LUMINOUS study confirms the effectiveness of ranibizumab for the treatment of neovascular age-related macular degeneration in real-world clinical practice. Visual acuity gains were higher among patients receiving a greater number of ranibizumab injections, particularly with three loading doses and lower BLVA levels. No new safety signals with ranibizumab were identified.
Supplemental Digital Content is Available in the Text.
Key words: anti-VEGF therapy, LUMINOUS, effectiveness, neovascular age-related macular degeneration, ranibizumab, observational study, real-world, treatment-naive, visual acuity
Abstract
Purpose:
To evaluate the effectiveness, safety, and treatment patterns of ranibizumab 0.5 mg in treatment-naive patients with neovascular age-related macular degeneration enrolled in LUMINOUS study.
Methods:
This 5-year, prospective, multicenter, observational study recruited 30,138 adult patients (treatment-naive or previously treated with ranibizumab or other ocular treatments) who were treated according to the local ranibizumab label.
Results:
Six thousand two hundred and forty-one treatment-naive neovascular age-related macular degeneration patients were recruited. Baseline (BL) demographics were, mean (SD) age 75.0 (10.2) years, 54.9% females, and 66.5% Caucasian. The mean (SD) visual acuity (VA; letters) gain at 1 year was 3.1 (16.51) (n = 3,379; BLVA, 51.9 letters [Snellen: 20/92]) with a mean (SD) of 5.0 (2.7) injections and 8.8 (3.3) monitoring visits. Presented by injection frequencies <3 (n = 537), 3 to 6 (n = 1,924), and >6 (n = 918), visual acuity gains were 1.6 (14.93), 3.3 (16.57), and 3.7 (17.21) letters, respectively. Stratified by BLVA <23 (n = 382), 23 to <39 (n = 559), 39 to <60 (n = 929), 60 to <74 (n = 994), and ≥74 (n = 515), visual acuity change was 12.6 (20.63), 6.7 (17.88), 3.6 (16.41), 0.3 (13.83), and −3.0 (11.82) letters, respectively. The incidence of ocular/nonocular adverse events was 8.2%/12.8% and serious adverse events were 0.9%/7.4%, respectively.
Conclusion:
These results demonstrate the effectiveness and safety of ranibizumab in treatment-naive neovascular age-related macular degeneration patients.
Neovascular age-related macular degeneration (nAMD) is the leading cause of legal blindness in developed countries, particularly in the elderly population.1–3 Overall, it accounts for 8.7% of total blindness worldwide.1–7 During the past decade, there have been significant advances in the management of nAMD with the introduction of anti–vascular endothelial growth factor (anti-VEGF) therapy, the current standard of care for nAMD.5,8,9
Ranibizumab (Lucentis; Novartis Pharma AG, Basel, Switzerland, and Genentech, Inc, South San Francisco, CA), an anti-VEGF agent, is approved for the treatment of patients with nAMD, and visual impairment because of diabetic macular edema, macular edema secondary to retinal vein occlusion (RVO; branch RVO and central RVO), and choroidal neovascularization (CNV) secondary to pathologic myopia choroidal neovascularization in the European Union (EU), the United States, and many other countries worldwide.8,10,11 Recently, it was also approved in the EU for its use in adults with visual impairment because of CNV associated with causes other than nAMD or myopia choroidal neovascularization.8 The efficacy and safety profile of ranibizumab is well-established across all approved indications based on data from several randomized clinical trials (RCTs) and is further supported by over 3.7 million patient-treatment years of exposure.8,10,12–22 Likewise, the effectiveness of ranibizumab in real-world settings has also been demonstrated, but these data have been limited to either specific regions, countries, or small patient populations.23–35 Hence, in this 1-year report, we have summarized our observations/findings from the large patient population both at global level and at country level and correlated these findings with the established efficacy and safety profile of ranibizumab in the treatment of patients with nAMD.
LUMINOUS (NCT01318941) was a large, prospective, observational trial designed to evaluate the long-term effectiveness, safety, and treatment pattern outcomes with intravitreal ranibizumab treatment in routine clinical practice across five approved indications (nAMD, diabetic macular edema, branch RVO, central RVO, and myopic CNV) over 5 years.36 Here, we present global and country-specific 1-year efficacy data for the treatment-naive patients with nAMD enrolled in this study.
Methods
Study Design
LUMINOUS was a 5-year, prospective, observational, multicenter, open-label, single-arm, global study. The period between study start and completion was predefined as 5 years in the protocol. The study was conducted from March 2011 to April 2016 at 488 clinical sites across 42 countries.36
Patients with any of the approved indications as per the ranibizumab label were enrolled. They were treated with intravitreal ranibizumab 0.5 mg according to the local product label at outpatient ophthalmology clinics. Novartis did not supply ranibizumab 0.5 mg to the patients who were enrolled. The enrolled patients were not compensated for their participation in the study visits. However, patients were reimbursed based on their reimbursement policy in the country they lived. As patients were recruited over time and the calendar time point of study completion was preset, follow-up time varied according to the entry dates. The minimum potential follow-up for each patient was defined as 1 year in the protocol. Visits took place at a frequency determined by the investigator. Data from all visits were documented in the electronic case report form. However, it was recommended to capture data in electronic case report form at every visit or at a minimum of every 3 months. Physicians were encouraged to follow-up with patients who had not been seen in the clinic for at least 6 months since the last visit, to capture data. Patients not seen at least once per year or who were switched to another anti-VEGF therapy were discontinued from the study.
The first eye treated during the study was considered the primary treated eye. If both eyes were first treated on the same date, or if both eyes were pretreated, the eye with the earliest diagnosis date was considered the primary treated eye. If both eyes had the same diagnosis date, one eye was chosen randomly as the primary treated eye.
The study protocol was reviewed and approved by an Independent Ethics Committee or Institutional Review Board for each center. The study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices issued by the International Society for Pharmacoepidemiology, with any applicable national guidelines, and ethical principles laid down in the Declaration of Helsinki. Patients provided written informed consent. The study is registered with clinicaltrials.gov as NCT01318941.36
Study Population
Consenting adult (age ≥18 years) patients, who were either treatment-naive or previously treated with ranibizumab or another ocular therapy for any of the approved indications included in the local product label, were enrolled. Patients were excluded if they were participating in other investigational studies or if they had received systemic or ocular anti-VEGF therapy other than ranibizumab 90 days or 30 days before enrollment, respectively.
Assessments
Demographic and baseline characteristics were collected at baseline, including ocular and nonocular medical history, primary indication for initiation of ranibizumab treatment, and previous ocular treatments/therapies. Baseline lesion characteristics for the patients with nAMD were optional and collected if available. These were presented using standard descriptive statistics and were presented by pretreatment status, indication, and period.
Effectiveness assessments included visual acuity (preferably best-corrected visual acuity) evaluation by each participating physician as a part of routine care practice using Early Treatment Diabetic Retinopathy Study letters or Snellen charts or equivalent. To facilitate data analysis, Snellen fractions and decimals were converted to the Early Treatment Diabetic Retinopathy Study equivalent letter scores. It was recommended that the same method of visual acuity assessment be used throughout the study wherever possible. All adverse events (AEs), including serious AEs (SAEs), irrespective of suspected causal association that occurred during the study were collected.
Other assessments such as optical coherence tomography (central retinal thickness) and ocular examination (preinjection intraocular pressure [IOP]) were optional but included if the data were available.
The number of ranibizumab injections administered overall, over time, and the average time interval (in weeks) between consecutive injections, visit frequency, treatment patterns, unilateral (involving single eye)/bilateral (involving both eyes) treatments, and proportion of patients receiving ocular and nonocular concomitant medications were recorded.
Statistical Analysis
Because of the design of the study, 1-year data were available potentially for all patients, while the availability of data for subsequent years depended on the patient's study entry date. The effectiveness data are therefore presented here for the period up to 1 year. Effectiveness data after more than 1 year of ranibizumab treatment in patients with nAMD will be described in a separate publication.
All effectiveness and safety data were summarized descriptively. The enrolled set included all patients who signed the informed consent and had at least a baseline assessment. The safety set comprised patients in the enrolled set who were treated with at least one dose of ranibizumab during the study or before the start of the study and had at least one safety assessment after the first treatment. The primary treated eye set included all primary treated eyes in patients from the safety set and was the primary analysis set for effectiveness.
For treatment-naive eyes, the date of first on-study injection with ranibizumab was considered the baseline date (study Day 1). The primary effectiveness variable was the mean change in visual acuity Early Treatment Diabetic Retinopathy Study letter score from baseline presented by quarterly and yearly periods for the primary treated eye set. Effectiveness data are presented for patients in the primary treated eye set who provided baseline and Year 1 data. The mean change in visual acuity from baseline at Year 1 was presented by injection frequency during Year 1 (<3, 3–6, and >6), loading (at least three ranibizumab injections up to Day 100)/nonloading dose and baseline visual acuity category. Further visual acuity evaluations include the proportion of patients maintaining baseline visual acuity of ≥73 letters (good starting vision, or Snellen equivalent 20/40) at Year 1 and those achieving visual acuity of ≥73 from a baseline visual acuity of <73 letters (poor starting vision) at Year 1; and the proportions of patients with a visual acuity loss (defined as ≤0 letter change from baseline) or gain (defined as >0 letter change from baseline) of >0 to <5 letters, 5 to <10 letters, 10 to <15 letters, and ≥15 letters at Year 1. The number of injections and monitoring visits up to 1 year were summarized for patients with at least 365 days of participation in the study. Safety was assessed based on the incidence proportion, relationship, and severity of treatment-emergent ocular and nonocular AEs. Ocular AEs were assessed for the primary treated eye set and nonocular AEs were assessed for the safety set over 5 years.
Results
Study Enrollment, Patient Demographics, and Baseline Ocular Characteristics
The number of patients recruited and treated with ranibizumab (safety set) in the LUMINOUS study was 30,138 for all approved indications (nAMD, diabetic macular edema, branch RVO, central RVO, and myopic CNV) worldwide. This represented a more diverse study population than those normally enrolled in RCTs. Overall, 75.4% (n = 22,717) of the patients in the safety set had nAMD, of whom 6,241 were treatment-naive. At 1 year, 4,768 treatment-naive patients with nAMD remained in the LUMINOUS study. The most frequent reasons for study discontinuation were loss to follow-up (11.5%), followed by switch to another anti-VEGF (5.9%) (Table 1). The reasons for switching were not recorded. However, the authors also expected other factors to affect discontinuation like travel distances, the upcoming availability of further treatment options, and possibly varying follow-up practices. Not every patient remaining in the study at 1 year had a 1-year visual acuity value recorded (a value recorded within a 1.5-month window around 12 months); as per design of the study, visits were scheduled at the discretion of the investigator and could fall outside the 12-month window. In the primary treated eye set, 3,379 treatment-naive patients with nAMD provided baseline and 1-year visual acuity data. The safety set included 6,241 treatment-naive patients with nAMD.
Table 1.
Patient Disposition for Treatment-Naive Patients With nAMD
| Disposition, n (%) | Treatment-Naive Patients With nAMD, N = 6,234* |
| Patients with one eye treated in the study (primary treated eye) | 5,502 (88.3) |
| Patients ongoing in the study at Year 1 | 4,768 (76.5) |
| Patients who discontinued the study | 1,466 (23.5) |
| Reason for discontinuation | |
| AE | 10 (0.2) |
| Abnormal laboratory value | 0 |
| Abnormal test procedure result | 0 |
| Unsatisfactory therapeutic effect | 41 (0.7) |
| Subject's condition no longer requires study drug | 126 (2.0) |
| Subject withdrew consent | 87 (1.4) |
| Loss to follow-up | 715 (11.5) |
| Administrative problems | 38 (0.6) |
| Death | 47 (0.8) |
| Pregnancy | 0 |
| Switched to anti-VEGF other than ranibizumab | 369 (5.9) |
| Protocol deviation | 33 (0.5) |
Safety set.
Data collected until the last recorded follow-up date were used to perform the analyses (i.e., data for 5-year duration of the study).
For treatment-naive eyes, the date of first on-study injection with ranibizumab was considered the baseline date.
Patients with a baseline visit on or before March 2015 are included. Data for seven patients are missing from the total 6,241 enrolled treatment-naive patients with nAMD.
AE, adverse event; nAMD, neovascular age-related macular degeneration; VEGF, vascular endothelial growth factor.
At baseline, the mean (SD) age of patients was 75.0 (10.2) years, most were Caucasian and the majority were female (Table 2). More than 40% of all lesions were graded as predominantly classic, and over 60% of all lesions had a disc area size of >1 (Table 2). Pigment epithelial detachment was the most common nAMD subphenotype, followed by polypoidal choroidal vasculopathy, and retinal angiomatous proliferation (Table 2).
Table 2.
Baseline Demographic, and Ocular Characteristics for Treatment-Naive Patients With nAMD
| Characteristics | Treatment-Naive Patients With nAMD, N = 6,241* |
| Patient demographics | |
| Mean (SD) age, years | 75.0 (10.17) |
| Gender, n (%) | |
| Male | 2,813 (45.1) |
| Female | 3,428 (54.9) |
| Race, n (%) | |
| Caucasian | 4,152 (66.5) |
| Asian | 1,827 (29.3) |
| Native American | 11 (0.2) |
| Pacific Islander | 5 (0.1) |
| Black | 8 (0.1) |
| Other | 193 (3.1) |
| Baseline lesion characteristics | |
| Lesion type, % | |
| Predominantly classic | 2,723 (43.6) |
| All others | 3,455 (55.4) |
| Lesion size, % | |
| ≤1 DA | 2,362 (37.9) |
| >1 DA | 3,761 (60.3) |
| PED, % | 2,665 (42.7) |
| PCV, % | 572 (9.2) |
| RAP, % | 246 (3.9) |
| Ocular characteristics | |
| VA | |
| n† | 5,797 |
| Mean (SD) VA, ETDRS letters | 49.7 (21.92) |
| Snellen VA | 20/102 |
| CRT | |
| n† | 4,599 |
| Mean (SD) CRT, µm | 365.7 (142.50) |
| IOP | |
| n† | 4,539 |
| Mean (SD), mmHg | 15.4 (3.6) |
| Median time from diagnosis to first treatment, days | 12.0 |
Safety set.
For treatment-naive eyes, the date of first on-study injection with ranibizumab was considered the baseline date.
Data collected until the last recorded follow-up date was used to perform the analyses (i.e., data for 5-year duration of the study).
For 3,379 patients with baseline and 1 year data, the mean (SD) VA at baseline was 51.9 (21.0) letters.
Number of patients at enrollment.
Number of evaluable baseline patients.
CRT, central retinal thickness; DA, disc area; ETDRS, Early Treatment Diabetic Retinopathy Study; PCV, polypoidal choroidal vasculopathy; PED, pigment epithelial detachment; RAP, retinal angiomatous proliferation; SD, standard deviation; VA, visual acuity.
Patients had a broad range of comorbidities with hypertension, hypercholesterolemia, and diabetes being the most frequent (see Table, Supplemental Digital Content 1, http://links.lww.com/IAE/B112). Ocular concomitant medications and significant nondrug therapies were reported for 22.2% of patients in the primary treated eye set and nonocular concomitant medications and significant nondrug therapies were reported for 53.7% of patients in the safety set.
Over 85% of treatment-naive patients with nAMD were recruited from 10 countries (see Table, Supplemental Digital Content 2, http://links.lww.com/IAE/B113). These were the United Kingdom (18.4%), Japan (12.7%), China (11.6%), Poland (9.4%), Russia (9.1%), Canada (8.8%), Slovakia (5.4%), Australia (4.0%), Germany (3.3%), and South Korea (2.4%; Figure 1). At the end of the LUMINOUS study, the highest discontinuation rates were observed in South Korea (81.60%), China (81.47%), Japan (60.51%), United Kingdom (60.44%), and Australia (50.20%). The main reasons in most of the cases were “lost to follow-up.” The highest rates of discontinuation owing to “lost to follow-up” were observed in China (70.3%) and South Korea (48.7%). “Patient switched to anti-VEGF other than ranibizumab” was the most frequent discontinuation reason in United Kingdom (30.0%), Japan (24.3%), and Australia (15.7%). Any patient who switched to another treatment was required to leave the study.
Fig. 1.
LUMINOUS study overview: Overall worldwide recruitment and countries enrolling the highest number of treatment-naive patients with nAMD. The pop-out boxes display the number (%) of treatment-naive patients with nAMD in the top 10 enrolling countries for this cohort. nAMD, neovascular age-related macular degeneration; UK, United Kingdom.
Efficacy Outcomes
The mean (SD) visual acuity gain from baseline at Year 1 for 3,379 treatment-naive patients with nAMD was 3.1 (16.5) letters from a baseline visual acuity letter score of 51.9 (21.0) (Snellen: 20/92). The mean visual acuity gain at Year 1 increased with the number of ranibizumab injections received during Year 1 in the study (Figure 2A). Similarly, the visual acuity gain was higher in patients who received a loading dose of three initial consecutive monthly ranibizumab injections (Figure 2B). The mean number of injections in these patients was numerically higher than the patients who did not receive a loading dose (Figure 2B). Stratified by baseline visual acuity category, vision was improved in patients in each baseline visual acuity category except for baseline visual acuity category of ≥74 letters (Snellen: 20/33) where there was a loss of 3.0 letters from baseline. Notably, visual acuity gains were greater in patients with lower baseline visual acuity (Figure 2C), whereas patients with better vision at baseline maintained their vision at or close to their starting levels.
Fig. 2.

Mean change in visual acuity from baseline to Year 1 by (A) injection frequency, (B) loading and nonloading dose, and (C) baseline visual acuity. Observed data set (primary treated eye set). Values in parentheses represent the Snellen visual acuity equivalent. *Total number of patients at enrollment from global cohort. †Total number of patients with baseline and Year 1 data from global cohort. ‡Number of evaluable patients with baseline and Year 1 data based on injection frequency category. **Number of evaluable patients with baseline and Year 1 data for 6 to 9 injection stratum. #Final visual acuity at Year 1. $Number of evaluable patients with baseline and Year 1 data based on the baseline visual acuity. Loading dose group is defined as the patients who received the three initial consecutive monthly ranibizumab injections up to Day 100. For treatment-naive eyes, the date of first on-study injection with ranibizumab was considered the baseline date. For the 1-year period, all patients with nonmissing baseline visual acuity and Year 1 visual acuity performed anywhere between Day 319 and Day 409 but who had been in the study for at least 365 days from baseline to the last follow-up date were included in the analysis. ETDRS, Early Treatment Diabetic Retinopathy Study.
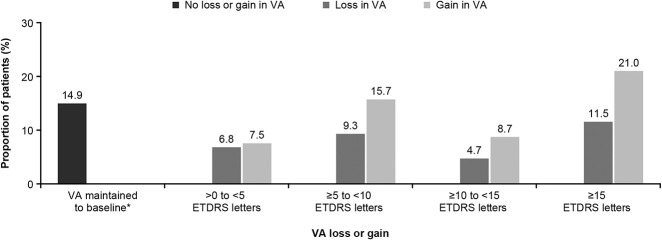
Overall, 15.6% (n = 527) of patients had a baseline visual acuity of ≥73 letters (Snellen: 20/35), and 73.8% (n = 389) of these patients maintained this good vision at Year 1 (see Figure, Supplemental Digital Content 3, http://links.lww.com/IAE/B114); of the 84.4% (n = 2,852) of patients with poorer baseline vision (visual acuity <73 letters [Snellen: 20/35]), 18.1% (n = 515) achieved visual acuity of ≥73 letters (Snellen: 20/35) at Year 1. Thus, of the total 3,379 treatment-naive patients with nAMD, 26.8% (n = 904) of patients had visual acuity of ≥73 letters (Snellen: 20/35) at 1 year, regardless of their baseline visual acuity. At Year 1, a majority of patients (52.9%; n = 1786) treated with ranibizumab had visual acuity gain (Figure 3). Visual acuity was maintained at baseline levels (0 letters loss) in 14.9% (n = 504) of patients (Figure 3). Visual acuity loss of >0 to <15 letters from baseline was reported in over one-fifth (20.8%; n = 701) of patients treated with ranibizumab, whereas the proportion of patients with a visual acuity loss of ≥15 letters was 11.5% (n = 388; Figure 3).
Fig. 3.
Proportion of patients with loss or gain in visual acuity at Year 1. *Includes patients with 0 letters loss. Observed data set (primary treated eye set). Data shown for 3,379 patients with baseline and Year 1 data from global cohort. For treatment-naive eyes, the date of first on-study injection with ranibizumab was considered the baseline date. For the 1-year period, all patients with nonmissing baseline visual acuity and Year 1 visual acuity performed anywhere between Day 319 and Day 409 but who had been in the study for at least 365 days from baseline to last follow-up date were included in the analysis. ETDRS, Early Treatment Diabetic Retinopathy Study; nAMD, neovascular age-related macular degeneration.
Treatment Exposure and Visits
The mean (SD) number of ranibizumab injections up to Year 1 was 5.0 (2.7), and the mean number of monitoring visits was 8.8 (3.3). The mean number of injections was comparable between each of the baseline visual acuity categories (range: 4.3–5.5 injections; Figure 2C). Overall, 72.9% of patients received six or fewer injections in the first year and 35.5% of patients received three or fewer (see Figure, Supplemental Digital content 4, http://links.lww.com/IAE/B115).
Country-Specific Analysis of Ranibizumab Treatment Patterns and Associated Outcomes
Similar to the global cohort, an improvement in visual acuity at Year 1 was observed across the 10 countries that enrolled the greatest number of patients (Figure 4). Highest visual acuity gains were seen in patients from South Korea (9.8 letters) and Japan (6.0 letters), with a mean of 5.3 and 4.0 injections, respectively, followed by Australia (4.5 letters) but with a greater (8.7) mean number of injections in the first year. Moderate gains (range: 2.3–3.0 letters) were seen in patients from Western and European countries with 4.0 to 7.5 mean number of injections, whereas the patients from China and Russia had much lower gains with a low number of injections (China: 1.1 letters with 2.9 injections; Russia: 1.6 letters with 2.7 injections). Countries whose patients had a higher baseline visual acuity generally achieved a greater final visual acuity at Year 1 (range: 4.0–8.7 injections), in contrast to those with lower baseline, especially China and Russia, where the patients received <3 mean injections (Figure 4). The median time from diagnosis of nAMD to the first treatment was within 15 days in all the highest recruiting countries except Russia and Slovakia, where the median time was more than a month.
Fig. 4.
Visual acuity outcomes at Year 1: country-specific analyses. Observed data set (primary treated eye set). Values in parentheses represent the Snellen visual acuity equivalent. N = total number of patients with baseline and Year 1 data from global cohort; n = number of evaluable patients from top 10 recruiting countries (treatment-naive nAMD) with highest evaluable baseline and Year 1 data. For treatment-naive eyes, the date of first on-study injection with ranibizumab was considered the baseline date. For the 1-year period, all patients with nonmissing baseline visual acuity and Year 1 visual acuity performed anywhere between Day 319 and Day 409 but who had been in the study for at least 365 days from baseline to the last follow-up date were included in the analysis. ETDRS, Early Treatment Diabetic Retinopathy Study.
Safety Outcomes
Over 5 years, across all treatment-naive patients with nAMD (n = 6,241), ocular AEs were reported in 8.24% (n = 514) of patients in the primary treated eye set; the most common were cataract (1.81%; n = 113) followed by increase in IOP (0.53%, n = 33) and conjunctival hemorrhage (0.53%; n = 33; Table 3). The ocular AEs suspected to be related to ranibizumab and/or ocular injection were reported in 2.28% (n = 142) of eyes, of which 0.74% (n = 46) were reported to be related to ranibizumab alone. In all, 4.95% (n = 309), 2.6% (n = 160), and 0.72% (n = 45) of patients had mild, moderate, and severe ocular AEs, respectively. Nonocular AEs were reported in 12.75% (n = 796) of patients in the safety set; the most common were fall (0.71%; n = 44), urinary tract infection (0.66%; n = 41), and lower respiratory tract infection (0.64%; n = 40; Table 3). Incidence of nonocular AEs suspected to be related to ranibizumab and/or ocular injection was low (0.71%; n = 44), all of which were reported to be related to ranibizumab alone. In all, 3.78% (n = 236), 3.81% (n = 238), and 5.16% (n = 322) of patients had mild, moderate, and severe nonocular AEs, respectively.
Table 3.
Proportion of Patients With Ocular and Nonocular Adverse Events for Total Treatment-Naive Patients With nAMD
| Preferred Term, n (%) | Treatment-Naive Patients With nAMD, N = 6,241* |
| Ocular AEs, total | 514 (8.24) |
| Cataract | 113 (1.81) |
| IOP increased | 33 (0.53) |
| Conjunctival hemorrhage | 33 (0.53) |
| Conjunctivitis | 26 (0.42) |
| Eye pain | 22 (0.35) |
| Visual acuity reduced | 22 (0.35) |
| Dry eye | 19 (0.30) |
| Retinal hemorrhage | 19 (0.30) |
| Blepharitis | 17 (0.27) |
| Posterior capsule opacification | 16 (0.26) |
| Glaucoma | 15 (0.24) |
| Vitreous floaters | 14 (0.22) |
| Ocular hypertension | 14 (0.22) |
| Visual impairment | 12 (0.19) |
| Vision blurred | 11 (0.18) |
| Endophthalmitis | 11 (0.18) |
| Corneal abrasion | 10 (0.16) |
| Retinal pigment epithelial tear | 10 (0.16) |
| Nonocular AEs, total | 796 (12.75) |
| Fall | 44 (0.71) |
| Urinary tract infection | 41 (0.66) |
| Lower respiratory tract infection | 40 (0.64) |
| Pneumonia | 34 (0.55) |
| Cerebrovascular accident | 28 (0.45) |
| Hypertension | 23 (0.37) |
| Influenza | 22 (0.35) |
| Atrial fibrillation | 21 (0.34) |
| Bronchitis | 20 (0.32) |
| Dyspnoea | 19 (0.30) |
| Osteoarthritis | 19 (0.30) |
| Nasopharyngitis | 18 (0.29) |
| Myocardial infarction | 17 (0.27) |
| Angina pectoris | 17 (0.27) |
| Cough | 16 (0.26) |
| Dizziness | 15 (0.24) |
| Anaemia | 14 (0.22) |
| Transient ischaemic attack | 14 (0.22) |
| Headache | 13 (0.21) |
| Basal cell carcinoma | 13 (0.21) |
| Cardiac failure | 12 (0.19) |
| Constipation | 11 (0.18) |
| Lung neoplasm malignant | 11 (0.18) |
| Back pain | 10 (0.16) |
| Diarrhoea | 10 (0.16) |
| Pain in extremity | 10 (0.16) |
| Vomiting | 10 (0.16) |
| Sciatica | 10 (0.16) |
Primary treated eye set for ocular AEs; Safety set for nonocular AEs.
n = number of patients.
Ocular and nonocular AEs ≥10 in number are shown.
Safety set comprised patients in the enrolled set who were treated with at least one dose of ranibizumab during this study or before the start of the study and had at least one safety assessment after the first treatment.
A patient with multiple occurrences of an AE is counted once per preferred term.
Data collected until the last recorded follow-up date was used to perform the analyses (i.e., data for overall duration of the study).
For treatment-naive eyes, the date of first on-study injection with ranibizumab was considered the baseline date.
Number of patients at enrollment.
The incidence of ocular SAEs was 0.91% (n = 57) in the primary treated eye set; the most common ocular SAE was endophthalmitis reported in 0.18% (n = 11 or 1 case per 3,628 injections) of treatment-naive patients with nAMD. Second most common ocular SAEs were retinal hemorrhage and cataract (each 0.10%; n = 10; Table 4). Ocular SAEs leading to discontinuation of ranibizumab treatment were reported in 0.1% (n = 9) of patients. The incidence of nonocular SAEs was 7.4% (n = 462) in the safety set; pneumonia (0.47%; n = 29) and cerebrovascular accidents (0.45%; n = 28) were the most common nonocular SAEs followed by myocardial infarction (0.27%; n = 17; Table 4). Nonocular SAEs leading to discontinuation of ranibizumab treatment were reported in 2.8% (n = 174) of patients. Death was reported in 0.83% (n = 52) of patients; none were suspected to be related to the study treatment by the investigator. No new safety signals in addition to the well-characterized safety profile of ranibizumab were identified in this population.
Table 4.
Proportion of Patients With Ocular and Nonocular Serious Adverse Events for Total Treatment-Naive Patients With nAMD
| Preferred Term, n (%) | Treatment-Naive Patients With nAMD, N = 6,241* |
| Ocular SAEs, total | 57 (0.91) |
| Endophthalmitis | 11 (0.18) |
| Retinal hemorrhage | 6 (0.10) |
| Cataract | 6 (0.10) |
| Retinal pigment epithelial tear | 5 (0.08) |
| Macular hole | 5 (0.08) |
| Retinal detachment | 4 (0.06) |
| nAMD | 4 (0.06) |
| Vitreous hemorrhage | 2 (0.03) |
| Glaucoma | 2 (0.03) |
| Iridocyclitis | 2 (0.03) |
| Blindness | 1 (0.02) |
| Open-angle glaucoma | 1 (0.02) |
| IOP increased | 1 (0.02) |
| Conjunctival hemorrhage | 1 (0.02) |
| Macular fibrosis | 1 (0.02) |
| Subretinal hematoma | 1 (0.02) |
| Dry age-related macular degeneration | 1 (0.02) |
| Eye hemorrhage | 1 (0.02) |
| Retinal injury | 1 (0.02) |
| Retinal vein thrombosis | 1 (0.02) |
| Vitritis | 1 (0.02) |
| Nonocular SAEs, total | 462 (7.4) |
| Pneumonia | 29 (0.47) |
| Cerebrovascular accident | 28 (0.45) |
| Myocardial infarction | 17 (0.27) |
| Atrial fibrillation | 16 (0.26) |
| Fall | 15 (0.24) |
| Angina pectoris | 12 (0.19) |
| Lung neoplasm malignant | 11 (0.18) |
| Cardiac failure | 10 (0.16) |
| Transient ischemic attack | 10 (0.16) |
| Dyspnea | 10 (0.16) |
| Death | 52 (0.83) |
Primary treated eye set for ocular SAEs; safety set for nonocular SAEs.
n = number of patients.
All ocular SAEs are shown. Nonocular SAEs ≥10 in number are shown, except for death which is mentioned for all patients.
Safety set comprised patients in the enrolled set who were treated with at least one dose of ranibizumab during this study or before start of study and had at least one safety assessment after the first treatment.
A patient with multiple occurrences of an SAE is counted once per preferred term.
Data collected until the last recorded follow-up date were used to perform the analyses (i.e., data for overall duration of the study).
For treatment-naive eyes, the date of first on-study injection with ranibizumab was considered the baseline date.
Number of patients at enrollment.
Ocular AE rates by country ranged from 2.1% (Russia) to 17.5% (Slovakia and United Kingdom), where cataract was observed most frequently. Slovakia and United Kingdom were also the countries with the highest rates of nonocular AEs (22.3% in Slovakia; 34.8% in United Kingdom), with no particular AEs occurring more frequently than the others in Slovakia and a slight predominance of fall in United Kingdom. The lowest nonocular AE rate (1.7%) was reported in China. The rates of ocular SAEs by country ranged from 0% to 2.1% (United Kingdom).This may be because of different reporting practices and the study situation, where possibly more examinations were performed than would have been done in a routine clinical practice.
Discussion
LUMINOUS is the first large-scale, multi-indication, prospective, observational, postmarketing study of ranibizumab, enrolling more than 30,000 patients across 42 countries worldwide. The results from the present analysis of the treatment-naive nAMD cohort from this study demonstrate the effectiveness of ranibizumab during the first year of treatment in these patients, particularly in those who received a higher number of ranibizumab injections and had adequate treatment at the start of therapy (loading dose). Patients with higher baseline visual acuity demonstrated better visual outcomes at 1 year, although the mean visual acuity gains were higher in those with lower baseline visual acuity. These results are consistent with previous real-world studies showing a “ceiling effect” in visual acuity gains for patients with good presenting vision27,37,38 and further support the common observation that baseline visual acuity is a major factor in predicting visual outcome.
In the LUMINOUS study, ranibizumab treatment resulted in a mean visual acuity gain of 3.1 letters (n = 3,379) at 1 year with a mean of 5.0 injections in treatment-naive patients with nAMD across all countries. These results are comparable with observational studies of ranibizumab that reported mainly preservation of vision in nAMD patients in the real-world after 1 year.23,25,27,34,35 In the LUMIERE study, conducted in France, the mean visual acuity gain was 3.2 letters (N = 551) with a mean of 5.1 injections, whereas in the German WAVE study, the mean visual acuity change from baseline was 0.02 logMAR (N = 2,467) with a mean of 4.3 injections.23,35 In another real-life retrospective, observational study (AURA), in which nAMD patients from Canada, France, Germany, Ireland, Italy, the Netherlands, the United Kingdom, and Venezuela were assessed, the mean visual acuity gains were 2.4 letters (N = 1,695) with a mean of 5.0 injections over 1 year.25,34
In an observational study from the Fight Retinal Blindness! registry that captured data from 1,140 treatment-naive patients with nAMD from Australia during routine clinical practice, the visual acuity gains were comparatively higher (4.7 letters) with a mean of 7.0 injections.39
Injection frequencies in LUMINOUS and these other real-world studies are lower than those seen in prospective RCTs and lower than anticipated; as according to the ranibizumab product label, even under a treat and extend (T&E) regimen, nAMD patients would receive at least seven injections in the first year if there was no disease activity after the first three loading doses were administered.8 The potential undertreatment of patients with fewer injections in routine clinical settings23,25,27,34,35,37 is particularly apparent when comparing the robust visual improvement seen in pivotal clinical trials such as ANCHOR (11.3 letters) and MARINA (7.2 letters) in which monthly ranibizumab treatment was administered18,40 or when comparing the visual acuity outcomes reported in several RCTs with an individualized treatment approach (T&E and pro re nata [PRN]) of ranibizumab.41–47 Thus, the suboptimal functional outcomes with relatively low injection numbers suggest that patient management would be likely to improve substantially in real-world settings with more consistent monitoring and more frequent treatment or proactive intervention. The variation in treatment response in treatment-naive patients with nAMD from LUMINOUS may also be attributed to the heterogeneous patient population, with diverse ocular and baseline characteristics and the presence of sundry comorbidities, many of which would have excluded them from participation in RCTs.
Visual acuity gains appear to be mostly related to injection frequency as evident here from the progressive increase with increased number of injections (Figure 2A). These findings support the need for sufficient treatment (at least six injections on average) in real-world clinical settings to achieve substantial gains in visual acuity during the first year of treatment. Similarly, the better visual outcomes in patients who received an initial three consecutive monthly ranibizumab injections (“loading”) reinforce the need for early intense treatment to achieve the best possible visual outcomes, as also observed in previous studies in treatment-naive patients with nAMD.13,18,35,40
Studies have also shown that ranibizumab treatment stabilizes vision in nAMD patients presenting with relatively good visual acuity at baseline.27,37,38 In LUMINOUS, over 70% of patients with a baseline vision of ≥73 letters (Snellen: 20/35) maintained the good visual acuity levels after 1 year of ranibizumab treatment. This result suggests that treatment with ranibizumab over 1 year in real-world settings has the potential to further increase the proportion of treatment-naive nAMD patients maintaining good visual acuity (e.g., ≥73 letters [Snellen: 20/35]) with more rigorous treatment.
The treatment-naive patients with nAMD from LUMINOUS were treated in real-world clinical practice settings across various countries with different healthcare systems. Among the 10 countries enrolling the most treatment-naive patients with nAMD, the highest baseline visual acuity was observed in patients from Japan and the lowest in patients from China and Russia. The visual acuity gains in China and Russia were also the lowest among these 10 countries, but this may be attributed to a low mean number of injections (<3 injections), lower baseline visual acuity, limitations in patient access to treatment and the concomitant inability to treat patients immediately after diagnosis, and limited medical insurance because of possible limitations of the healthcare system in these countries.48 Highest visual acuity gains were observed in South Korea and Japan with 4.0 to 5.0 mean injections, whereas in Australia, where a T&E regimen is commonly used in clinical practice,49 better visual acuity gains (4.5 letters) were achieved with 8.7 mean injections. Many European countries (Poland, Germany, United Kingdom, and Slovakia) had only modest gains (2.3–3.0 letters) in vision with a comparable mean number of injections (range: 4:0–5.6 injections). The better visual outcomes in the Australian cohort may be because of higher injection frequency after the T&E regimen compared with the PRN regimen extensively practiced in Europe, mainly until 2014.49–51 Canada, where individualized treatment regimen such as T&E is largely used by practicing physicians, also showed a moderate gain of 2.5 letters with a higher mean number of injection (7.5 injections); incidentally, the baseline visual acuity of patients from Canada was lower compared with the other countries (except for China, Russia, and Poland).52 These variations in visual outcomes between countries reflect differences in the underlying healthcare systems including reimbursement of treatment, limited medical insurance coverage, and access to treatment and treatment regimen used. Furthermore, undertreatment of patients may be because of treatment cost, patient compliance and follow-up, and the clinician's decision to treat in subsequent visits. Identifying such barriers to the receipt of optimal or adequate therapy by clinics, with action to address these, could help to achieve improved outcomes.
A major challenge in routine clinical settings is the provision of adequate individualized treatment and monitoring to optimize patient visual outcomes. In LUMINOUS globally, 72.9% of treatment-naive patients with nAMD (who received ≤6 injections) were thus effectively undertreated in the first year of treatment.
Across all treatment-naive patients with nAMD, the frequency of ocular and nonocular SAEs and AEs over 5 years was low. Ocular SAEs and AEs leading to discontinuation of ranibizumab, and ocular AEs related to ranibizumab and/or injection were rare. Overall, the ocular AEs observed in LUMINOUS were consistent with those observed in the more restrictive RCT populations and consistent with the well-established safety profile of ranibizumab.8,13,18,24,40
The strength of the LUMINOUS study is its large sample size including patients with diverse demographics and baseline characteristics, long-term duration, prospective nature, and its real-world assessment of use of ranibizumab across multiple centers worldwide. The study was limited by a number of factors: first, the lack of a comparator arm; second, although the study enrolled a large number of patients, nearly 24% discontinued the study and efficacy data were available only for over 54% of the enrolled population at Year 1; finally, the flexible inclusion criteria, variable treatment schedules across regions, and inclusion of difficult-to-treat patients that could have resulted in unexplained variations in the outcomes.
To conclude, real-world evidence from the LUMINOUS study confirms the benefits of ranibizumab in treatment-naive patients with nAMD over 1 year. Visual acuity gains observed at Year 1 were greater in patients receiving a higher, more appropriate number of injections including three doses within the first 90 days of treatment (loading dose). Thus, prompt initiation of treatment is critical in improving visual outcomes. The baseline vision of ≥73 letters (Snellen: 20/35) was maintained in most of the patients after 1 year of ranibizumab treatment. LUMINOUS also highlights the diversity in visual acuity outcomes and treatment patterns in real-world clinical settings among countries. Globally and across regions, baseline visual acuity was an important predictor for overall visual acuity outcomes. There were no new safety signals with ranibizumab identified in this study. Further follow-up analyses of the LUMINOUS nAMD cohort are expected to provide additional long-term evidence on the benefit of ranibizumab treatment in real-world clinical practice.
Supplementary Material
Acknowledgments
The authors thank all the investigators (see Table, Supplemental Digital Content 5, http://links.lww.com/IAE/B116) for their valuable contribution toward this study, and study participants. The authors also thank Urvashi Bawane (Scientific Services Practice—Product Lifecycle Services, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for medical writing and editorial assistance toward the development of this article.
Footnotes
Supported and sponsored by Novartis Pharma AG and is registered with www.clinicaltrials.gov (NCT01318941). The sponsor participated in the study design, conducting the study, and data collection, management, analysis, and interpretation. Proprietary or commercial disclosures may be found after the references.
Data from this study were presented at the 17th European Society of Retina Specialists (EURETINA), Barcelona, Spain, September 7 to 10, 2017; the 7th European Association for Vision and Eye Research (EVER) Congress, Nice, France, September 27 to 30, 2017; the Asia-Pacific Academy of Ophthalmology (APAO) Congress, Hong Kong, China, February 8 to 11, 2018; the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, Honolulu, Hawaii, April 29 to May 03, 2018; the 18th European Society of Retina Specialists (EURETINA), Vienna, Austria, September 20 to 23, 2018; and the American Academy of Ophthalmology Congress, Chicago, IL, October 27 to 30, 2018.
F. G. Holz: Consultant—Acucela, Bayer, Boehringer-Ingelheim, Genentech, Heidelberg Engineering, LIN Bioscience, Novartis, Pixium, Roche, and Zeiss; Grants—Allergan, Carl Zeiss Meditec, Genentech, Heidelberg Engineering, Nightstar, Novartis, Optos, Pixium, and Roche; Lecture fees—Bayer, Genentech, Heidelberg Engineering, Novartis, Roche, and Zeiss. M. S. Figueroa: Consultant—Alcon, Allergan, Bayer, Novartis, Roche, and Zeiss. F. Bandello: Consultant—Allergan, Bayer, Boehringer-Ingelheim, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Sooft, Thrombogenics, and Zeiss; Board membership, expert testimony, and lecture fees—Allergan, Bayer, Boehringer-Ingelheim, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Sooft, Thrombogenics, and Zeiss; Y. Yang: Research grants—Alcon, Allergan, Alimera Sciences, Bayer, Thrombogenics; Consultant—Allergan, Alimera Sciences, Bayer, Pfizer; Honoraria from Alcon, Allergan, Alimera Sciences, Bayer, Novartis, Pfizer, and Thrombogenics; Advisory board—Novartis. M. Ohji: Grant—Alcon, Hoya Corp, Kowa Pharmaceutical, Novartis, Otsuka Pharmaceutical, Pfizer, Santen Pharmaceutical, Senju Pharmaceutical, and Topcon; Personal fees—Alcon, Allergan, Bayer, Bausch & Lomb, Carl Zeiss, Chugai Pharmaceutical, Chuo Sangio, Hoya Corp, Kowa Pharmaceutical, MSD, Novartis, Otsuka Pharmaceutical, Pfizer, RE Medical Inc, Santen Pharmaceutical, Senju Pharmaceutical, Sanwa Kagaku Kenkyusho, and Topcon. H. Dai: Research grants and nonfinancial support—Novartis. S. Sharma: Financial support—Alcon, Allergan, Bausch and Lomb, Bayer, Genentech, Health Canada, Novartis, Roche, and the Canadian Institutes of Health Research; Consultant—Alcon, Allergan, Bausch and Lomb, Bayer, Genentech, Health Canada, Novartis, Roche, and the Canadian Institutes of Health Research. C. Dunger-Baldauf: Employee—Novartis Pharma AG, Basel, Switzerland. S. Lacey: Employee—Novartis Pharmaceuticals UK Limited, Surrey, Frimley, Camberley, United Kingdom. W. Macfadden: Employee—Novartis Pharma AG, Basel, Switzerland. P. Mitchell: Consultant—Abbott, Allergan, Bayer, Genentech, Novartis, and Roche; Financial support—Abbott, Allergan, Bayer, Genentech, Novartis, and Roche. The remaining author has no conflict of interests to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
References
- 1.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA 2004;291:1900–1901. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–572. [DOI] [PubMed] [Google Scholar]
- 3.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 4.Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology 2010;117:921–927. [DOI] [PubMed] [Google Scholar]
- 5.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e116. [DOI] [PubMed] [Google Scholar]
- 6.Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol 2017;6:493–497. [DOI] [PubMed] [Google Scholar]
- 7.Holekamp NM. Overview of diabetic macular edema. Am J Manag Care 2016;22:s284–s291. [PubMed] [Google Scholar]
- 8.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–1431. [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi T, et al. The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology 2015;122:1402–1415. [DOI] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. Lucentis Prescribing Information. San Francisco, California, USA: Genentech, Inc; 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125156s111lbl.pdf. Accessed November 13, 2017. [Google Scholar]
- 11.Business Wire. FDA Approves Genentech's Lucentis (Ranibizumab Injection) for Myopic Choroidal. 2017. Available at: http://www.businesswire.com/news/home/20170105006494/en/FDA-Approves-Genentech%E2%80%99s-Lucentis%C2%AE-Ranibizumab-Injection-Myopic. Accessed November 13, 2017. [Google Scholar]
- 12.Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011;118:1594–1602. [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116:57–65.e55. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology 2014;121:209–219. [DOI] [PubMed] [Google Scholar]
- 15.Larsen M, Waldstein SM, Boscia F, et al. Individualized ranibizumab regimen driven by stabilization criteria for central retinal vein occlusion: twelve-month results of the CRYSTAL study. Ophthalmology 2016;123:1101–1111. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118:615–625. [DOI] [PubMed] [Google Scholar]
- 17.Prünte C, Fajnkuchen F, Mahmood S, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol 2016;100:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431. [DOI] [PubMed] [Google Scholar]
- 19.Tadayoni R, Waldstein SM, Boscia F, et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology 2016;123:1332–1344. [DOI] [PubMed] [Google Scholar]
- 20.Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 2014;121:682–692.e682. [DOI] [PubMed] [Google Scholar]
- 21.Lai TYY, Staurenghi G, Lanzetta P, et al. Efficacy and safety of ranibizumab for the treatment of choroidal neovascularization due to uncommon cause: twelve-month results of the MINERVA study. Retina 2018;38:1464–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Medicines Agency. Lucentis Assessment Report. 2016. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000715/WC500217894.pdf. Accessed November 14, 2017. [Google Scholar]
- 23.Finger RP, Wiedemann P, Blumhagen F, et al. Treatment patterns, visual acuity and quality-of-life outcomes of the WAVE study—a noninterventional study of ranibizumab treatment for neovascular age-related macular degeneration in Germany. Acta Ophthalmol 2013;91:540–546. [DOI] [PubMed] [Google Scholar]
- 24.Holz FG, Bandello F, Gillies M, et al. Safety of ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. Br J Ophthalmol 2013;97:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holz FG, Tadayoni R, Beatty S, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol 2016;100:1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim LN, Mehta H, Barthelmes D, et al. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 2016;36:1418–1431. [DOI] [PubMed] [Google Scholar]
- 27.Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014;121:1092–1101. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Downey L, Mehta H, et al. Resource use and real-world outcomes for ranibizumab treat and extend for neovascular age-related macular degeneration in the UK: interim results from TERRA. Ophthalmol Ther 2017;6:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertelmann T, Feltgen N, Scheffler M, et al. Vision-related quality of life in patients receiving intravitreal ranibizumab injections in routine clinical practice: baseline data from the German OCEAN study. Health Qual Life Outcomes 2016;14:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziemssen F, Voegeler J, Schmitz-Valckenberg S, et al. Dependency of BCVA and ranibizumab treatment frequency of DME patients in a real-life setting (OCEAN study). 2017. Abstract presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO); May 7–11, 2017; Baltimore, MD. [Google Scholar]
- 31.Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye (Lond) 2016;30:270–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaz-Pereira S, Marques IP, Matias J, et al. Real-world outcomes of anti-VEGF treatment for retinal vein occlusion in Portugal. Eur J Ophthalmol 2017;27:756–761. [DOI] [PubMed] [Google Scholar]
- 33.Vorum H, Olesen TK, Zinck J, Størling Hedegaard M. Real world evidence of use of anti-VEGF therapy in Denmark. Curr Med Res Opin 2016;32:1943–1950. [DOI] [PubMed] [Google Scholar]
- 34.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015;99:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen SY, Mimoun G, Oubraham H, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina 2013;33:474–481. [DOI] [PubMed] [Google Scholar]
- 36.ClinicalTrials.gov. Observe the effectiveness and safety of ranibizumab in real life setting (LUMINOUS). Available at: https://clinicaltrials.gov/ct2/show/NCT01318941. Accessed November 13, 2017.
- 37.Ross AH, Donachie PH, Sallam A, et al. Which visual acuity measurements define high-quality care for patients with neovascular age-related macular degeneration treated with ranibizumab? Eye (Lond) 2013;27:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams TA, Blyth CP. Outcome of ranibizumab treatment in neovascular age related macula degeneration in eyes with baseline visual acuity better than 6/12. Eye (Lond) 2011;25:1617–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillies MC, Walton R, Simpson JM, et al. Prospective audit of exudative age-related macular degeneration: 12-month outcomes in treatment-naive eyes. Invest Ophthalmol Vis Sci 2013;54:5754–5760. [DOI] [PubMed] [Google Scholar]
- 40.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–1444. [DOI] [PubMed] [Google Scholar]
- 41.CATT Research Group, Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. New Engl J Med 2011;364:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;121:2181–2192. [DOI] [PubMed] [Google Scholar]
- 43.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009;148:43–58.e41. [DOI] [PubMed] [Google Scholar]
- 44.Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 2015;122:146–152. [DOI] [PubMed] [Google Scholar]
- 45.Silva R, Berta A, Larsen M, et al. Treat-and-Extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology 2018;125:57–65. [DOI] [PubMed] [Google Scholar]
- 46.Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol 2007;143:679–680. [DOI] [PubMed] [Google Scholar]
- 47.Toalster N, Russell M, Ng P. A 12-month prospective trial of inject and extend regimen for ranibizumab treatment of age-related macular degeneration. Retina 2013;33:1351–1358. [DOI] [PubMed] [Google Scholar]
- 48.Wu B, Li J, Lin H, et al. Different strategies for the treatment of age-related macular degeneration in China: an economic evaluation. J Ophthalmol 2016;2016:7689862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston RL, Carius HJ, Skelly A, et al. A retrospective study of ranibizumab treatment regimens for neovascular age-related macular degeneration (nAMD) in Australia and the United Kingdom. Adv Ther 2017;34:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teper S, Nowińska A, Lyssek-Boroń A, et al. Neovascular form of age-related macular degeneration—current management in Poland and in Europe [in Polish]. Pol Merkur Lekarski 2014;37:56–60. [PubMed] [Google Scholar]
- 51.Ziemssen F, Eter N, Fauser S, et al. Retrospective investigation of anti-VEGF treatment reality and effectiveness in patients with neovascular age-related macular degeneration (AMD) in Germany: treatment reality of ranibizumab for neovascular AMD in Germany [in German]. Ophthalmologe 2015;112:246–254. [DOI] [PubMed] [Google Scholar]
- 52.Rousseau C, Saad R, Davies B, et al. Real-world treatment patterns of ranibizumab among patients with retinal diseases in Canada: 5 Years of data. Value Health 2015;18:A428. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.