilation appropriate for this phase (d). Vagina, normal proestrus (e & f). The vaginal epithelium consists of four layers (double arrows) (e). The four layers include the stratum germinativum (SGerm), stratum granulosum (SG), stratum corneum (SC) and stratum mucification (SM) (f).
Figure 3.

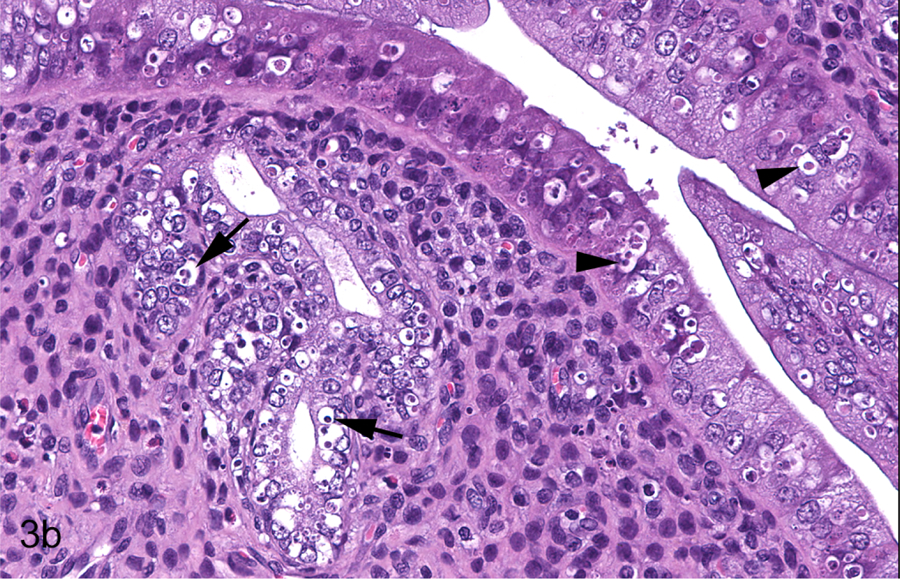




Young adult female Hsd:SD rat, PND 91, Study A, 2500 ppm. All sections stained with hematoxylin and eosin. Longitudinal uterus, normal estrus (a & b). Endometrium is lined by plump, tall columnar cells (arrow); some endometrial glands exhibit dilation (arrowhead) appropriate for this phase (a). Apoptosis is observed in the luminal (arrowheads) and glandular epithelium (arrows) (b). Transverse uterus, normal estrus (c & d). Endometrium is lined by plump, tall columnar cells (arrowhead); some endometrial glands exhibit dilation (arrow) appropriate for the phase (c). Apoptosis is observed in the luminal (arrowheads) and glandular epithelium (arrows) (d). Vagina, normal estrus (e & f). The well-developed stratum corneum is variably attached (arrow) and detached (arrowhead) (e). The stratum corneum (SC) is focally separated (arrow) from the underlying stratum granulosum (SG) (f).
Figure 4.






Mature adult female Hsd:SD rat, PND 250, Study A, 2500 ppm. All sections stained with hematoxylin and eosin. Longitudinal uterus, normal estrus (a & b). The uterine lumen is lined by tall columnar epithelial cells (arrow) (a). Apoptosis of luminal (arrows) and glandular (arrowheads) epithelial cells is present (b). Transverse uterus, normal estrus (c & d). The uterine lumen is lined by tall columnar epithelial cells (arrow) (c). Apoptosis of luminal (arrows) and glandular (arrowheads) epithelial cells is present (d). Vagina, normal estrus (e & f). The well-developed stratum corneum is variably attached (arrow) and detached (arrowhead) (e). The stratum corneum (SC) is separated (arrow) from the underlying stratum granulosum (SG) (f).
Figure 5.
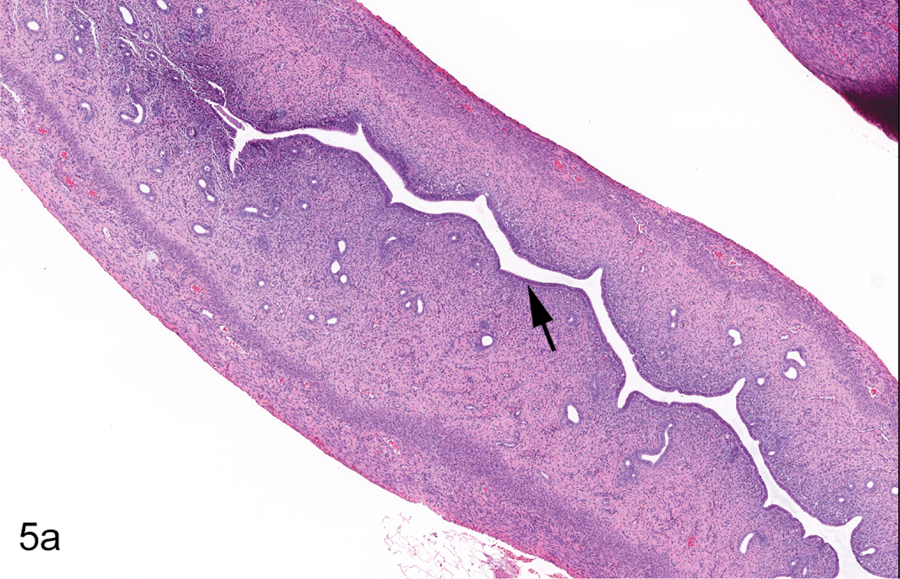





Young adult female Hsd:SD rat, PND 93, Study A, 0 ppm. All sections stained with hematoxylin and eosin. Longitudinal uterus, normal metestrus (a & b). The endometrial epithelium is reduced in height (arrow) compared to that of estrus (Figure 3a) (a). Apoptosis (arrowhead) and mitoses (arrows) may be present concurrently in the luminal and/or glandular epithelium (b). Transverse uterus, normal metestrus (c &d). The lumen is narrow and the epithelium reduced in height (arrow) compared to estrus (c). Apoptosis (arrowheads) and mitosis (arrow) of endometrial cells occur concurrently (d). Vagina, normal metestrus (e & f). There is complete dehiscence and loss of the stratum corneum (arrow) (e). Polymorphonuclear inflammatory cells are present within the degenerating stratum germinativum (SGerm) (arrows) (f).
Figure 6.






Mature adult female Hsd:SD rat, PND 248, Study A, 0 ppm. All sections stained with hematoxylin and eosin. Longitudinal uterus, normal metestrus (a & b). The endometrial epithelium lining the lumen (arrow) is reduced in height as compared to normal estrus (a). Apoptosis (arrowheads) and mitosis (arrow) of endometrial cells occur concurrently (b). Transverse uterus, normal metestrus (c & d). The endometrial epithelium lining the lumen (arrow) is reduced in height as compared to normal estrus. Remodeling of the metrial gland post-pregnancy is present (asterisk) (c). Apoptosis (arrowheads) and mitosis (arrow) of endometrial cells occur concurrently (d). Vagina, normal metestrus (e & f). There is complete dehiscence and loss of the stratum corneum (arrow) (e). Polymorphonuclear inflammatory cells are present within the degenerating stratum germinativum (SGerm) (arrows); desquamated epithelial cell (arrowhead) (f).
Figure 7.






Young adult female Hsd:SD rat, PND 91, Study A, 0 ppm. All sections stained with hematoxylin and eosin. Longitudinal uterus, normal diestrus (a & b). Uterus is small and inactive with a slit-like lumen (arrow) (a). Uterine lumen (arrowhead) and endometrial glands (arrows) are lined by cuboidal to low columnar epithelium (b). Transverse uterus, normal diestrus (c & d). Uterus is small and inactive with a slit-like lumen (arrow) (c). Uterine lumen (arrowhead) and endometrial glands (arrows) are lined by cuboidal to low columnar epithelium (d). Vagina, normal diestrus (e & f). Vaginal epithelium is low and consists of the stratum germinativum (arrow) (e). Stratum germinativum (SGerm) includes the stratum basale (arrowhead) and stratum spinosum (arrow) (f).
The results of the microscopic review for CEH in uteri from female Hsd:SD rats taken from the archived NTP reproductive and developmental toxicity studies are in Tables 4 and 5. Only cases confirmed to be CEH based upon the diagnostic features developed for this review and that of the INHAND document and NTP NNLA9,10 were included in Tables 4 and 5. Generally, the authors agreed with uterine lesions originally diagnosed as CEH in longitudinal and transverse sections of uteri. The authors also added diagnoses of CEH in longitudinal and transverse uteri, originally diagnosed as normal or cyst (only), based upon the diagnostic features developed in this review and that of the INHAND document and NTP NNLA.9,10 Occasionally, CEH was only evident in the longitudinal sections of uterus. Original and/or current study diagnoses of CEH ranged from minimal to moderate in severity in longitudinal and transverse sections of uteri from Study A (Figures 9a–11d) and minimal in severity in Studies B and C. CEH in longitudinal sections of rat uteri was a diffuse lesion that was occasionally more severe in the uterine horns near the bifurcation of the fused horns. Generally, there was agreement in CEH severity between the longitudinal and transverse uterine sections; however, the lesion was occasionally more severe in the longitudinal section of uterus.
Table 4.
Microscopic Evaluation for Cystic Endometrial Hyperplasia (CEH) in Longitudinal and Transverse Sections of Uterus in Harlan Sprague Dawley (Hsd:SD) Rats Previously Diagnosed with or without CEH
| Study | Gen1 | PPM2 | Animal | Age (Days)3 | Estrous Stage | Diagnosis: CEH | ||
|---|---|---|---|---|---|---|---|---|
| Original | New | |||||||
| Longitudinal | Transverse | |||||||
| A | F0 | 0 | 22 | 275 | Persistent Estrus | Mild | Moderate | Moderate |
| 0 | 36 | 276 | Persistent Estrus | Mild | Moderate | Moderate | ||
| 0 | 46 | 276 | Diestrus | -4 | - | - | ||
| 0 | 8 | 275 | Late Proestrus | - | - | - | ||
| 0 | 40 | 276 | Proestrus | - | - | - | ||
| 0 | 2 | 275 | Late Estrus | - | - | - | ||
| 0 | 26 | 275 | Metestrus | - | - | - | ||
| 0 | 30 | 276 | Early Diestrus | - | Minimal | Minimal | ||
| F1cP3 | 0 | 1100 | 256 | Late Estrus | Minimal | Mild | Minimal | |
| 0 | 1102 | 248 | Metestrus | Minimal | - | - | ||
| 0 | 1168 | 247 | Early Estrus | Minimal | Minimal | Minimal | ||
| 0 | 1178 | 246 | Early Estrus | Minimal | Minimal | Minimal | ||
| 0 | 1180 | 250 | Proestrus | Minimal | Minimal | - | ||
| 0 | 1198 | 241 | Metestrus | Mild | Mild | Mild | ||
| 0 | 1112 | 250 | Late Metestrus | - | - | - | ||
| 0 | 1082 | 250 | Metestrus | - | - | - | ||
| 0 | 1042 | 251 | Early Metestrus | - | - | - | ||
| 0 | 1096 | 248 | Early Estrus | - | - | - | ||
| 0 | 1056 | 252 | Late Metestrus | - | Minimal | Minimal | ||
| 0 | 1154 | 254 | Diestrus | - | - | - | ||
| F1cNP4 | 0 | 1008 | 91 | Late Metestrus | Minimal | Minimal | Minimal | |
| 0 | 1038 | 91 | Metestrus | Minimal | Minimal | Minimal | ||
| 0 | 1064 | 91 | Late Estrus | Mild | Mild | Mild | ||
| 0 | 1146 | 91 | Metestrus | Minimal | Minimal | Minimal | ||
| 0 | 1006 | 91 | Late Proestrus | - | - | - | ||
| 0 | 1158 | 93 | Proestrus | - | - | - | ||
| 0 | 1050 | 91 | Late Metestrus | - | - | - | ||
| B | F1 | 0 | 1084 | 127 | Diestrus | Minimal | Minimal | Minimal |
| 0 | 1158 | 132 | Repetitive Pseudopregnancy | Minimal | - | - | ||
| 0 | 1060 | 128 | Early Estrus | - | - | - | ||
| 0 | 1092 | 128 | Diestrus | - | - | - | ||
Gen = Generation
PPM = Parts per million
Age of animal at termination
”-“ = Not diagnosed
Table 5.
Microscopic Evaluation for Cystic Endometrial Hyperplasia (CEH) in Longitudinal and Transverse Sections of Uterus in Harlan Sprague Dawley (Hsd:SD) Rats Previously Diagnosed with or without Endometrial Cyst
| Study | Gen1 | PPM2 | Animal | Age (Days)3 | Estrous Stage | Diagnosis: CEH | ||
|---|---|---|---|---|---|---|---|---|
| Original | New | |||||||
| Longitudinal | Transverse | |||||||
| A | F0 | 2500 | 98 | 275 | Early Estrus | -4 | - | - |
| 0 | 32 | 276 | Early Proestrus | - | Minimal | Minimal | ||
| 0 | 38 | 276 | Late Metestrus | - | - | - | ||
| 2500 | 108 | 275 | Metestrus | - | Minimal | - | ||
| F1cP2 | 750 | 1514 | 248 | Metestrus | - | Minimal | Minimal | |
| 2500 | 1602 | 255 | Late Proestrus | - | - | - | ||
| 2500 | 1634 | 250 | Estrus | - | - | - | ||
| 2500 | 1718 | 251 | Late Metestrus | - | - | - | ||
| 0 | 1090 | 245 | Proestrus | - | - | - | ||
| 0 | 1156 | 250 | Diestrus | - | - | - | ||
| 0 | 1022 | 254 | Early Diestrus | - | Minimal | - | ||
| 0 | 1132 | 248 | Early Metestrus | - | Mild | Mild | ||
| 0 | 1130 | 250 | Late Proestrus | - | - | - | ||
| 750 | 1526 | 250 | Late Metestrus | - | - | - | ||
| F1cP2 | 750 | 1396 | 254 | Early Estrus | - | Mild | Minimal | |
| 2500 | 1604 | 254 | Early Proestrus | - | - | - | ||
| 2500 | 1664 | 255 | Early Proestrus | - | Minimal | Minimal | ||
| F1cNP3 | 0 | 1138 | 91 | Late Proestrus | - | - | - | |
| 0 | 1172 | 93 | Estrus | - | Minimal | - | ||
| 0 | 1194 | 91 | Early Diestrus | - | - | - | ||
| 2500 | 1608 | 91 | Estrus | - | - | - | ||
| 0 | 1136 | 91 | Early Proestrus | - | - | - | ||
| 0 | 1026 | 91 | Late Metestrus | - | - | - | ||
| 0 | 1020 | 91 | Early Proestrus | - | Minimal | Minimal | ||
| C | F1 | 0 | 1026 | 131 | Repetitive Pseudopregnancy | - | - | - |
| 0 | 1074 | 129 | Diestrus | - | - | - | ||
| 0 | 1170 | 133 | Late Diestrus | - | - | - | ||
| 1000 | 1478 | 127 | Diestrus | - | - | - | ||
| 6000 | 1942 | 129 | Late Proestrus | - | - | - | ||
| 0 | 1080 | 83 | Early Proestrus | - | - | - | ||
| 0 | 1090 | 130 | Diestrus | - | - | - | ||
| 0 | 1084 | 126 | Repetitive Pseudopregnancy | - | - | - | ||
| 0 | 1076 | 125 | Repetitive Pseudopregnancy | - | - | - | ||
| 1000 | 1014 | 133 | Metestrus | - | - | - | ||
| 6000 | 1056 | 128 | Late Metestrus | - | - | - | ||
| 0 | 1094 | 83 | Proestrus | - | Minimal | Minimal | ||
| 6000 | 2036 | 124 | Diestrus | - | - | - | ||
Gen = Generation
PPM = Parts per million
Age of animal at termination
”-“ = Not diagnosed
Figure 9.

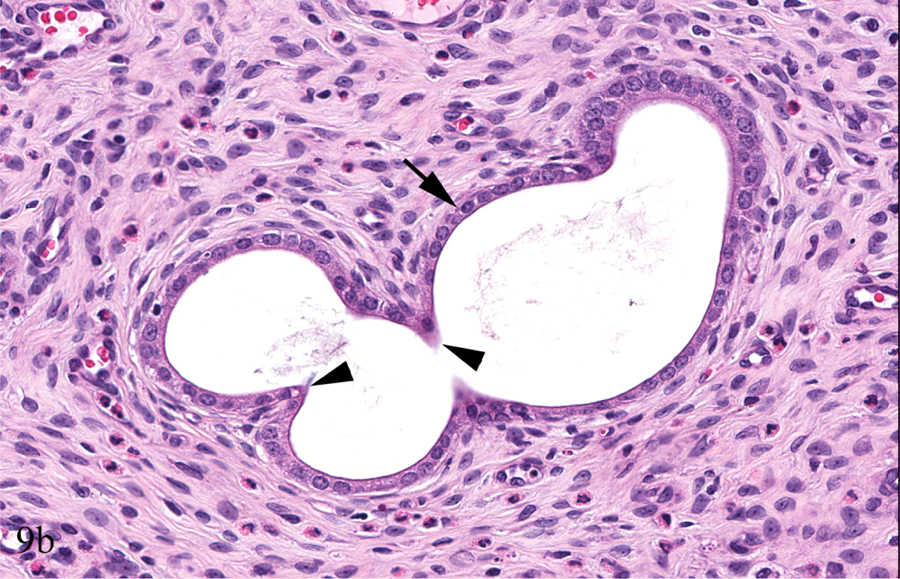


Young adult female Hsd:SD rat, PND 91, Study A, 0 ppm. Longitudinal uterus, cystic endometrial hyperplasia (CEH), minimal (a & b). There is a minimal, diffuse increase in the number of dilated (arrows) and occasionally tortuous (arrowheads) endometrial glands (a). Minimally tortuous and dilated gland is lined by normal low cuboidal epithelium (arrow) with invaginations/evaginations (arrowheads) (b). Transverse uterus, CEH, minimal (c & d). Endometrial glands are minimally increased and dilated (arrows); the change is less evident than that of the longitudinal section of uterus (Figure 9a) (c). Minimally dilated endometrial glands are lined by normal low cuboidal epithelium (arrows) (d).
Figure 11.




Mature adult female Hsd:SD rat, PND 275, Study A, 0 ppm. Longitudinal uterus, cystic endometrial hyperplasia (CEH), moderate (a & b). Diffusely, endometrial glands are moderately increased, dilated (arrow) and/or occasionally tortuous (arrowhead). The endometrial stroma is also increased (asterisk) (a). Moderately tortuous and dilated glands are lined by cuboidal to low columnar epithelium (arrowheads); there is occasional cellular crowding and bulging into the glandular lumen (arrow) (b). Transverse uterus, CEH, moderate (c & d). Other than the moderately dilated gland, the change is more focal and less evident than in the longitudinal section of uterus. The endometrial stroma is also increased (asterisk) (c). The variably dilated endometrial glands are lined by low cuboidal epithelial cells (arrows) (d).
The following diagnostic features for CEH in longitudinal sections of uterus were compiled from the current review of female Hsd:SD rats from the three archived NTP reproductive and developmental toxicity studies and that of the INHAND document and the NTP NNLA9,10:
Diagnostic Features: Cystic Endometrial Hyperplasia in Longitudinal Rat Uterus
Cystic endometrial hyperplasia is typically a diffuse change characterized by an increased number of proliferating glands generally confined to the endometrium.
Endometrial glands involved are variably dilated, but amount of dilation generally increases with increasing severity, usually becoming cystic.
Increased numbers of dilated glandular profiles are typically individualized and may be minimally tortuous or branched showing regions of epithelial evaginations or invaginations.
Endometrial glands involved are lined by a single layer of eosinophilic cuboidal to columnar epithelium which may become attenuated with increasing severity of dilation.
Dilated endometrial glands may be separated by normal, increased or decreased amounts of endometrial stroma.
Increased mitoses, cellular atypia of the endometrial gland epithelium and/or adenomyosis are generally not observed.
Discussion
For the National Toxicology Program (NTP) toxicology and carcinogenicity studies, uterine findings have historically been based on the traditional NTP histopathology review of a transverse histological section through each uterine horn 0.5 cm from the cervix of the uterus.2,5 In the NTP 2-year Wistar Han study of TBBPA, longitudinal histological sectioning of residual uterine tissue revealed additional non-neoplastic lesions, preneoplastic lesions, and uterine tumors in all groups, some of which were not identified upon original transverse inspection of the uterine tissues (e.g. atypical endometrial hyperplasia, adenoma or adenocarcinoma).2,3,17 This prompted a new directive to review both longitudinal and transverse histological sections of the female reproductive tract, including uterus body, uterine horns, cervix and vagina, in their subsequent rodent studies.1,2,3,17 The protocol for removal of the uterus currently being used by the NTP obtains both longitudinal and transverse histological sections (Supplemental Figure S1).
Despite the new directive, very little to no reference material is available for the phases of the normal estrous cycle, much less spontaneous and inducible lesions, in longitudinal sections of rat or mouse uteri. To begin to address the relative absence of reference material for longitudinal sections of rodent uteri, the authors evaluated the known, common background lesion in the uterus of rodents, cystic endometrial hyperplasia (CEH), in longitudinal uterine sections from female Harlan Sprague Dawley (Hsd:SD) rats.7–13 Hsd:SD rats with or without an original diagnosis of CEH or cyst (only) were evaluated from three archived NTP reproductive and developmental toxicity studies. Existing diagnostic criteria for CEH available in the INHAND and NTP NNLA9,10 were applied to these Hsd:SD rats and a list of diagnostic features for CEH in the longitudinal uterus was developed to aid in diagnosis. Because CEH was occasionally more severe or only evident in the longitudinal sections of uterus, the authors concluded that an atlas of the normal estrous cycle was needed for the longitudinal rat uterus to compare and contrast CEH with estrous cycle phase changes observed in the cycling rats in the NTP MOG and RACB studies. An atlas illustrating the normal physiological changes of the estrous cycle in longitudinal uterus would likely have been helpful at the time Studies A, B, and C were originally conducted.
Normal physiological microscopic changes associated with the estrous cycle phases were considered in the diagnosis of CEH in the current review. Generally, there was agreement between the diagnostic features for CEH in longitudinal sections of rat uteri observed in this review with the criteria available in the INHAND document and the NTP NNLA.9,10 CEH was typically a diffuse change in the longitudinal uterus, occasionally more pronounced towards the region of the fused horns, but remained confined to the normal morphological boundaries of the endometrium. The main histologic feature lacking in CEH from the current review was the increase in the number of mitotic figures reported in the INHAND document.9 Any mitotic figures seen in this review were considered appropriate for the phase of the estrous cycle in that female rat.11 Only minimal to moderate CEH was observed in longitudinal and transverse sections of uterus from rats in the current review. Marked CEH was not observed in longitudinal and transverse sections of rat uterus from this study most likely due to the age of the rats evaluated (~3 or ~9 months) and the filtering out of background normal physiological estrous cycle changes. The degree of endometrial gland hyperplasia and dilation did not meet this level of severity according to the diagnostic features developed for this review and that of the INHAND and NTP NNLA.,9,10 The main differences between minimal and moderate CEH were the number of glands and the degree of glandular dilation, which was often more prominent in the uterine horns near the bifurcation of the uterine body. Generally, there was agreement in CEH incidence and severity between the longitudinal and transverse sections of rat uteri from this review, although occasionally the lesion was more severe or only evident in the longitudinal section of uterus. These occasional discrepancies highlight the importance of reviewing longitudinal and transverse sections of rodent uteri, as lesions could be missed in the transverse section alone.
To the best of our knowledge, this is the first report focusing on the application of existing diagnostic criteria to CEH in longitudinal histological sections of uteri from Hsd:SD rats. Little difference was noted between the diagnostic features developed for CEH in this review and that of the INHAND and NTP NNLA9,10, demonstrating the likely applicability of these references to longitudinal sections of rat uterus. Reference to the diagnostic features developed for CEH in longitudinal sections of rat uterus from this review, as well as the atlas of the normal estrous cycle in longitudinal uterine sections from young and mature adult rats, should facilitate the evaluation of longitudinal uterine sections in toxicology and carcinogenicity studies in this strain of rat. The occasional demonstration of CEH lesions in longitudinal sections that were less severe or not evident in transverse sections of the Hsd:SD rat uteri, during this review, underscores the importance of increased “real estate” in the diagnosis of uterine lesions. Longitudinal uterine sections allowed us to better appreciate CEH as a typically diffuse lesion and decreased the likelihood of missing CEH lesions that may not be present in a single transverse section. The evaluation of both longitudinal and transverse sections of uteri provides a better representation of the 3-dimensional tissue, thereby improving diagnostic accuracy and consistency. The few incidences of persistent estrus and repetitive pseudopregnancy observed in older females underscores the importance of evaluating uterus in younger cycling rats to avoid these potentially confounding age-related changes. It is also important to take into consideration the phase of the estrous cycle when evaluating the rat uterus for CEH, as normal physiological changes could be misconstrued as lesions. Hopefully, the information from this study will open the door to future publications on spontaneous and induced lesions in longitudinal sections of rat and mouse uterus.
Supplementary Material
Figure S1. The protocol for removal of the rodent uterus currently being used by the NTP obtains longitudinal sections of the two free portions of uterine horn (1), two transverse sections of uterine horn (2), and a longitudinal section of uterine body with attached portions of uterine horn (3).12,22 The corresponding H&E-stained histological sections are on the right. Figure S1 was modified from Keane KA et al.22 The image was created by Beth Mahler and David Sabio.
Figure 10.




Mature adult female Hsd:SD rat, PND 241, Study A, 0 ppm. Longitudinal uterus, cystic endometrial hyperplasia (CEH), mild (a & b). There is a mild, diffuse increase in the number of dilated (arrowheads) and tortuous (arrows) endometrial glands (a). Mildly dilated and/or tortuous endometrial glands (arrows) are lined by normal cuboidal epithelium with invaginations/evaginations (arrowheads) (b). Transverse uterus, CEH, mild (c & d). Endometrial glands are mildly increased and dilated (c), but the change is less evident than in the longitudinal section of uterus (Figure 10a) (c). Mildly dilated endometrial glands are lined by normal low cuboidal epithelium (arrows), which is sometimes invaginated (arrowhead) (d).
Acknowledgements
The authors wish to acknowledge the following people for their contributions to this original article: Emily Singletary (EPL) for scanning of slides; Dr. Ronald Herbert (NIEHS) for provision of the original supplemental figure illustration; and David Sabio (EPL) and Beth Mahler (EPL) for modification of the original and creation of a new supplemental figure image. The authors would also like to thank Drs. Amy Brix and Erin Quist for their critical review of this manuscript. This work was funded, in part, by the Intramural Research Program of the NIH, NIEHS and DNTP. NIH IRP: ES103319-04 (2019) Toxicologic Pathology Evaluations for the National Toxicology Program https://intramural.nih.gov/search/searchview.taf?ipid=110659&ts=1575385967.
Footnotes
Declaration of Conflicting Interest Statement
The authors declare no real, perceived or potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Elmore SA, Chen VS, Hayes-Bouknight S, et al. Proceedings of the 2016 National Toxicology Program Satellite Symposium. Toxicol Pathol. 2017; 45(1):11–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Toxicology Program. NTP Technical Report on the Toxicology Studies of Tetrabromobisphenol A (CAS NO. 79-94-7) in F344/NTac Rats and B6C3F1/N Mice and Toxicology and Carcinogenesis Studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] Rats and B6C3F1/N Mice (Gavage Studies). Research Triangle Park, NC: National Toxicology Program, U.S. NTP TR 587, NIH Publication No. 14–5929. National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services. [Google Scholar]
- 3.Elmore SA. Longitudinal residual evaluations for identifying uterine proliferative lesions. National Institute of Environmental Health Sciences NTP Technical Reports Peer Review Meeting; May 2014; RTP, NC, U.S. National Toxicology Program website. https://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2014/may/presentations/03uteruspathelmore_508.pdf. Accessed 30 June 2019. [Google Scholar]
- 4.National Toxicology Program. Historical Controls. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Research Triangle Park, NC: National Toxicology Program website. https://ntp.niehs.nih.gov/results/dbsearch/historical/index.html. Accessed 25 July 2019 Edinburgh, U.K.: Saunders Elsevier; 2012: 101–121. [Google Scholar]
- 5.National Toxicology Program. Specifications for the conduct of studies to evaluate the toxic and carcinogenic potential of chemical, biological and physical agents in laboratory animals for the National Toxicology Program (NTP). National Toxicology Program website. https://ntp.niehs.nih.gov/ntp/test_info/finalntp_toxcarspecsjan2011.pdf. Accessed 30 June 2019.
- 6.Ruehl-Fehlert C, Kittel B, Morawietz G, et al. Revised guides for organ sampling and trimming in rats and mice, part 1: a joint publication of the RITA*) and NACAD*) groups. Exp Toxic Pathol. 2003; 55:91–106. [DOI] [PubMed] [Google Scholar]
- 7.Dixon D, Vidal JD, Leininger JR, Jokinen MP. Oviduct, uterus, and vagina In: Suttie AW, Bradley AE, Leininger JR, eds. Boorman’s Pathology of the Rat: Reference and Atlas, 2nd Edition. Cambridge, MA: Academic Press; 2018:537–559. [Google Scholar]
- 8.Davis BJ, Dixon D, Herbert RA. Ovary, oviduct, uterus, cervix and vagina In: Maronpot RR, Boorman GA, Gaul BW, eds. Pathology of the Mouse: Reference and Atlas, 1st Edition. Vienna, IL: Cache River Press; 1999: 409–443. [Google Scholar]
- 9.Dixon D, Alison R, Bach U et al. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J Toxicol Pathol. 2014; 27(3–4 Suppl):1S–107S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willson G, Cimon KY, 2015. Uterus – uterus, endometrium – hyperplastic, cystic In: Cesta MF, Herbert RA, Brix A, Malarkey DE, Sills RC, Eds. National Toxicology Program Nonneoplastic Lesion Atlas. National Toxicology Program website. https://ntp.niehs.nih.gov/nnl/female_reproductive/uterus/enhypc/index.htm. Accessed 30 June 2019.
- 11.Westwood FR. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol. 2008; 36(3):375–384. [DOI] [PubMed] [Google Scholar]
- 12.Willson G, Cimon KY, 2015. Female reproductive system – uterus In: Cesta MF, Herbert RA, Brix A, Malarkey DE, Sills RC, Eds. The National Toxicology Program Nonneoplastic Lesion Atlas. National Toxicology Program website. https://ntp.niehs.nih.gov/nnl/female_reproductive/uterus/authors/uterus_authors_and_reviewers.htm. Accessed 30 June 2019.
- 13.Leininger JR, Jokinen MP. Oviduct, uterus and vagina In: Boorman GA, Eustis SL, Elwell MR, Montongery CA, MacKenzie WF, Eds. Pathology of the Fischer Rat. San Diego, CA: Academic Press; 1990: 443–460. [Google Scholar]
- 14.National Toxicology Program. Specifications for the conduct of studies to evaluate the reproductive and developmental toxicity of chemical, biological and physical agents in laboratory animals for the National Toxicology Program (NTP). National Toxicology Program website. https://ntp.niehs.nih.gov/ntp/test_info/finalntp_reprospecsmay2011_508.pdf. Accessed 30 June 2019.
- 15.Creasy D Reproduction of the Rat, Mouse, Dog, Non-human Primate and Minipig In: McInnes EF. Background Lesions in Laboratory Animals: A Color Atlas, 1st Edition. Edinburgh, U.K.; 2012:114. [Google Scholar]
- 16.Klaunig JE, Dekant W, Plotzke K, Scialli AR. Biological relevance of Decamethylcyclopentasiloxane (D5) induced rat uterine endometrial adenocarcinoma tumorigenesis: mode of action and relevance to humans. Regul Toxicol Pharmacol. 2016; 74: S44–S56. [DOI] [PubMed] [Google Scholar]
- 17.Wikoff DS, Rager JE, Haws LC, Borghoff SJ. A high dose mode of action for Tetrabromobisphenol A-induced uterine adenocarcinomas in Wistar Han rats: a critical evaluation of key events in an adverse outcome pathway framework. Regul Toxicol Pharmacol. 2016; 77: 143–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The protocol for removal of the rodent uterus currently being used by the NTP obtains longitudinal sections of the two free portions of uterine horn (1), two transverse sections of uterine horn (2), and a longitudinal section of uterine body with attached portions of uterine horn (3).12,22 The corresponding H&E-stained histological sections are on the right. Figure S1 was modified from Keane KA et al.22 The image was created by Beth Mahler and David Sabio.


