Abstract
Objectives
The use of opioid analgesics for pain management in hospitalized patients is associated with a high risk of adverse events, including respiratory depression which may lead to respiratory arrest and death. Patients who experience opioid-related adverse drug reactions (ADRs) have been shown to experience longer and more costly hospital stays and have a higher risk of requiring a readmission after discharge. In this study, we report on the impact of the introduction of Wesley Medical Center's Safe Medication Practice Protocol on opioid-related ADRs.
Methods
A retrospective, pre-post cohort study using electronic health records combined with manual chart review was undertaken at the Wesley Medical Center, a 760-bed tertiary care facility. The Safe Medication Practice Protocol incorporating a smart infusion pump system with capnography monitoring was implemented in May 2010 hospital-wide. The number and severity of ADRs and the duration of opioid treatment were compared between the pre (2007-April 2010) and post (May 2010–2014) periods.
Results
A total of 139,734 (pre-period) versus 267,573 (post-period) patients received opioid treatment during the hospital stay. Compared with the pre-period, the post-period resulted in a 79.2% reduction in the number of severe adverse reactions (3.08 vs 0.64 per 10,000 patients treated with opioid, P < 0.0001) as well as a shorter duration of opioid treatment (average 2.05 vs 1.37 days, P < 0.0001).
Conclusions
Implementing education, revisions to patient-controlled analgesia policies and procedures, and capnography monitoring with patient-controlled analgesia pause is associated with significantly lower rates of severe ADRs and shorter opioid treatment duration.
Key Words: capnography, end-tidal carbon dioxide, analgesia, patient-controlled analgesia, respiratory depression, postoperative care
There has been an increasing focus on the use of opioid analgesics for pain management in the United States, with the rate of opioid analgesic-related overdose deaths quadrupling between 1999 and 2011.1 The use of opioid analgesics for pain management in hospitalized patients is associated with a high risk of adverse events, including respiratory depression which may lead to respiratory arrest and death.2 It has been estimated that opioid-related adverse events account for up to 16% of all inpatient drug-related adverse events,3 with 0.5% of all postoperative patients experiencing respiratory depression.4 In a recent study using the Anesthesia Closed Claims Project database, an estimated 92 of 357 acute pain claims within a malpractice database may have been due to respiratory depression; of those, 77% resulted in severe brain damage, with the vast majority of cases occurring within 24 hours of opioid initiation and could have been prevented with improved monitoring.5 The risk of opioid-related adverse events within an inpatient setting applies to both nurse-administered analgesia and patient-controlled analgesia (PCA). Strategies aimed at reducing avoidable opioid-related adverse events such as respiratory depression could result in better patient outcomes and significant cost savings. Patients experiencing opioid-related adverse events have been shown to be associated with a 55% increase in length of hospital stay,6 approximately $21,000 increase in cost of care,6 and a 36% increase in risk of 30-day readmission.6
In 2002, Wesley Medical Center (WMC) introduced a number of measures to control opioid-associated adverse reactions including revisions to the policies and procedures for PCA use, restriction of meperidine use, limit in intramuscular (IM) opioid use, assessment of whether patients were opioid naive or opioid tolerant, and patient family education. However, it was found in 2008 that despite the introduction of these measures, total opioid-induced adverse events and respiratory reactions continued to rise. Therefore, the medication safety committee chose to target pain management as their single focus in 2008 to decrease opioid-induced respiratory adverse drug reactions (ADRs), decrease code blues and transfers to intensive care unit, and improve patient outcomes. A number of measures were introduced as part of the Safe Medication Practice Protocol (SMPP) which included staff education, sleep apnea risk assessment documentation and communication, revisions to PCA policies and procedures, and revisions to preprinted PCA orders. In addition, a smart infusion pump system with continuous capnography to monitor end-tidal carbon dioxide (ETCO2) was introduced for use with all PCA patients, epidural patients, and patients considered at high risk of respiratory depression. The smart infusion pump system incorporates a PCA pause functionality automatically stopping opioid delivery to those patients who are identified as compromised. In this study, we sought to evaluate the impact of the introduced measures and the introduction of continuous capnography monitoring to manage opioid-induced respiratory events and especially severe adverse events and code blue events in all inpatient and outpatient patients who were treated with opioids.
METHODS
Study Design
We conducted a retrospective, pre-post, cohort study at WMC, a 760-bed tertiary care teaching facility with a level I trauma unit located in Wichita, Kansas. All adults and children (except neonates) treated within an inpatient or outpatient setting at WMC between January 2007 and December 2014 and received opioid (all routes) treatment were included in this study. Patients treated within the outpatient cardiovascular procedure unit, outpatient heart unit, or the outpatient radiology unit were excluded. Exposure to opioids was obtained from patient administration records. Opioid-related ADRs were identified from either standard ADR reports, voluntary reports completed by the pharmacist, or evidence in the patient's administration record that naloxone (an opioid reversal agent) had been administered. Adverse drug reactions were recorded in the Meditech Electronic Medical Record IT system which further triggered a chart review to extract additional information on the incident. As this study was considered a quality improvement initiative through the hospital safety committee, WMC institutional review board approval was not required.
Capnography
Smart infusion pump systems with continuous capnography monitoring were adopted in May 2010 to replace pulse oximetry to monitor respiratory depression among PCA patients, epidural patients, and patients considered at high risk of respiratory depression. Capnography is a noninvasive monitoring device which provides continuous measurement of exhaled carbon dioxide concentration in patients, allowing for rapid identification of ventilation problems (hypoventilation and hyperventilation) and sleep apnea by audible alarm. If a patient's respiratory status fell below defined thresholds, the smart pump would alarm and stop opioid infusion. These smart infusion pumps that are regularly used to deliver various IV solutions and medications (including PCA) can be integrated with capnography monitoring through the acquisition of dedicated ETCO2 modules. The costs of these modules are amortized over time (years of useful pump life) and number of patients monitored per year. Each patient requires a disposable ETCO2 catheter (sensor) that costs approximately US$8 to $12.
Safe Medication Practice Protocol
The SMPP included the development and implementation of a sleep apnea risk assessment model to identify patients at high-risk of opioid-induced respiratory depression (Obstructive Sleep Apnea Assessment)7 and introducing dosing parameters for opioids and educating patients, nurses, and physicians about pain management. The policy interventions within the SMPP were implemented starting November 2009, as continued measure of quality improvement.
Outcomes
All opioid-related ADRs had to have evidence of administration of naloxone and decreased respiratory rate or central nervous system depression, which was corrected upon treatment with naloxone. Each ADR was classified as mild, moderate, or severe as per the following definitions:
-
Mild — any one of the following:
-
o
Decreased level of consciousness (LOC) (observed by physician, nurse, respiratory therapist, or pharmacist as patient lethargy, confusion, or altered mental status)
-
o
Decreased respiratory rate (≤10 breaths per minute)
-
o
-
Moderate — any two of the following:
-
o
Decreased LOC (defined as above)
-
o
Decreased respiratory rate (≤10 breaths per minute)
-
o
Decreased O2 saturations (<94%)
-
o
Increased ETCO2 level by 10 mmHg from baseline or exceeding 60 mmHg
-
o
Supplemental oxygen
-
o
Rapid response activation
-
o
Increased length of hospital stay (by ≥1 day)
-
o
-
Severe — any three of the following:
-
o
Decreased LOC (defined as above)
-
o
Absent or decreased respiratory rate (≤10 breaths per minute)
-
o
Decreased O2 saturations (<94%)
-
o
Increased ETCO2 level by 10 mmHg from baseline or exceeding 60 mmHg
-
o
Supplemental oxygen
-
o
Rapid response activation or code blue
-
o
Intubation
-
o
Intensive care unit stay of one or more days
-
o
Noninvasive ventilated patients with supplemental O2 were considered moderate, but if those patients progressed to requiring intubation, they were classified as severe. Adverse drug reactions were recorded using the Meditech Electronic Medical Record IT system.
Statistical Analysis
Data were split into 2 time periods: before and after hospital-wide adoption of smart infusion pump systems with capnography (ie, January 2007–April 2010 vs May 2010–December 2014). We report total opioid-treated patients, overall days patients were hospitalized (irrespective of opioid exposure), total patient days of opioid exposure (calculated as sum of days exposed to opioids across all opioid-treated patients), in the precapnography and postcapnography periods. Average opioid treatment duration, calculated as the cumulative duration of treatment divided by the total number of opioid patients, was reported in the precapnography and postcapnography periods as well as yearly over the study period (2007–2014). The rate of mild, moderate, severe, and all opioid-related ADRs per 10,000 patients was calculated as the number of opioid-treated patients who have experienced an ADR divided by the total number of opioid-treated patients, multiplied by 10,000. The proportion of ADRs that were mild, moderate, severe, or code blue events was also calculated. Adverse drug reaction rates before and after the introduction of capnography monitoring were compared using χ2 test. Statistical analysis was performed using SAS v9.2.
RESULTS
Table 1 provides details on opioid use during the pre- and post-capnography periods. The number of patients treated with opioids was 139,734 patients in the precapnography and 267,573 patients in the postcapnography period. The total number of opioid-treated days was 286,434 in the pre-period versus 367,177 days in the post-period. There was a reduction in average duration of opioid treatment after May 2010; the average duration was 2.05 days during the precapnography period in comparison to 1.37 during the postcapnography period (P < 0.0001). Average duration of opioid treatment decreased steadily over the study period, as shown in Figure 1 (trending P < 0.0001).
TABLE 1.
Opioid Use Before and After Hospital-Wide Adoption of Capnography
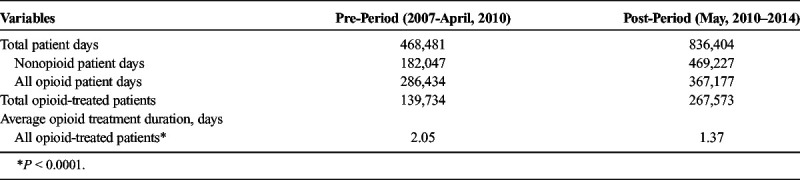
FIGURE 1.
Average duration of opioid treatment among all opioid-treated patients. Dotted line indicates point at which smart infusion pump system with capnography for monitoring end tidal carbon dioxide (ETCO2) was introduced.
Table 2 shows a nonsignificant increase in the overall ADR rates from 14.1 per 10,000 patients during the precapnography period to 15.4 per 10,000 patients during the postcapnography period (P = 0.3149). Mild and moderate opioid-related ADR rates increased from 5.1 and 5.9 per 10,000 patients during the precapnography period to 8.1 and 6.7 per 10,000 patients during the postcapnography period, respectively (P < 0.0001 for both comparisons). However, there was a 79% reduction in severe ADR rates during this period with severe ADR rates decreased from 3.1 per 10,000 patients during the precapnography period to 0.6 per 10,000 patients during the postcapnography period (P < 0.0001). The relative proportion of severe ADRs decreased from approximately 30% before the introduction of capnography monitoring to less than 10% per year after implementation of capnography, to nearly 0% by the end of the study period in 2014 (trending P < 0.01; Fig. 2). Similarly, the relative proportion of code blue events decreased from approximately 40% before the introduction of capnography monitoring to just more than 10% per year after introduction, to nearly 0% by the end of the study period (trending P < 0.01).
TABLE 2.
Mild, Moderate, Severe, and Overall Opioid-Related ADR Rates per 10,000 Opioid-Treated Patients

FIGURE 2.
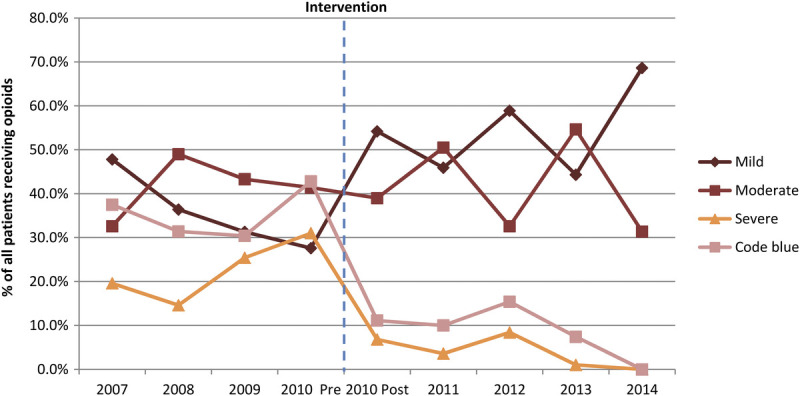
Distribution of ADR severity among patients with an opioid-related ADR from 2007 to 2014. Dotted line indicates point at which smart infusion pump system with capnography for monitoring end tidal carbon dioxide (ETCO2) was introduced.
DISCUSSION
In this study, a combination of smart infusion pump system with continuous capnography monitoring (replacing pulse oximetry) to monitor respiratory depression and pain management policy and procedure changes were used to reduce ADRs rates in hospitalized patients receiving opioids. We observed a decrease in the average duration of opioid treatment per patient, which is likely due to increased focus on safety concerns associated with opioid treatment.8 The study herein also found that rates of severe opioid-related ADRs fell significantly after the adoption of continuous capnography monitoring, whereas the rates of mild and moderate ADRs increased, which might be due to automated monitoring that captured more early mild or moderate warning signs and symptoms that prevented severe ADRs. Specifically, our finding of increased rates of mild and moderate ADRs in the postcapnography period may be due to improved detection (whereas the ADR assessment and recording procedures at Wesley did not change during the study period) of reduced respiratory rates from the use of continuous capnography monitoring during the postimplementation period. Indeed, a number of publications have reported on the increased sensitivity of capnography monitoring in detecting respiratory depression in comparison to pulse oximetry or by the treating physician,9–11 particularly in PCA patients. For example, in one study of 634 patients receiving PCA, there were 9 events of respiratory depression with all events being identified through capnography and none through pulse oximetry.11 All of these events occurred within the first 24 hours of PCA administration.11 Thus, our results are consistent with other studies suggesting capnography may be more effective than pulse oximetry at detecting early mild and moderate ADRs, which may explain the higher rate of these events after the introduction of capnography.
Although the rate of mild and moderate ADRs increased after the introduction of capnography, the rate of severe ADRs decreased. The increased detection of mild and moderate events may have resulted in the avoidance of more severe events. In the aforementioned study of 634 patients, of the 9 patients identified by capnography to have respiratory depression, the early alarm detection and alarm resulted in the rapid response team being called on 4 occasions, the attending physician being called on 3 occasions, with the nurse stimulating the patient in the other 2 cases. The study concluded that capnographic monitoring leading to the early identification of these 9 patients resulted in the prevention of serious ADRs.11 Thus, through the early detection of opioid-related ADRs, more serious consequences of respiratory depression such as respiratory arrest or events leading to an intensive care unit stay or the prolongation of a hospitalization may have been averted in our patients.
The findings of this study must be interpreted in the context of its limitations. First, because of the ecological design of the study, no statistical adjustments could be made for differences that might have existed between patients treated before and after the hospital-wide adoption of capnography. However, substantial different patient population admitted in the same hospital is unlikely; nevertheless, we cannot completely rule out that differences in case mix may exist between patients treated before and after the implementation of capnography. Lastly, although unlikely, the pre-post study design has inherent biases which do not allow us to rule out the possibility that there may have been other changes taking place at WMC which may have contributed to the observed changes in opioid use and opioid-related ADRs.
CONCLUSIONS
To our knowledge, this is the first study to evaluate the impact on opioid-related ADRs by monitoring respiratory depression with smart pump infusion systems with continuous capnography monitoring and PCA pause functionality. The implementation of a safe medication practice protocol that included staff education, pain management policies and procedures change, and the adoption of smart pumps with capnography monitoring and PCA pause functionality resulted in significantly lower rates (nearly 0%) of severe opioid-related ADRs and shorter opioid treatment. The increased rates of mild and moderate ADRs are likely secondary to the capnography monitoring sensitivity to detect early respiratory depression events compared with pulse oximetry. Hospitals seeking to reduce severe opioid-related ADRs may consider replacing pulse oximetry with smart infusion pump systems with continuous capnography monitoring among opioid-treated patients which will further strengthen the safeguards for these patients. Future studies should investigate the cost savings associated with adoption of the technology.
Footnotes
Conflicts of interest and sources of funding: Manuscript development and editorial support was provided by Analytica Laser with funding from Becton, Dickinson and Company (BD). Statistical analysis was provided by Xiaowu Sun, PhD (former BD employee), and Ying P. Tabak, PhD (currently a BD employee). Data retrieval support was provided by Jim Rosendale, MBA-IS, Pharmacy Automation Supervisor, Wesley Medical Center, Wichita, KS.
REFERENCES
- 1.Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths involving opioid analgesics: United States, 1999–2011. NCHS Data Brief. 2014;166:1–8. [PubMed] [Google Scholar]
- 2.Vila H Jr. Smith RA Augustyniak MJ, et al. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg. 2005;101:474–480, table. [DOI] [PubMed] [Google Scholar]
- 3.Davies EC Green CF Taylor S, et al. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One. 2009;4:e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Joint Commission. Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1–5. [PubMed] [Google Scholar]
- 5.Lee LA Caplan RA Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122:659–665. [DOI] [PubMed] [Google Scholar]
- 6.Kessler ER Shah M Gruschkus SK, et al. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33:383–391. [DOI] [PubMed] [Google Scholar]
- 7.Nagappa M Liao P Wong J, et al. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015;10:e0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute for Safe Medication Practices. Patient-controlled analgesia: making it safer for patients. [Institute for Safe Medication Practices Web site] 2009. Available at: https://www.ismp.org/profdevelopment/PCAMonograph.pdf. Accessed October 18, 2017.
- 9.Maddox RR Williams CK Oglesby H, et al. Clinical experience with patient-controlled analgesia using continuous respiratory monitoring and a smart infusion system. Am J Health Syst Pharm. 2006;63:157–164. [DOI] [PubMed] [Google Scholar]
- 10.Hutchison R, Rodriguez L. Capnography and respiratory depression. Am J Nurs. 2008;108:35–39. [DOI] [PubMed] [Google Scholar]
- 11.McCarter T Shaik Z Scarfo K, et al. Capnography monitoring enhances safety of postoperative patient-controlled analgesia. Am Health Drug Benefits. 2008;1:28–35. [PMC free article] [PubMed] [Google Scholar]


