Abstract
Pulmonary lymphangioleiomyomatosis accounts for the majority of cadaveric lung transplantation cases. Post-transplantation management is continuingly necessary not only to prevent the progression of LAM but also to address complications. A woman with lymphangioleiomyomatosis underwent cadaveric lung transplantation. She developed post-operative native lung hyperinflation and hemoptysis with cavity shadow in the native lung on computed tomography. Isolated Aspergillus from her sputum and positive Aspergillus galactomannan antigen in the blood led to a diagnosis of aspergillosis. Despite the reduction of hemoptysis by antifungal medication, she developed fatal hemoptysis. An autopsy showed an Aspergillus fungal mass in the bronchus in the native lung whilst the lung graft was free from lymphangioleiomyomatosis lesions. Endobronchial aspergilloma was suggested to be a cause of hemoptysis. This fatal clinical course suggested that hemoptysis due to endobronchial aspergilloma in the native lung should have been considered native lung pneumonectomy as a further intervention.
Keywords: Fatal Hemoptysis, Pulmonary Aspergillosis, Native Lung Hyperinflation, Single Lung Transplantation, Lymphangioleiomyomatosis
INTRODUCTION
Pulmonary lymphangioleiomyomatosis (LAM) is a rare disease that occurs more commonly in woman of reproductive age. It is a systemic disease that typically results in lung destruction with cystic changes caused by LAM-cell proliferation and invasion. Although LAM also affects the lymphatic system, associated pelvic masses, retroperitoneal masses, and pulmonary lesions are markedly progressive and determine the prognosis. While medical management can be attempted in order to control the lung function decline, lung transplantation is ultimately the last resort for patients with advanced disease.
The present patient underwent cadaveric left lung transplantation surgery for LAM. Post-operative complications, including endobronchial aspergilloma, occurred in the native hyper-inflated lung, eventually resulting in fatal hemoptysis. We herein report her clinical course, including radiological lung images, and the pathological findings at the autopsy.
CASE PRESENTATION
A 45-year-old woman was taken to the hospital by ambulance due to hemoptysis. She had consulted our hospital at 29 years of age due to dyspnea, which had been gradually worsening since she first noticed it at 26 years of age. Chest computed tomography (CT) showed lung hyperinflation and multiple cystic lesions in her lungs. A transbronchial lung biopsy confirmed the diagnosis of LAM. She was administered medroxyprogesterone acetate and leuprorelin acetate; however, her dyspnea had worsened, accompanied by a decline in the lung function. She therefore underwent cadaveric left lung transplantation surgery at 40 years of age. Post-operative treatment was initiated with immunosuppressive drugs, including mycophenolate mofetil and tacrolimus.
Several months after lung transplantation, she developed dyspnea on exertion and needed home oxygen therapy (HOT). Chest X-ray and CT showed that the lung graft was compressed by the hyperinflated native right lung. Five years after transplantation, she began complaining of hemoptysis. She was taken to the hospital by ambulance due to increasing hemoptysis. On admission, 2 L/min of oxygen administration was needed to maintain the oxygen saturation; however, uncontrolled tachypnea and bleeding led to tracheal intubation and mechanical ventilation to maintain her airway. CT showed a cavity in the right middle lobe and a high-density tumor shadow with air crescent sign filling the inside of the cavity (Fig. 1A and 1B), suggesting a fungal infection was responsible for the bleeding. We performed bronchial artery embolization (BAE) to control the sustained hemoptysis, and she was able to be discharged on the 13th hospital day. Blood Aspergillus galactomannan antigen was positive, and Aspergillus spp. was isolated from her sputum. We diagnosed her with pulmonary aspergilloma in the right lung cavity. She was administered voriconazole but had to later discontinue it due to drug-induced renal and liver injury. She underwent BAE due to hemoptysis again the following year after her discharge. Repeated hemoptysis as well as respiratory failure due to the native lung hyperinflation was considered to require surgery. We arranged for right pneumonectomy to remove the native lung and then administered itraconazole to control the infectious lesions by Aspergillus. Since the hemoptysis was well controlled, she expressed a desire to observe her symptoms with medical treatment and postpone surgery. The next year, however, the hemoptysis worsened frequently again, prompting us to reschedule pneumonectomy. However, before pneumonectomy, she developed massive hemoptysis leading to cardiac arrest, showing asystole on arrival to our hospital. Spontaneous circulation was recovered by resuscitation, but she died of hypoxic encephalopathy on the 3rd hospital day. We performed an autopsy with consent from her family.
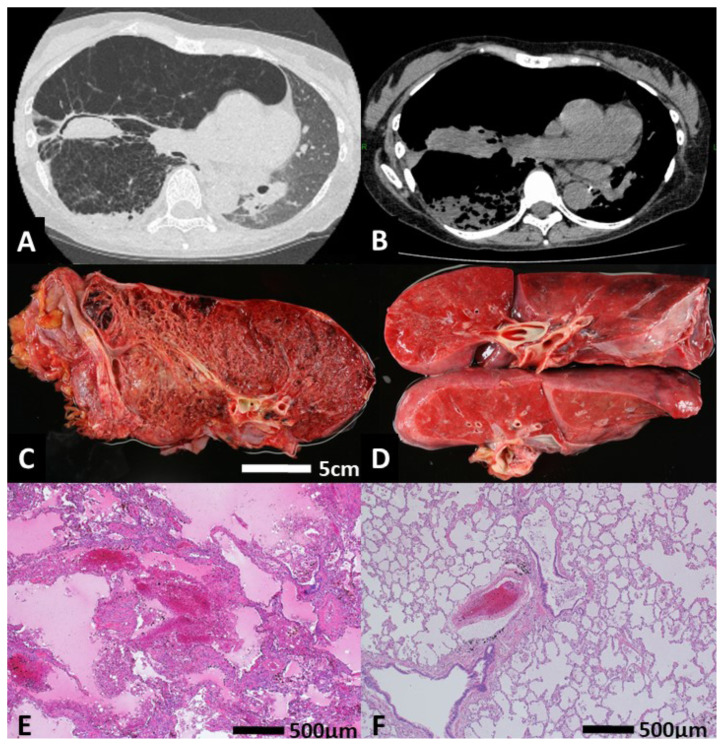
Figure 1.
(A, B) Chest CT findings at the first admission due to hemoptysis. (C) Macroscopic findings of a right native lung sample at the autopsy. Multiple cystic lesions due to LAM were noted. (D) Macroscopic findings of a left lung graft sample at the autopsy. (E) Hematoxylin and eosin (H&E) staining for the microscopic examination of a right native lung sample at the autopsy. The alveolar structure was extensively destroyed, and new bleeding was noted. (F) H&E staining for the microscopic examination of a left lung graft sample at the autopsy. No findings of LAM were noted, and the alveolar structure was intact.
Autopsy findings
We noted left rib fractures, subcutaneous hematoma, erosion of tracheal mucosa, and bloody foam discharge in the trachea and main bronchi due to the resuscitation procedures.
The macroscopic findings showed congestive changes in the bilateral lungs with an increased weight (right lung was 665 g, left lung was 790 g). The right lung showed multiple cystic lesions and a devastated structure due to LAM. There was little normal lung structure left in the right native lung (Fig. 1C and Fig. 2A). In the right middle lobe, brown plugs that resembled fungus balls had obstructed the dilated bronchus. The left lung graft showed no significant abnormalities, including LAM legions or bleeding sources other than congestion (Fig. 1D).
Figure 2.
(A) Macroscopic finding of Aspergillus clusters in the right ectatic bronchus. (B) Periodic acid-Schiff staining of the microscopic findings of Aspergillus clusters in the right ectatic bronchus. The upper left of the image is the bronchial mucosa.
The microscopic findings showed that the right lung alveolar structure was extensively destroyed due to LAM progression, which was characterized by cystic dilation of the alveolar spaces and marked thickening of the alveolar walls due to abnormal alveolar smooth muscle proliferation. There was an Aspergillus fungal mass of 25 mm in size in the right cystically dilated bronchus (Fig. 1E and Fig. 2B). In the left lung graft, congestive findings, which had been caused by cardiac arrest and the subsequent resuscitation procedures, were observed; however, neither findings of LAM nor obstructive bronchiolitis were noted (Fig. 1F). There was a little bone marrow embolism in the left graft pulmonary artery due to the resuscitation procedures (Figure not shown).
DISCUSSION
Aspergillus spp. can cause various pulmonary diseases, depending on the immune status of patients and the underlying lung disease. Endobronchial aspergilloma is a rare presentation of pulmonary aspergillosis, caused by an overgrowth of aspergillus species with a non-invasive form in endobronchial lumen (1).
In the present case, Aspergillus infection in the native hyperinflated lung resulted in fatal hemoptysis. The autopsy findings of the patient showing granulomatosis with Aspergillus clusters filling the ectatic bronchi in the native lung, which was considered as endobronchial aspergilloma. Although the exact point of hemorrhaging was unclear, it seemed that the rupture of the bronchial artery damaged by inflammation at the ectatic bronchus induced hemoptysis. With the predilection of Aspergillus to invade blood vessels, angioinvasive aspergillosis such as invasive pulmonary aspergillosis commonly leads to areas of infarction and hemorrhage in the lungs. The symptoms are intense pleuritic chest pain, sudden dyspnea, tachypnea as well as hemoptysis as in this case (2).
Recent case review about endobronchial aspergilloma showed that hemoptysis and cough were the most common symptoms. It also showed that 70% of those had history of lung diseases, such as pulmonary tuberculosis, lung cancer, foreign bodies, lung resection, and that half of those had non-pulmonary comorbidities including diabetes (3). A nest structural change causing the airflow stasis are important factors to help Aspergillus colonize in the airway, where fungal growth occurs and develop respiratory symptoms (4,5).
In present case, operative indication was postponed due to the low preoperative lung function as well as ameliorating but limited effect of antifungal treatment on the symptoms caused by the lesion that was considered to be pulmonary aspergilloma. The effect of antifungal treatment was limited seemingly because of the fungal lesions in the ectatic bronchi. Antifungal therapy was employed as a suboptimal treatment while awaiting surgery. And indeed, the hemoptysis was temporarily reduced to some extent by itraconazole; however, unpreventable massive hemoptysis indicates earlier need to decide further interventions. Optimal treatment of endobronchial aspergilloma has not yet been established. Massive lesion and frequent bleeding in this case had needed further intervention.
There have been no reports on endobronchial aspergilloma as a fatal complication after lung transplantation. The autopsy enables us to make comparison between radiological images and pathological findings as well as between native disease lung and intact lung graft at the autopsy. Our case indicates the necessity for further studies on post-organ-transplantation endobronchial aspergilloma, especially focusing on the postoperative prevention as well as additional surgical indication such as native lung pneumonectomy.
REFERENCES
- 1.Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest. 2002;121:1988–1999. doi: 10.1378/chest.121.6.1988. [DOI] [PubMed] [Google Scholar]
- 2.Amchentsev A, Kurugundla N, Saleh A. Aspergillus-related lung disease. Respiratory Medicine CME. 2005;1:205–215. [Google Scholar]
- 3.Huang D, Li B, Chu H, Zhang Z, Sun Q, Zhao L, Xu L, Shen L, Gui T, Xie H, Zhang J. Endobronchial aspergilloma: A case report and literature review. Experimental and therapeutic medicine. 2017;14:547–554. doi: 10.3892/etm.2017.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma JE, Yun EY, Kim YE, Lee GD, Cho YJ, Jeong YY, Jeon KN, Jang IS, Kim HC, Lee JD, Hwang YS. Endobronchial aspergilloma: report of 10 cases and literature review. Yonsei medical journal. 2011;52:787–792. doi: 10.3349/ymj.2011.52.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung SW, Kim MW, Cho SK, Kim HU, Lee DC, Yoon BK, Jeong JP, Ko YC. A Case of Endobronchial Aspergilloma Associated with Foreign Body in Immunocompetent Patient without Underlying Lung Disease. Tuberculosis and respiratory diseases. 2013;74:231–234. doi: 10.4046/trd.2013.74.5.231. [DOI] [PMC free article] [PubMed] [Google Scholar]