Abstract
Quantification of steroid hormones in fish is an important step for toxicology and endocrinology studies. Among the hormone analysis techniques, liquid chromatography tandem mass spectrometry (LC-MS/MS) has widely been used for measuring hormones in various biological samples. Despite all improvements in the technique, detection of several hormones in a low volume of serum or plasma is still challenging. We developed a robust method for simultaneous quantification of 14 steroid hormones including corticosterone, cortisol, 11-ketotestosterone, progesterone, testosterone, 17OH-progesterone, aldosterone, dihydrotestosterone, estrone, 17β-estradiol, estriol, ethinylestradiol, levonorgestrel and equilin from volumes as low as 10 μL serum or plasma in a short run by LC-MS/MS. The lowest limit of detection in 10 μL serum was 0.012 ng/mL measured for cortisol, progesterone, testosterone, 17OH-progesterone and estrone. Use of high (25 times more) serum volume improved detection limit of hormones by 2–40 times. The method was compared with the radioimmunoassay technique in which testosterone and 17β-estradiol were highly correlated with R2 of 0.95 and 0.96, respectively. We validated the method by measuring four selected hormones, in low and high plasma volumes of largemouth bass (Micropterus salmoides). In addition, we developed a method to quantify hormones in whole body fish homogenates of small fish and compared the values to plasma concentrations, using fathead minnow (Pimephales promelas). Calculated concentrations of the hormones in plasma were consistent with those in the homogenate and 11-ketotestosterone and 17β-estradiol were significantly different in males and females. The ability to measure hormones from whole body homogenates was further evaluated in two model small fish species, zebrafish (Danio rerio) and juvenile silverside (Menidia beryllina). These results suggest that whole tissue homogenate is a reliable alternative for hormone quantification when sufficient plasma is not available.
Keywords: Fish, LC-MS/MS, Plasma, Steroid hormone
1. Introduction
Measuring steroid hormones in biological samples of aquatic animals is a key factor for endocrine related research in toxicology. Various procedures with diverse advantages and disadvantages have been developed for quantification of steroid hormones. Radioimmunoassay (RIA) and enzyme-linked immunosorbent assays (ELISA) are well-known approaches for hormone measurements in biological samples (Boggs et al., 2016; Kuhnz et al., 1993), but these require the availability of specific high affinity antibodies. Detection of hormones as low as picograms per mL with high sensitivity using small volumes of plasma or serum are advantages of these techniques. However, antibody cross-reactivity, limited dynamic range, matrix interferences, detection of a single hormone per assay, and lack of internal standards to calculate recovery limits the application of these techniques (Penning et al., 2010; Wang et al., 2016). Alternatively, mass spectrometry approaches are well developed to enhance detection and accuracy of analysis. Gas chromatography mass spectrometry is a technique for fast and precise measurement of steroid hormones. However, the requirement of extra steps in sample preparation such as derivatization to increase volatility and reduce polarity and thermal instability of steroid hormones (Bowden et al., 2009; Rambaud et al., 2007; Wang et al., 2012; EPA, 2007) has limited the application of this technique for hormone analysis.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is an alternative mass spectrometry-based approach that has been used extensively to detect hormones in various biological samples (Bertin et al., 2015; Boggs et al., 2016; Gaikwad et al., 2013). Rapid, robust and simultaneous detection of several steroid hormones in the same analysis makes this technique more applicable. However, a disadvantage has been the requirement for relatively high concentrations of hormone and higher volumes of plasma, up to 2 ml for some analyses (Tai and Welch, 2004). It has been possible to get around the concentration issue for estrogens, especially 17β-estradiol (E2), by derivatization with a variety of reagents to increase ionization of these hormones and to improve the limit of detection (LOD). A diverse range of derivatizing reagents has been developed with different specificities and efficiencies (Boggs et al., 2016). Ranges of E2 concentration in blood varies widely and it is much lower in humans (Häkkinen et al., 2018; Yuan et al., 2019) than in aquatic animals (Adeogun et al., 2018). Therefore, there is a need to optimize the technique to improve LOD for E2, appropriately.
To optimize and validate the hormone quantification approach from low volume serum or plasma (10 μL), we used several fish species. Besides the importance of monitoring hormone levels in aquatic animals, fish is a unique model, because plasma and whole body can be compared for hormone measurements. Plasma or serum are preferred biological samples to monitor hormone content as blood can directly contact all target tissues (Schultz et al., 2013). The amount of plasma that can be obtained from small fish, such as zebrafish, is very low (about 10 μL), which may hamper hormone quantification. An alternative technique is to use the whole body or carcass of a fish to quantify hormones in small fish or juveniles. We have used a diverse selection of small fish model species, including fathead minnow (FHM) (Pimephales promelas), zebrafish (Danio rerio) and silversides (Menidia beryllina) to determine the efficiency of hormone detection from low volumes of plasma or whole carcass. In addition, largemouth bass (LMB) (Micropterus salmoides), a relatively large fish was used for collection of larger volumes (245 μL) of plasma. Despite several valuable publications, to the best of our knowledge, there is no comprehensive hormone analysis procedure for hormone quantification from low volume of plasma or carcass.
In this study, a panel for quantification of 14 steroid hormones was developed, which can widely be used for detection of hormones in a low volume of serum or plasma. The method was validated by quantification of selected hormones in plasma or whole tissue of fish species and by comparing to results from RIA. We demonstrated a new technique to measure hormone concentration in a low volume of plasma and the efficiency of the technique was confirmed by comparing the results with high volumes of plasma. For the first time, we developed and validated the approach of using small fish whole body extracts for hormone quantification when plasma is not sufficient. These new techniques can widely be applied for quantification of hormones in various biological samples, especially juvenile or small model fish.
2. Material and Methods
2.1. Chemicals and reagents
HPLC grade methanol (CAS No: 67-56-1, minimum purity: 99.9%) and methyl tert-butyl ether (MTBE, CAS 1634-04-4, 99.9%) were purchased from Fisher Scientific (Optima, MA). Ammonium fluoride (CAS 12125-01-8, 99.99%) and protease inhibitor cocktail were acquired from Sigma-Aldrich (St. Louis, MO). Water was provided from Milli-Q ultrapure water system with 18 MΩ.cm resistivity (Millipore Milli-Q, MA). Phosphate-buffered saline tablet (PBS) was purchased from Research Products International Corp. (Mt. Prospect, IL). To make calibration curves, corticosterone (CAS 50-22-6, 98% purity), cortisol (CAS 50-23-7, 99%), 11-ketotestosterone (11-KT, CAS 564-35-2, 100%), estrone (E1, CAS 53-16-7, 99%), 17 β-estradiol (E2, CAS 50-28-2, 98%), progesterone (CAS 85-045-4, 98%), dihydrotestosterone (DHT, CAS 521-18-6), estriol (E3, CAS 50-27-1, 99%), ethinylestradiol (EE2, CAS 57-63-6), 17-OH-progesterone (CAS 68-96-2, 95%), equilin (CAS 474-86-2) and aldosterone (CAS 52-39-1, 95%) were purchased from Sigma-Aldrich (St. Louis, MO). Testosterone (T, CAS 58-22-0, 98%) was obtained from Steraloids, Inc. (Newport, RI) and levonorgestrel (CAS 797-63-7, 100%) was purchased from Cayman chemical (Ann Arbor, MI). Six deuterium-labelled internal standards, cortisol-d7 (CAS 50-23-7, 89%) and aldosterone-d7 (CAS 52-39-1, 98%) (Santa Cruz biotechnology, MN), progesterone-d9 (CAS 15775-74-3) (TRC, Toronto, Canada), 17β-E2-d5 (CAS 50-28-2), EE2-d4 (CAS 350820-06-3, 98%) (CDN isotopes, Quebec, Canada) and levonorgestrel-d6 (99%) (Cayman chemical, MI) as well as a 13C steroid hormone, 17OH-Progesterone-13C3 (CAS 1356154-92-1) (Sigma-Aldrich, MO) were used as internal standards. Dansyl chloride (CAS 605-65-2, 99%) was purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Preparation of calibration solutions, quality controls and internal standards
Pure steroid hormones, including corticosterone, cortisol, 11-KT, progesterone, T, 17OH-progesterone, aldosterone, DHT, E1, E2, E3, EE2, levonorgestrel and equilin were combined to prepare a calibration working solution in methanol (Supplemental Table 1). Calibration samples were prepared by spiking 2970 μL double charcoal stripped, delipidized human serum (Golden West Diagnostics, CA) with 30 μL of the working solution and serially diluting with the serum to obtain an 11-point calibration curve for each hormone. Ranges of calibration curves were 0.0003 to 20 ng/mL for progesterone, 17-OH-progesterone, T, DHT and aldosterone; 0.003 to 200 ng/mL for corticosterone, cortisol, 11-KT and equilin; 0.006 to 400 ng/mL for E1, E2 and levonorgestrel; 0.007 to 500 ng/mL for E3 and EE2. The upper concentration of each standard hormone was diluted by 4, 20 and 200 times using the charcoal stripped serum to make high, middle and low-quality controls, respectively.
All calibration solutions and quality controls were prepared in the same volume of control plasma as the designated plasma samples. For the low plasma volume, standards added to 10 ul of the control plasma varied by hormone ranging from 0.12 to 5 pg analyte for the low end to 200 to 5,000 pg analyte for the high end. For the higher plasma volume, standards added to 245 μL of control plasma ranged from 0.0735 to 6.125 pg for the lower end of the calibration to 4,900 to 122,500 ng for the upper end of the calibration. Internal standards were dissolved in methanol and mixed to prepare working solutions. Final concentrations of the internal standards in the working solution were 1 μg/mL for cortisol-d7 and aldosterone-d7; 0.2 μg/mL for progesterone-d9; 0.5 μg/mL for 17OH-progesterone-13C3; 5 μg/mL for 17β-E2-d5 and levonorgestrel and 10 μg/mL for EE2-d4. To check the background effect and calculation of signal to noise, both water and doubly charcoal-stripped human serum were considered as blank samples.
2.3. Hormone extraction
Steroid hormones were extracted using MTBE according to Van Der Gugten (2012) with minor modifications. Briefly, 245 μL (for samples designated as high volume) serum, plasma or homogenate was spiked with 5 μL of the internal standard working solution. Then, 500 μL MTBE was added, followed by vortex-mixing for 30 sec and centrifuging at 1,100 X g for 10 min at 4 °C. The upper organic phase was carefully transferred to an autosampler glass vial. To improve the extraction efficiency, the extraction was repeated by adding 200 μL MTBE to the plasma, centrifuging and collecting the upper phase. The collected solutions were pooled and evaporated using a nitrogen evaporator (Organomation, N-EVAP 112, MA) under a gentle stream of high-purity nitrogen and reconstituted in 50 μL of methanol prior to analysis by LC-MS/MS.
Extractions from 10 μL plasma were performed using a small scale extraction procedure with a 100 μL glass vial insert with a bottom spring (Thomas Scientific, NJ). This modification in the extraction step helps to avoid potential loss during the extraction. Plasma was spiked with 2 μL internal standard mix and 20 μL MTBE was added, followed by vortex-mixing, centrifuging and upper phase collection. A second round of extraction was performed using 15 μL MTBE and both upper phase extracts were pooled directly into a new glass vial insert. The extracts were dried and then reconstituted in 12 μL of methanol prior to LC-MS/MS analysis. The narrow shape of the insert allows reconstitution of the dried extract using as low as 12 μL methanol, which is about 4 times lower than the reconstitution solution used for high volume samples (50 μL). This helps to avoid dilution and ultimately leads to better detection of hormones for low volume samples. In the small-scale process, we recommend using long pipette tips for efficient reconstitution.
2.4. E2 Derivatization
To improve ionization efficiency and the detection limit of E2, extracts were derivatized, following the steps described by Nelson and et al. (2004) with modifications to adapt to small scale. Extracts were initially injected into LC-MS/MS to detect all hormones and then derivatization was performed on the remaining extracts in the glass vial inserts. The extracts were dried and re-dissolved in 50 μL sodium bicarbonate buffer (100 mM, pH 10.5) and then 50 μL of 1 mg/mL dansyl chloride in acetone was added. Each sample was vortex-mixed for 1 min and incubated in a heating block at 60 °C for 10 min. Samples were kept on ice to cool down, dried and reconstituted using 25 μL methanol. To remove particulates, samples were centrifuged at 1000 X g for 30 sec and the supernatant was transferred to a new glass vial insert using long pipette tips and injected into the LC MS/MS.
2.5. Ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC- MS/MS)
Samples were analyzed using an ultra-high-performance liquid chromatography (UHPLC, Shimadzu Co., Kyoto, Japan) coupled to a Triple Quadrupole Linear Ion Trap (QTrap 6500, AB Sciex, CA). Chromatographic separation was performed using an Eclipse plus C18 column, 2.1× 100 mm, 3.5 μm (Agilent, CA). A binary gradient was applied in which 0.1 mM ammonium fluoride in water and 100% methanol were used as mobile phase A and B, respectively. The linear gradient started at 10% solvent B and increased to 98% within 12.5 min and remained at the level for 30 sec. Then, mobile phase B was reduced to 10% within 1.5 min. Flow rate was set to 0.3 mL.min−1 with the total running time of 15 min. Eight μL of each sample was injected into the LC and as it eluted, transferred to the mass spectrometer via electrospray ionization. The instrument was run under scheduled multiple reaction monitoring (MRM) method and two transitions with the exact retention times of each hormone were considered for quantification and qualification. Declustering potential, collision energy and collision cell exit potential were optimized and introduced in the method (Table 1).
Table 1:
List of steroid hormones, MRMs and settings for data acquisition.
| Hormone* | Parental ions (m/z) | Product ions (m/z) | Retention Time (min) | DP (volts) | CE (volts) | CPX (volts) |
|---|---|---|---|---|---|---|
| Corticosterone | 347 | 121/105 | 8.4 | 80 | 32 | 12 |
| Cortisol | 363 | 309/121 | 7.2 | 80 | 32 | 9 |
| 11-Ketotestosterone | 303 | 259/121 | 7.4 | 60 | 34 | 12 |
| Progesterone | 315 | 109/97 | 10.8 | 80 | 32 | 14 |
| Testosterone | 289 | 109/97 | 9.5 | 100 | 27 | 12 |
| 17OH-Progesterone | 331 | 109/97 | 9.8 | 90 | 28 | 9 |
| Aldosterone | 361 | 343/315 | 5.8 | 90 | 25 | 23 |
| Dihydrotestosterone | 291 | 255/91 | 10.5 | 80 | 21 | 16 |
| Levonorgestrel | 313 | 245/109 | 9.9 | 130 | 25 | 12 |
| Equilin | 267 | 223/143 | 9.0 | −105 | −44 | −15 |
| Estrone | 269 | 145/143 | 9.2 | −130 | −48 | −17 |
| Estradiol | 271 | 183/145 | 9.5 | −140 | −56 | −20 |
| Estriol | 287 | 171/145 | 5.2 | −120 | −49 | −17 |
| Ethinylestradiol | 295 | 145/143 | 9.4 | −130 | −56 | −20 |
Two transitions were considered for each hormone, one for quantitation and the second for qualification. DP, Declustering potential; CE, Collision energy; CPX, Collision cell exit potential.
E1, E2, EE2 and equilin were monitored in negative ion mode and the rest of the hormones as well as derivatized E2 were detected in positive ion mode. Curtain gas and collision gas were set at 35 kPa and 12 kPa, respectively. In positive and negative ion modes, ion spray voltages were 5250 V and 4400 V and temperatures were set at 550 °C and 500 °C, respectively. Blank samples made up of methanol were interspersed between samples to wash the instrument and to avoid carryover.
2.6. Method validation, accuracy calculation and data analysis
Duplicates of quality controls, as well as blank and zero calibrator (blank spiked with internal standards) were prepared to verify the precision and accuracy of detection. Calibration solutions were prepared in three different rounds (inter-assay) to check the day-to-day precision and in triplicate (intra-assay) to determine the within experiment variability. The linearity of calibration standard curves was confirmed by plotting the ratio of analyte area to internal standard area versus the known spiked concentration. A correlation coefficient above 0.997 was considered as acceptable for calibration curves. The accuracy of the method was calculated by dividing the observed concentration by the spiked concentration and was expressed as a percentage (Matuszewski et al., 2003). The lowest detectable amount of each hormone was considered as the lower limit of detection (LLOD). The lowest concentration that was quantitatively determined with an accuracy range of 85–115% was considered as the lower limit of quantification (LLOQ) (FDA, 2018; Koal et al., 2012). To calculate recovery percentage, relative peak area of each analyte from the extracted solution was divided by the relative peak area of the neat standard and expressed as a percentage. Concentrations of hormones in plasma were expressed as nanogram per mL. For whole fish extractions, data were normalized based on the milligram protein per mL of whole body homogenate of fish.
Liquid chromatography and mass spectrometry instruments were operated and data was acquired using Analyst software ver. 1.6.2 (AB Sciex). Generation of calibration curves and quantitation of hormones were performed using MultiQuant software ver. 3.0.1. Various options in the software were tested for optimal analysis in which selection of appropriate peak smoothing significantly improved detection limit of non-derivatized E2. A weighting factor of 1/x was applied to the data (Markham et al., 2014; Vitku et al., 2015) and the linearity of the standard curves was evaluated using correlation coefficient. Coefficient of variations (CV), defined as the ratio of the standard deviation to the mean and represented as a percentage, was used to express the precision and repeatability of the analyses (Blue et al., 2018; Gaikwad, 2013). To check the precision and repeatability of quality controls, acceptable CVs were determined as 15% for quality controls and 20% for LLOQ, following the guideline outlined by the US Food and Drug Administration (FDA, 2018). Data processing and preparation of graphs were performed using Microsoft Excel.
2.7. Sample preparation and hormone extraction from fish
All fish species, including LMB, FHM, silverside and zebrafish were collected from the aquatic facility in the Center for Environmental and Human Toxicology at the University of Florida. Fish take down process or blood collection were under anesthetic condition, following the Institutional Animal Care and Use Committee (IACUC) protocol of the University of Florida. To have a more reliable hormone analysis, only healthy and reproductive adults of LMB, FHM and zebrafish were selected. In these species, fish sex was verified accurately using visual inspection of gonads with a dissecting microscope. Weight and length of all fishes were recorded prior to hormone extraction. Four hormones, cortisol, 11-KT, T and E2, which are generally used for endocrinology studies, were selected for validation of the method in fish samples. An additional hormone, DHT, was measured in silverside samples.
2.8. Plasma Preparation
Blood samples were collected from two species, LMB and FHM. Using a heparinized syringe, blood was collected from the caudal vein of 10 LMB (5 males and 5 females) and centrifuged for 15 min at 600 g to separate plasma and aliquoted into 245 μL and 10 μL for hormone extraction. The plasma volume of 245 μL was selected based on our general lab protocol to compare with 10 μL (average volume of available plasma in small fish species). Blood from 8 FHM (4 Male and 4 Female) was obtained using heparinized micro-hematocrit capillary tubes (Fisher Scientific, PA) after cutting the tail. Plasma was separated by centrifugation for 10 min using a micro hematocrit centrifuge (IEC, MA) and a 10 μL aliquot per fish was used for hormone extraction. All samples were kept in −80 °C until use.
2.9. Whole fish body
Zebrafish (4 males and 4 females) and FHM as explained above, were ground to powder in liquid nitrogen separately and resuspended in PBS (which contains 137 mM sodium chloride, 2.7 mM potassium chloride and 11.9 mM phosphate) with 10% protease inhibitor cocktail. Six Juvenile silversides were individually homogenized in PBS with protease inhibitor cocktail using a Tissue Tearor Homogenizer (IKA T10, USA). The required volume of PBS and protease inhibitor (in μL) for homogenization was calculated as 1.2, 2.2 and 22 times the fish weight in milligrams for FHM, zebrafish, and juvenile silversides, respectively. After centrifugation at 400 X g for 5 min at 4 °C, 245 μL of the supernatant was immediately used for hormone analysis. In order to normalize hormone concentrations, total protein content in the fresh whole fish homogenates was measured in a 96 well plate using a Bradford Assay (Coomassie Plus TM, Thermo Scientific) using bovine albumin (BSA) as a standard. The 10 μL supernatant were diluted 1:10 and 1:100 with PBS and compared to a BSA standard curve (0, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 mg/mL). Samples and standards were run in triplicate wells.
2.10. Radioimmunoassay for hormone measurement
To compare the efficiency of the LC-MS/MS method with the RIA technique, 65 frozen pallid sturgeon plasma samples (33 males and 32 females) were provided from Bozeman Fish Technology Center (MT, USA). For LC-MS/MS analysis, hormones were extracted from 245 μL plasma as described above. For RIA analysis, hormones were extracted from the fish plasma following the method of Fitzpatrick et al. (1987). Concentrations of T in males and E2 in females were measured in duplicate by RIA using a competitive binding assay as described by Webb et al. (2002). Steroid levels were validated by verifying that serial dilutions were parallel to standard curves. The intra- and inter-assay coefficients of variation for all assays in RIA were less than 5% and 10%, respectively.
3. Results
Simultaneous quantitation of 14 hormones, including corticosterone, cortisol, 11-KT, progesterone, 17OH-progesterone, aldosterone, T, DHT, E1, E2, E3, EE2, levonorgestrel and equilin was performed using LC-MS/MS. Total ion chromatogram of hormones represents peak intensities vs. time in a single run of the sample (Fig. 1). Calibration curves of hormones were generated for 10 or 245 μL serum volumes. To make a calibration solution, double charcoal stripped, delipidized human serum was compared with 10% methanol in water. Although both solutions were free of background noise or interfering peaks, human serum was used, because it provided a matrix that was similar to our samples. The calibration curve was optimized using 11 concentrations of each hormone and provided a wide range of concentrations with high accuracy. Curve linearity improved when results were weighted (1/x) for regression analysis both for low and high-volume samples. Inspection of peaks in blanks (methanol or Milli-Q ultrapure water) and zero calibrator confirmed that all hormones of interest were free of interfering peaks, background noise and carryovers. Linear equations for all hormones, both in low and high-volume serum had a minimum correlation coefficient of 0.997, indicating excellent linearity and precision of quantification (Table 2). Variation percentage of each calibration point, as an indicator of accuracy was less than ±15%.
Figure 1. Total ion chromatogram of steroid hormones.
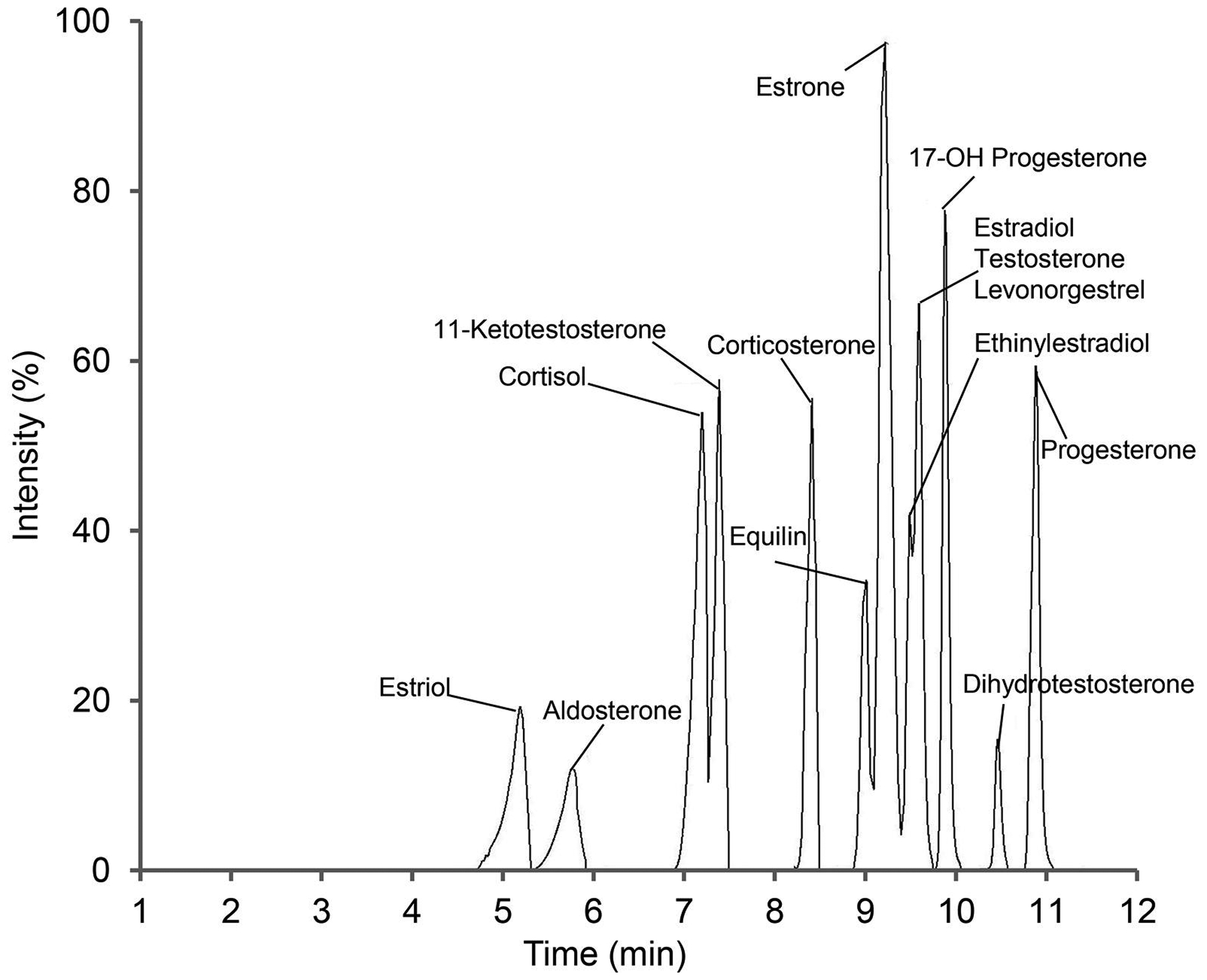
Hormones were extracted from double charcoal stripped, delipidized human serum, using MTBE after spiking with internal standards and injected into the LC-MS/MS. Fourteen hormones were chromatographically separated from a high concentration of quality control sample (5 to 125 ng/mL depends on the hormone) using a C18 column in 12 min. A binary gradient was applied with mobile phase A containing 0.1 mM ammonium fluoride in water and mobile phase B, 100% methanol. The instrument was run under the scheduled MRM method.
Table 2.
Calibration curve regression equations and lower limit of quantification (LLOQ) of steroid hormones in high and low volume plasma.
| Hormone | Regression equation | LLOD (ng/ml) | LLOQ (ng/ml) | |||
|---|---|---|---|---|---|---|
| 10 μL | 245 μL | 10 μL | 245 μL | 10 μL | 245 μL | |
| Corticosterone | y=0.0024x+3.35e–4 (r = 0.998) | y=0.028x+0.001 (r = 0.997) | 0.5 | 0.025 | 0.5 | 0.05 |
| Cortisol | y=0.013x+0.033 (r = 0.999) | y=0.124x+0.012 (r = 0.999) | 0.012 | 0.006 | 0.025 | 0.006 |
| 11-KT | y=0.014x+3.156e–4 (r = 1) | y=0.153x+0.0102 (r = 0.998) | 0.025 | 0.012 | 0.05 | 0.012 |
| Progesterone | y=0.035x–0.0012 (r = 1) | y=0.403x+0.0027 (r = 1) | 0.012 | 0.0003 | 0.012 | 0.0003 |
| Testosterone | y=0.016x–2.17e–4 (r = 0.998) | y=0.114x-4.01e–4 (r = 0.999) | 0.012 | 0.0006 | 0.012 | 0.001 |
| 17OH-Progesterone | y=0.011x+4.91e–5 (r = 0.999) | y=0.119x+1.84e–4 (r = l) | 0.012 | 0.001 | 0.012 | 0.001 |
| Aldosterone | y=0.0098x–8.72e–4 (r = l) | y=0.131x+0.0015 (r = 0.999) | 0.05 | 0.005 | 0.05 | 0.005 |
| Dihydrotestosterone | y=0.013x–7.87e–5 (r = l) | y=0.116x+6.76e–4 (r = 0.999) | 0.125 | 0.005 | 0.125 | 0.012 |
| Levonorgestrel | y=0.018x+3.456e–4 (r = 1) | y=0.104x+4.39e–4 (r = 1) | 0.025 | 0.012 | 0.05 | 0.012 |
| Equilin | y=0.0061x+3.76e–4 (r = 0.997) | y=0.02x–2.597e–4 (r = l) | 0.05 | 0.003 | 0.05 | 0.012 |
| Estrone | y=0.0062 x+0.025 (r = 0.999) | y=0.067x+0.0278 (r = l) | 0.012 | 0.006 | 0.025 | 0.006 |
| Estradiol* | y=0.0016x–8.03e–4 (r = 0.999) | y=0.017x+3.57e–4 (r = 0.998) | 0.05 | 0.006 | 0.25 | 0.025 |
| Estriol | y=9.14e–4 x+3.3e–5 (r = 0.999) | y=0.007x+7.16e–5 (r = l) | 0.125 | 0.025 | 0.25 | 0.025 |
| Ethinylestradiol | y=0.0011x+2.5e–5 (r = l) | y=0.012x+1.77e–5 (r = 1) | 0.125 | 0.007 | 0.125 | 0.015 |
Data are based on the quantification of derivatized Estradiol.
We examined the absolute limit of quantitation of the instrument to verify the feasibility of using 10 μL plasma. The limit of quantitation value was lower for detected hormones analyzed in positive ionization mode such as progesterone, 17OH-Progesterone and T. The LLOD and LLOQ were determined for each hormone in triplicate and compared in 10 or 245 μL human serum. The LLOD in 10 μL serum was 0.012 ng/mL, which was calculated for cortisol, progesterone, T, 17OH-progesterone and estrone. Use of 245 μL serum improved LLOD of hormones by 2–40 times, reducing the LLOD for progesterone to 0.0003 ng/mL and T to 0.0006 ng/mL, well below 1 pg/mL. LLOQ ranges for 10 μL serum were as follow: 0.5 ng/mL for corticosterone; 0.25 ng/mL for E2, and E3; 0.125 ng/mL for DHT, and EE2; 0.05 ng/mL for aldosterone, equilin, and, 11-KT; 0.025 ng/mL for cortisol, and E1; 0.012 ng/mL for progesterone, T and, 17-OH progesterone. The quantitation limits have also been improved 2–40 times, when the initial serum volume increased to 245 μL (Table 2).
Among estrogens, separation of E1 and E2 is more challenging because of the following reasons: First, they elute from the C18 column, used in this experiment, in close proximity (9.2 and 9.5 min retention time, respectively) and due to their similar structures, they share product ions (Nelson et al., 2004; Wang et al., 2016). Second, concentration of E1 is usually 2–3 times higher than E2 (Wang et al., 2016). The low ionization efficiency and the overlap in retention times can be alleviated by derivatization. By derivatization, signal intensity increased six times leading to improvement in the lower limit of detection by 10 times (Fig. 2). Although it has been proven that derivatization can significantly improve detection limit, here, we demonstrate that changing peak picking criteria, as described below, improves LLOD and LLOQ, as well. In all analyses for derivatized and underivatized hormones, the Gaussian smoothing option was set at 2 in MultiQuant software to have a reasonably smooth peak. This degree of smoothing (or more) causes overlapping of E1 and E2 peaks when the concentration gradually decreases from 1 to 0.006 ng/mL. Reducing smoothing to zero clearly separates E1 and E2 peaks and it was verified by both precise retention time and excellent correlation coefficient (>0.997) of calibration curves (Fig. 3). This result clearly shows that selection of the right Gaussian smoothing option is critical for accurate quantitation.
Figure 2: Relative intensities of 17β-estradiol (E2) peaks with or without derivatization.
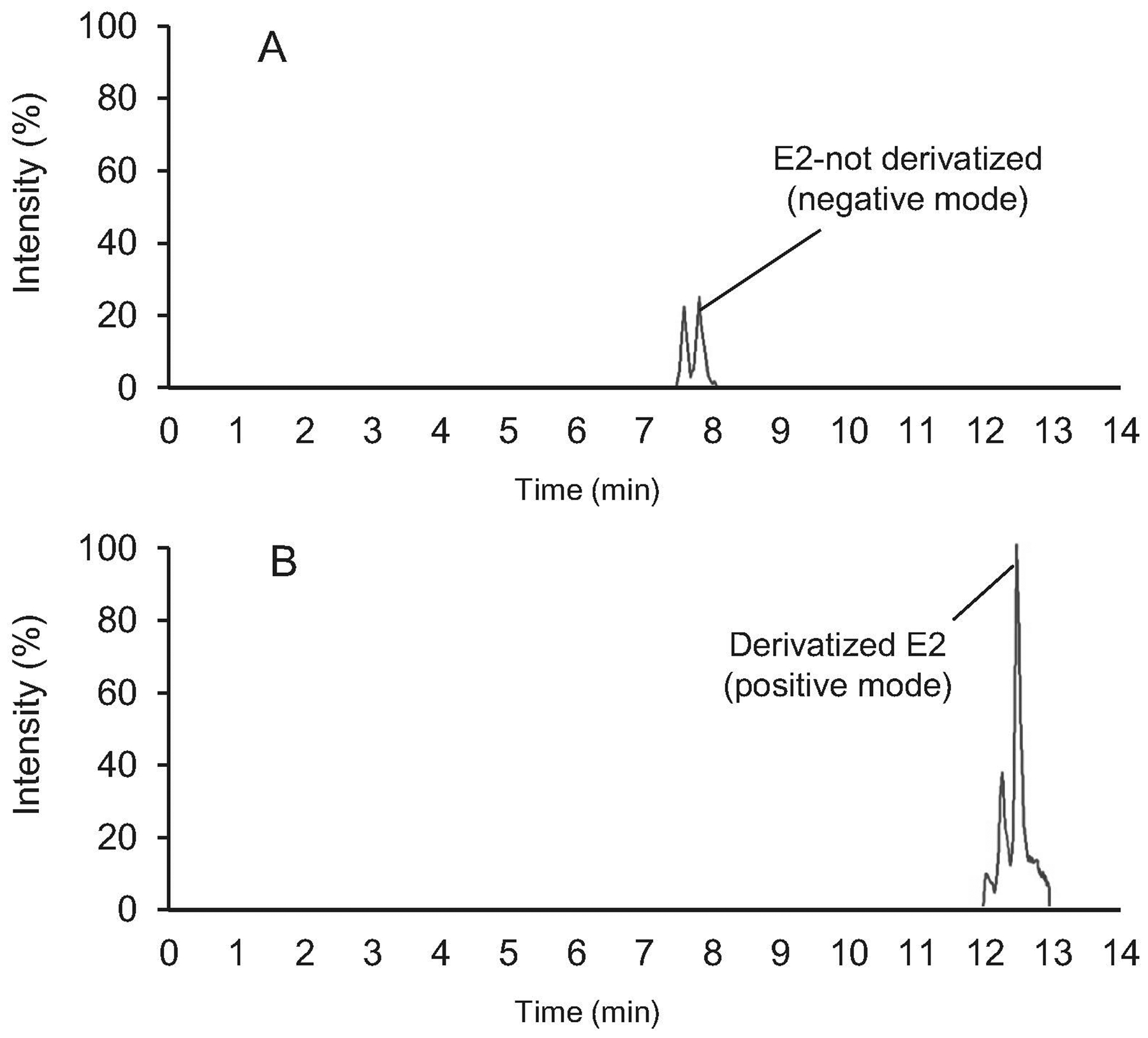
E2 (0.1 ng/mL) was spiked into double charcoal stripped, delipidized human serum and extracted using MTBE. The E2 peak was acquired in negative ion mode (A) and then the remainder of the sample was dried, reconstituted in sodium bicarbonate buffer, derivatized using dansyl chloride (B) and reanalyzed by LC-MS/MS in positive ion mode.
Figure 3. Effect of peak smoothing on separation of estrone (E1) and 17β-estradiol (E2).
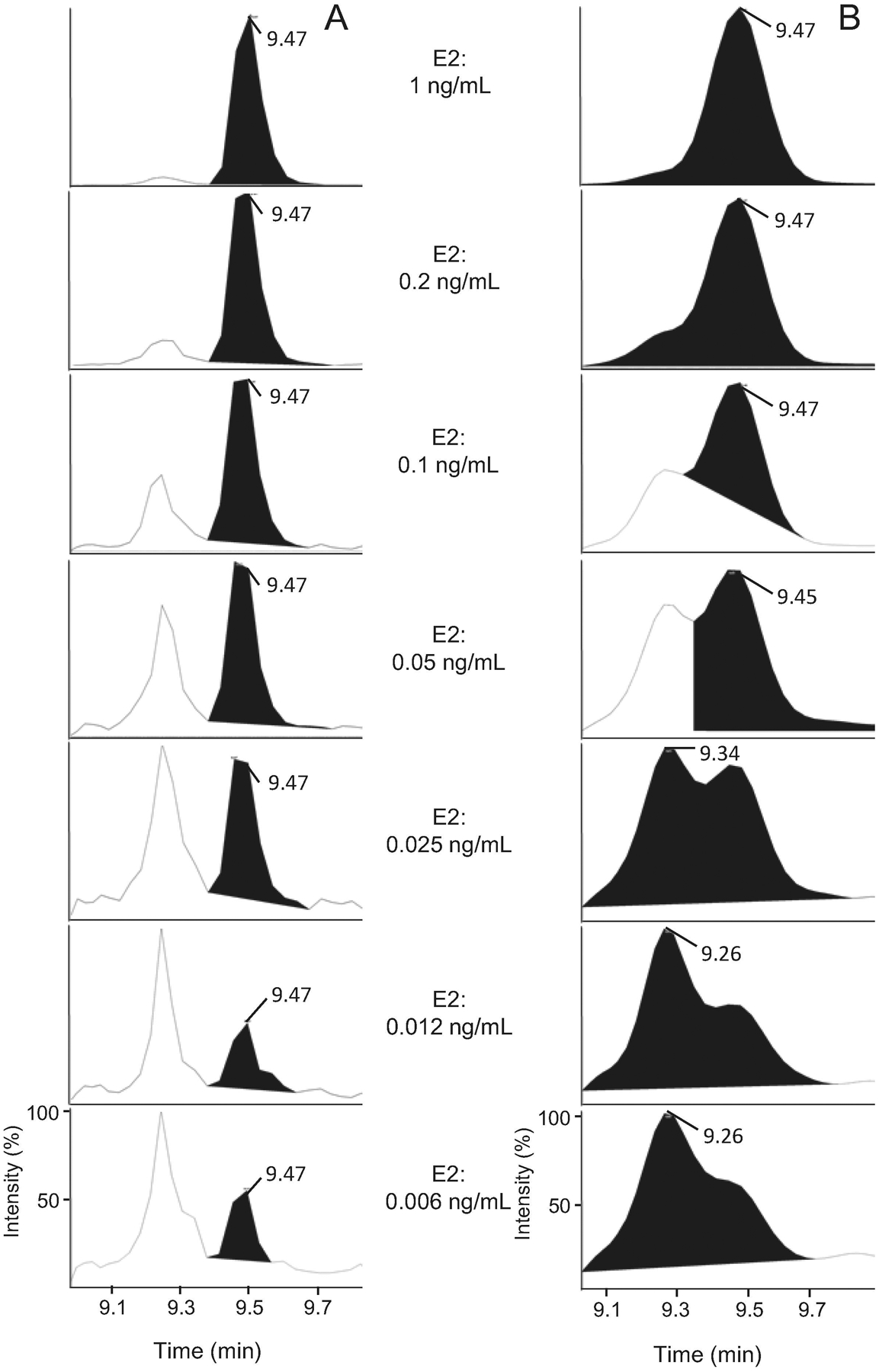
Calibration samples were prepared by spiking working solution into serum followed by serially dilution and analyzing with LC-MS/MS. Peaks of E1 and E2 in the ranges of 1 to 0.006 ng/mL were selected and smoothing value of 0 (A) and 2 (B) were compared in MultiQuant software.
In high, middle and low concentration quality controls, CV percentages of inter- and intra-assays were compared for 10 and 245 μL plasma volumes (Fig. 4). The CV percentage was <10% (mainly < 5%) for all hormones, when 245 μL plasma was used. Decreasing plasma volume to 10 μL, did not change the CV range for high concentration quality controls. In the middle or low concentration quality controls, the CV was < 20% for most hormones except for 11-KT, cortisol and estrone, where the CV was < 22% (Fig. 4). Variability of the extraction process in 10 μL samples can be a reason for increasing the CV percentage in the middle or low concentration quality controls. The recovery percentage of all hormones was calculated for high- and low-quality controls in high- and low-volume samples (Table 3). Sample volume (high vs. low) and hormone concentration (high QC vs. Low QC) did not affect the percentage of recovery.
Figure 4. Coefficient of variation for quality control samples for low- and high-volume extracts.
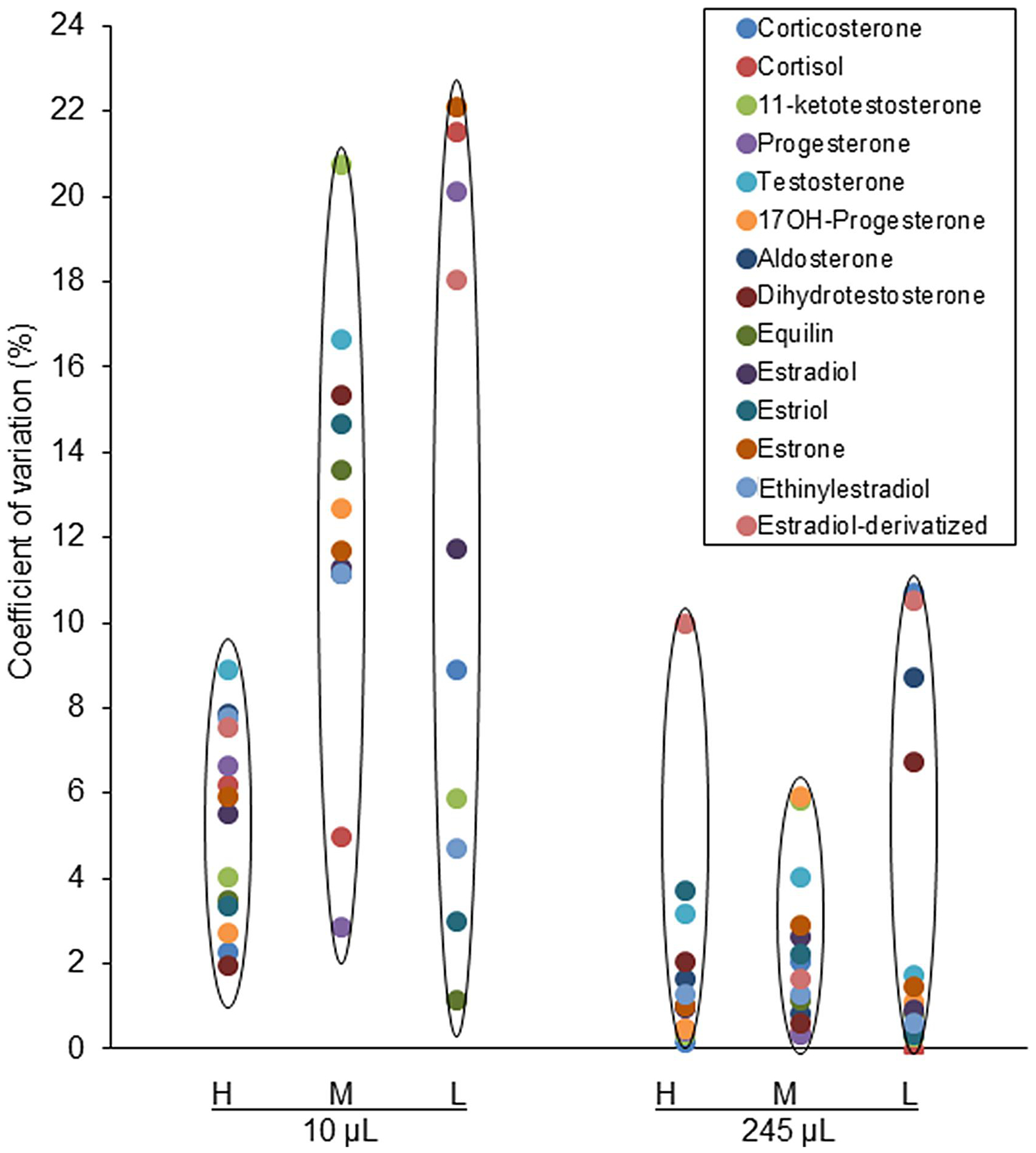
Accuracy and precision of quantification of all hormone were evaluated in triplicate by calculating coefficient of variation percentage (CV%) for High (H), middle (M) and low (L) quality controls for 10 and 245 μL double charcoal stripped, delipidized human serum.
Table 3.
Recovery percentage of some hormones in high- and low-volume plasma for quality controls
| Hormone | High volume (250 μL) | Low volume (10 μL) | ||
|---|---|---|---|---|
| High QC | LowQC | High QC | LowQC | |
| Cortisol | 109.7 | 116.2 | 116.8 | 88.1 |
| ll-KT | 113.5 | 98.9 | 80.2 | 60.2 |
| Progesterone | 96.8 | 118.3 | ND | 113 |
| Testosterone | 71.1 | 78 | 94.1 | ND |
| 17OH-Progesterone | 107.8 | 97.1 | 111.7 | ND |
| Aldosterone | 80.4 | 84.2 | 110.7 | ND |
| Dihydrotestosterone | 79 | 71.3 | ND | ND |
| Equilin | 111.5 | 119 | 111.9 | 103.2 |
| Estrone | 83.9 | 77 | 84.7 | ND |
| Estradiol* | 116.1 | 125.9 | 119.3 | 87.8 |
| Estriol | 113.9 | 109.9 | 60.1 | 58 |
| Ethinylestradiol | 92.8 | 95.2 | 107 | 114.4 |
Data are based on the quantification of derivatized Estradiol. ND, not determined.
Efficiency of hormone quantification in low plasma volume was evaluated by comparing low and high volumes of LMB plasma. Weight and full length of LMB, the largest fish in the current study, were recorded before hormone extraction. Despite the similar length, the average body mass of the male (155 g) was higher than the female (135 g) (Fig. 5). Concentration of cortisol, 11-KT, T and E2 were calculated in both 10 μL and 245 μL plasma. Results of the quantification were consistent in all hormones in terms of the differences in males and females (Table 4). The concentration of hormones was slightly but not significantly higher in low volume plasma except for E2 where there seemed to be a difference of E2 quantitation depending on the volume of plasma extracted as well as on whether derivatization was used, as was the case for the 10 ul samples. Enhancement in ionization efficiency of derivatized E2 may cause overestimation. In addition, the internal standard used was deuterated and this may be responsible for the increased standard error observed for low-volume plasma (Szarka et al., 2013).
Figure 5. Weight and size of fish species and type of samples used for hormone quantification.
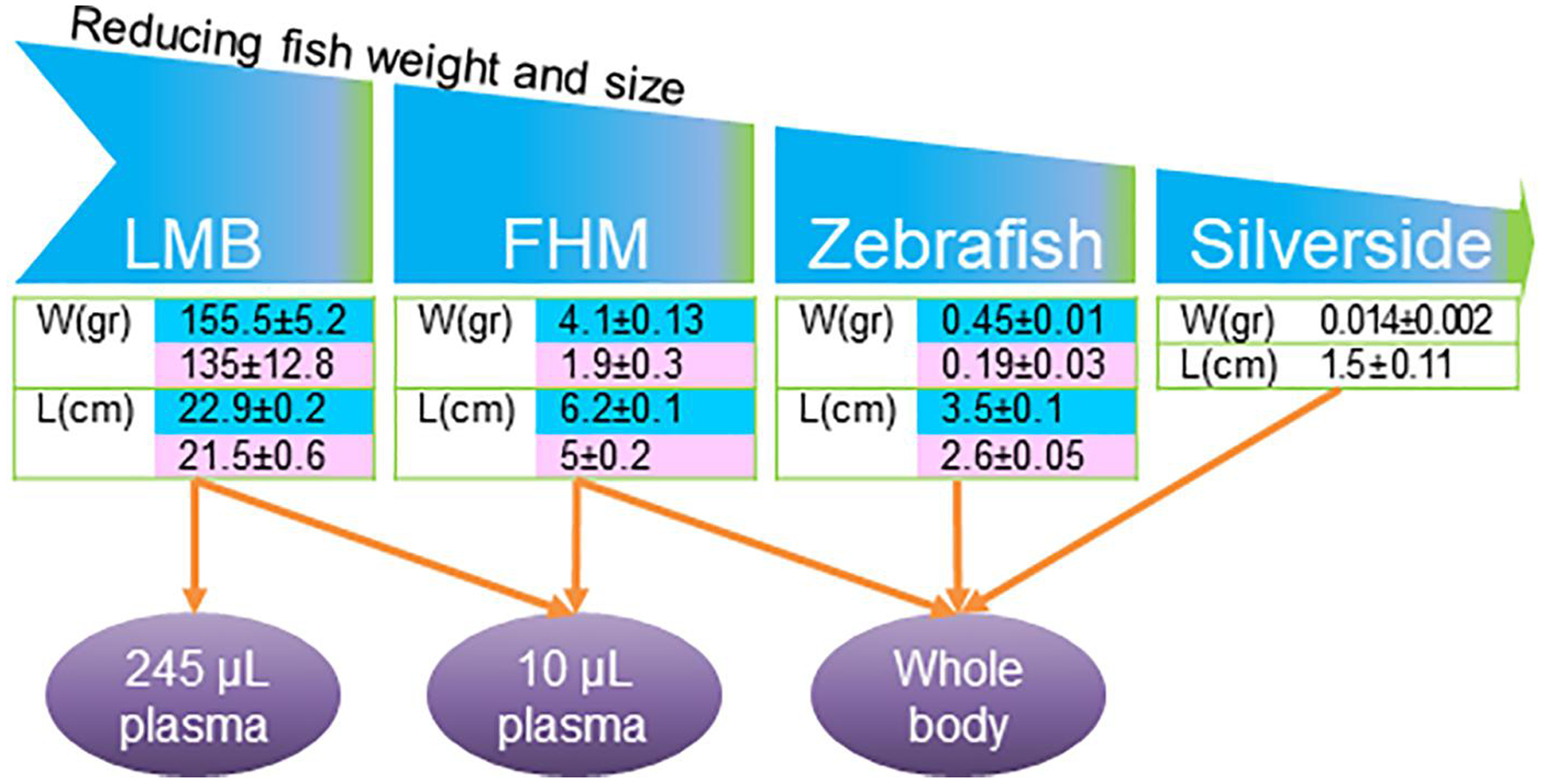
Largemouth bass, LMB (Micropterus salmoides), fathead minnow, FHM (Pimephales promelas), zebrafish (Danio rerio) and silverside (Menidia beryllina) were used for hormone quantification using 245 or 10 μL plasma volume or whole body. Data in the tables are weight (W) and length (L) of male (blue cell) and female (pink cell) fish.
Table 4.
Comparison of hormone quantification in LMB using 245 and 10 μL plasma (ng/mL).
| Hormones | Male | Female | ||
|---|---|---|---|---|
| 245 μL | 10 μL | 245 μL | 10 μL | |
| Cortisol | 41.1±3.9 | 43.5±4.9 | 91.6±21.1* | 104.3±21.4* |
| 11-KT | 0.12±0.05** | 0.15±0.06** | - | - |
| Testosterone | 0.1+0.03 | 0.14±0.04 | 0.06±0.02 | 0.08±0.04 |
| E2# | 0.87±0.2 | 3.1±1.5 | 1.7±0.8 | 3±1.3 |
Data are mean of 5 plasma samples ±SE. No peaks were observed in female for 11-KT and the concentration can be considered as zero.
Indicate significant differences between the calculated values (p ≤0.05 and p ≤0.01, respectively) for males and females measured in similar volumes.
Indicate significant differences between the calculated values (p ≤0.05 and p ≤0.01, respectively) for males and females measured in similar volumes.
Only 10 μL plasma samples were derivatized using dansyl chloride.
Plasma samples were expressed as ng/mL, but for the whole-body calculation, they were expressed as pg/mg protein. Comparison of the hormone concentration calculations for two methods confirmed the consistency of the calculation. 11-KT was significantly higher in males, both in tissue homogenate and in plasma. E2 in female whole-body homogenate was significantly higher than in plasma (Fig. 6). Unsurprisingly, the concentrations were not exactly similar in plasma and body homogenate because: 1) The protein concentration of each sample affects the final calculation in the body homogenate. 2) The calculated hormone contents in body homogenate are from the whole carcass, not from plasma alone. Regardless of these differences, whole tissue homogenate is a reliable alternative for hormone quantification when enough plasma is not available.
Figure 6. Concentration of hormones in FHM plasma and whole-body homogenate.
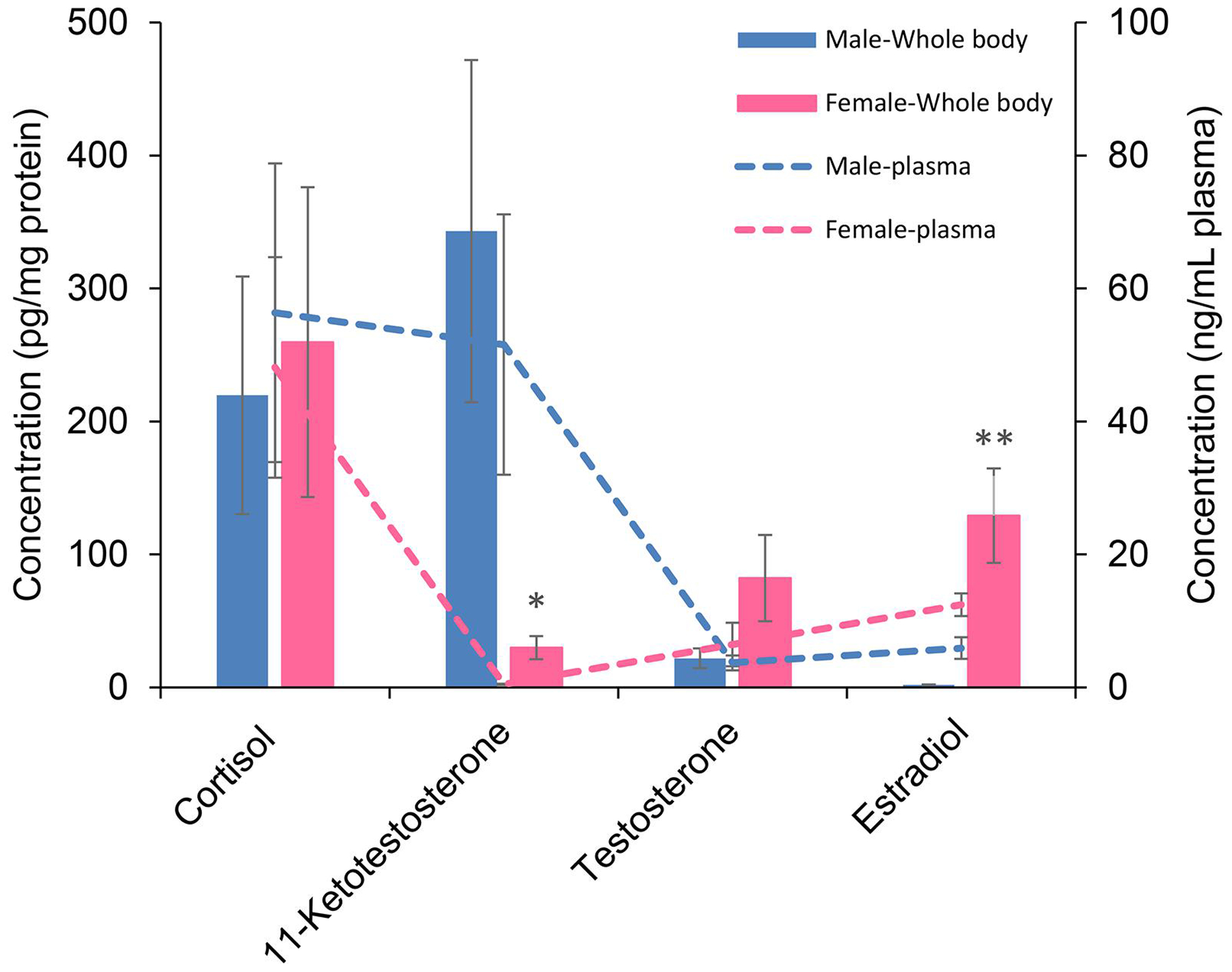
Cortisol, 11-ketotestosterone, testosterone and 17 β-estradiol, quantified in 10 μL plasma and whole body homogenate were compared in a single graph. Data are mean of four replicates of 10 μL plasma (dashed-line graph) or whole body (bar graph) ±SE. Right and left Y-axis are concentration of hormones in plasma (ng/mL) and whole body (pg/mg protein), respectively. * and ** indicate significant differences (p ≤0.05 and p ≤0.01, respectively) between male and female.
Collection of enough plasma from zebrafish for hormone analysis is difficult, although, it is a model fish for various biological and toxicological studies. In this study, whole tissue homogenate was used for measuring hormones in zebrafish. 11-KT and E2 concentrations were not significantly different in males and females. However, both cortisol and T were lower in females. We measured an additional hormone, 17OH-progesterone, in whole homogenates and while there was no detectable hormone in males, it was relatively high in females (Fig. 7). The smallest fish samples used in the study for hormone quantification was juvenile silversides with an average weight of 14 mg (Fig. 5) and they were not sexually differentiated. T could not be detected in this stage; however, DHT was quantified with an average of 5.96±1.2 pg/mg protein. Concentration of cortisol, 11-KT and derivatized E2 were 114±19.9, 0.54±0.09 and 7.8±2.4 pg/mg protein, respectively.
Figure 7. Quantification of hormones in zebrafish whole body homogenate.
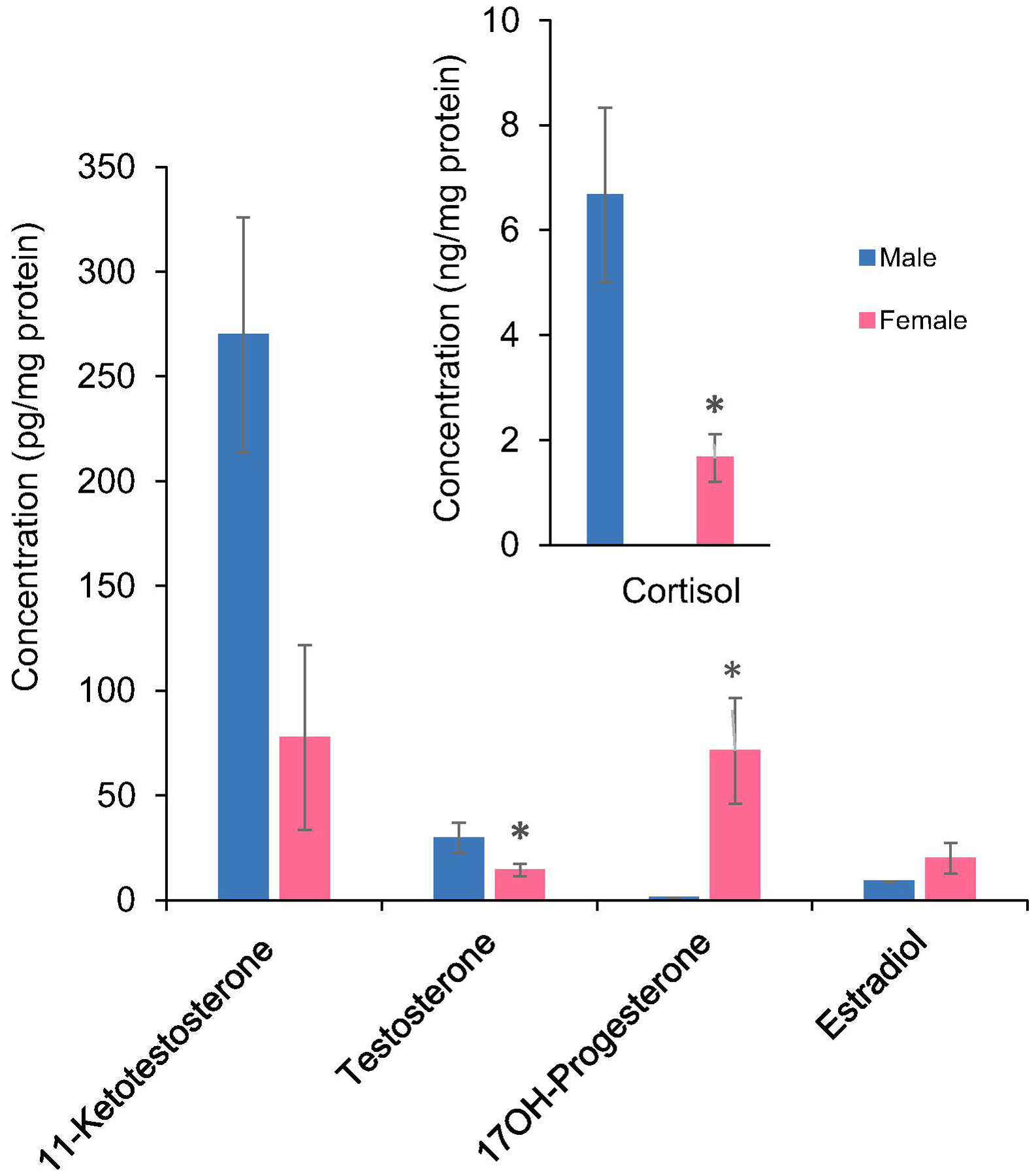
Cortisol, 11-ketotestosterone, testosterone, 17OH-progesterone and 17 β-estradiol were measured in male and female zebrafish. Data are mean of four replicates normalized based on pg/mg protein ±SE. * indicates significant differences (p ≤0.05) between males and females.
To compare LC-MS/MS and RIA methods, T and E2 were calibrated for each method, separately. While, LLOD was 0.0025 ng/mL and 0.03 ng/mL for T and E2 in LC-MS/MS technique, it was 0.2 ng/mL and 0.1 ng/mL, respectively in RIA (Table 5). Values of two hormones, T and E2, were compared in fish plasma samples using LC-MS/MS and RIA techniques. Data of LC MS/MS and RIA for T (Fig. 8a) and E2 (Fig. 8b) in males and females, respectively, were plotted. The values were highly correlated with the correlation coefficient (R2) of 0.95 and 0.96 for T and E2, respectively. This result confirms the validity and accuracy of the LC-MS/MS technique for hormone quantification.
Table 5.
Detection limits and calibration curve equations in LC-MS/MS and RIA techniques.
| LC-MS/MS | Radioimmunoassay | |||
|---|---|---|---|---|
| T ng/mL | E2 ng/mL | T ng/mL | E2 ng/mL | |
| Calibration range | 0.0025 to 20 | 0.03 to 100 | 0.20 to 250 | 0.10 to 100 |
| LRE (R2) | y = 0.9961x +0.0135 (1) | y = 1.0801x + 0.0367 (0.999) | y = −0.4584x + 1.0671 (0.996) | y = −0.4671x + 1.3556 (0.995) |
T, testosterone; E2, 17β-estradiol; LRE, Linear Regression equation; R2, correlation coefficient
Figure 8. Comparison of the values of testosterone (T) and 17β-estradiol (E2) measured by radioimmunoassay and LC-MS/MS.
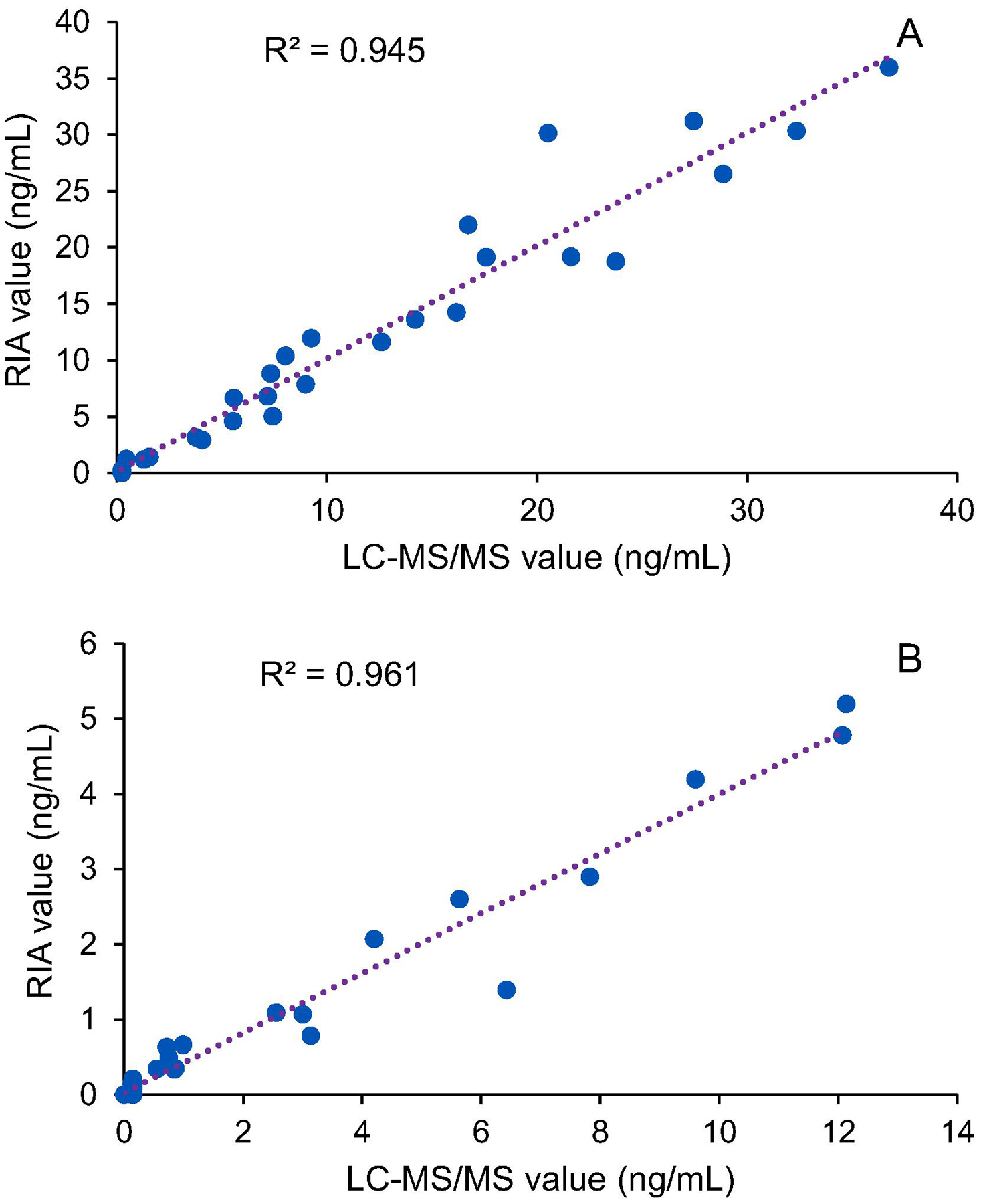
The values of T (a) and E2 (b) in male and female pallid sturgeon plasma were plotted. Data are mean of duplicates of concentration of hormones (ng/mL). Samples with not-detected E2 were removed from the plot b. R2 is the correlation coefficient of each plot.
4. Discussion
The LC-MS/MS approach is a robust technique that has been used for quantification of steroid hormones. It has advantages of fast and accurate hormone detection without the limitations of antibody-based approaches. The ability to detect several hormones simultaneously in a short time makes this technique more interesting. In this study, 14 steroid hormones were chromatographically separated in 12 min and data were acquired in positive and negative ion modes using the MRM method. Two product ions were used for quantitation and qualification for each hormone and validity of data were confirmed only when both transitions were seen.
We developed an improved technique for simultaneous detection of a panel of hormones in low volume of serum or plasma, i.e. 10 μL, as that it is hard to obtain more than this amount by traditional bleeding protocols. The results were compared to extractions with 245 μL plasma, which is the standard volume of plasma for this approach. Linearity of calibration for standard curves along with the accuracy and CV percentage, using both low and high plasma volumes, confirmed the validity of the results. Review of literature showed a similar accuracy, CV and LOQ using different or similar matrices. Bertin et al. (2015) detected seven steroid hormones in the monkey brain using the same instruments as we used in this study. LLOQ was 0.05, 0.01, 0.004 and 0.001 ng/mL for T, DHT, E1, and E2, respectively. In a human serum study, LLOQ was 0.010 ng/mL for E2, EE2, and progesterone (Blue et al., 2018). In our study, E2 does not ionize readily by electrospray ionization and thus we were able to aid the ionization by derivatizing with dansyl chloride. This helped to quantify E2 from the same extract that was used for detection of other hormones. Changing the Gaussian smoothing option to zero can be considered as an alternate approach when the concentration of E2 is very low.
Four fish species, LMB, FHM, zebrafish and silversides were selected for hormone analysis because of their impacts in scientific studies. LMB, is a relatively large fish with economic value for the sport-fishing industry and ecological value as an apex predator in the freshwater environment (Martyniuk et al., 2013). FHM, is a freshwater small fish model in North America (Ankley and Villeneuve, 2006). This fish along with other small fishes such as zebrafish (Danio rerio) and Japanese medaka (Oryzias latipes) are commonly used as toxicology research models around the world (Ankley and Johnson, 2004; Parrott et al., 2012). Silversides, is an estuarine species, commonly found along the east coast of North America, and is an excellent model species to study endocrine disrupting compounds in a marine environment. This species is susceptible to increasing temperatures and pollutants as it affects their sex determination (Duffy et al., 2009; Mehinto et al., 2018). Therefore, accurate quantification of hormones in juvenile silversides would be an important step for studies related to endocrine disrupting compounds. Fig 5, illustrates the types of samples used for hormone extraction.
In all fish species, concentrations of cortisol were relatively higher than other selected hormones. Cortisol in female LMB in high and low volume plasma averaged 91.6 and 104.3 ng/mL, respectively, which was significantly higher than the average in corresponding males (41.1 and 43.5 ng/mL, respectively). Cortisol was more consistently calculated in the high and low volume plasma. Absolute quantity of cortisol was calculated using cortisol-d7 as an internal standard. For 11-KT and T, calculations were based on surrogate internal standards, cortisol-d7 and 17-OH-progesterone, respectively. For the selected hormones, the variability among samples was quite low and CVs were less than 20%. For E2, the best results came from derivatized samples, but there could be slight differences in the derivatization efficiencies in low and high plasma volumes, which might affect the calculations and increase the standard errors. Nevertheless, we observed good reproducibility between the two extracted volumes for both males and females.
FHM was a perfect example to compare the concentration of hormone in low volume plasma against whole body homogenate. The fish size was big enough to collect 10 μL plasma and it was small enough to homogenize the whole body in a single vial. Trends in normalized data from various fish weights and plasma were almost equivalent both in male and in female. Blood volume as a percentage of body weight has been measured for some fishes and values from about 2% to 6.6% of body weight have been reported. The high value of 6.6% of body weight was reported for Chondrichthyes (Randall et al., 1970). Freshwater teleosts are on the lower end, with approximately 3% of body weight (Randall et al 1970). A recent estimate for total circulating blood volume in zebrafish is about 2–3.8% of body weight, depending on age (Zang et al., 2015). We normalized hormone concentrations to total protein within each homogenate to make it possible to compare measured hormone values among fish within a study and this is representative of the concentrations in blood.
In this study, we developed a robust method for simultaneous detection of 14 steroid hormones from low volume serum using LC-MS/MS. The method was validated by measuring hormones in high volume of serum. Detection and quantitation limits of hormones improved 2–40 times, when 25 times more serum was used as a high-volume serum. An alternate method of using whole body fish homogenates of small fish was developed and the results were verified using four fish species. Measuring hormones in juvenile small fish, before sexual differentiation is important for endocrinology and toxicology studies. These findings may open a new window for accurate quantification of hormones in small fish when small volumes of plasma are available.
Supplementary Material
Highlights.
A panel for quantification of 14 steroid hormones was developed from low volume plasma in a short time using LC-MS/MS.
We developed and validated the approach of using small fish whole body extracts for hormone quantification.
Four fish species, largemouth bass, fathead minnow, zebrafish and juvenile silverside were used in this study.
Acknowledgement
This project was made possible by U.S. National Institutes of Health (NIH) Shared Instrumentation (Grant 1S10OD018141-01A1 to N.D.D.). The authors would like to thank Dimitry Gorksky, U.S. Fish and Wildlife Service, Lower Great Lakes Fish and Wildlife Conservation Office for providing the Lake Sturgeon samples for comparison between LC-MS/MS and RIA. The findings and conclusions in the article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adeogun AO, Ibor OR, Imiuwa ME, Omogbemi ED, Chukwuka AV, Omiwole RA, Arukwe A, 2018. Endocrine disruptor responses in African sharptooth catfish (Clarias gariepinus) exposed to di-(2-ethylhexyl)-phthalate. Comp. Biochem. Physiol. C Toxicol. Pharmacol 213, 7–18. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Johnson RD, 2004. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. Inst. Lab. Anim. Res. J 45, 469–483. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Villeneuve DL, 2006. The fathead minnow in aquatic toxicology: past, present and future. Aquat. Toxicol 78, 91–102. [DOI] [PubMed] [Google Scholar]
- Bertin J, Dury AY, Ke Y, Ouellet J, Labrie F, 2015. Accurate and sensitive liquid chromatography/tandem mass spectrometry simultaneous assay of seven steroids in monkey brain. Steroids 98, 37–48. [DOI] [PubMed] [Google Scholar]
- Blue SW, Winchell AJ, Kaucher AV, Lieberman RA, Gilles CT, Pyra MN, Heffron R, Hou X, Coombs RW, Nanda K, Davis NL, Kourtis AP, Herbeck JT, Baeten JM, Lingappa JR, Erikson DW, 2018. Simultaneous quantitation of multiple contraceptive hormones in human serum by LC-MS/MS. Contraception 97, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs AS, Bowden JA, Galligan TM, Guillette LJ Jr., Kucklick JR, 2016. Development of a multi-class steroid hormone screening method using liquid chromatography/tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem 408, 4179–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden JA, Colosi DM, Mora-Montero DC, Garrett TJ, Yost RA, 2009. Enhancement of chemical derivatization of steroids by gas chromatography/mass spectrometry (GC/MS). J Chromatogr. B Analyt. Technol. Biomed. Life Sci 877, 3237–3242. [DOI] [PubMed] [Google Scholar]
- Duffy TA, McElroy AE, Conover DO, 2009. Variable susceptibility and response to estrogenic chemicals in Menidia menidia. Mar. Ecol. Prog. Ser 380, 245–254. [Google Scholar]
- EPA (US Environmental Protection Agency, 2007. Method 1698: Steroids and Hormones in Water, Soil, Sediment, and Biosolids by HRGC/HRMS. https://www.epa.gov/sites/production/files/2015-10/…/method_1698_2007.pdf. (Accessed 29 July 2019). [Google Scholar]
- FDA (US Food and drug administration)., 2018. Bioanalytical method validation guidance for industry Center for drug evaluation and research. https://www.fda.gov/regulatory-information/search-fda-guidance-documents (Accessed 29 May 2019). [Google Scholar]
- Fitzpatrick MS, Redding JM, Ratti FD, Schreck CB 1987. Plasma testosterone predicts the ovulatory response of coho salmon (Oncorhynchus kisutch) to gonadotropin-releasing hormone analog. Can. J. Fish. Aquat. Sci 44, 1351–1357. [Google Scholar]
- Gaikwad NW, 2013. Ultra performance liquid chromatography-tandem mass spectrometry method for profiling of steroid metabolome in human tissue. Anal. Chem 85, 4951–4960. [DOI] [PubMed] [Google Scholar]
- Häkkinen MR, Heinosalo T, Saarinen N, Linnanen T, Voutilainen R, Lakka T, Jääskeläinen J, Poutanen M, Auriola S, 2018. Analysis by LC- MS/MS of endogenous steroids from human serum, plasma, endometrium and endometriotic tissue. J. Pharm. Biomed. Anal 52, 165–172. [DOI] [PubMed] [Google Scholar]
- Koal T, Schmiederer D, Pham-Tuan H, Röhring C, Rauh M, 2012. Standardized LC-MS/MS based steroid hormone profile-analysis. J. Steroid Biochem. Mol. Biol 129, 129–138. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Louton T, Back DJ, Michaelis K, 1993. Radioimmunological analysis of ethinylestradiol in human serum. Validation of the method and comparison with a gas chromatographic/mass spectrometric assay. Arzneimittelforschung 43, 16–21. [PubMed] [Google Scholar]
- Markham D, Waechter J, Budinsky R, Gries W, Beyer D, Snyder S, Dimond S, Rajesh VN, Rao N, Connolly P, Neeley M, Hentges S, 2014. Development of a method for the determination of total bisphenol a at trace levels in human blood and urine and elucidation of factors influencing method accuracy and sensitivity. J. Anal. Toxicol 38, 194–203. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Prucha MS, Doperalski NJ, Antczak P, Kroll KJ, Falciani F, Barber DS, Denslow ND, 2013. Gene expression networks underlying ovarian development in wild largemouth bass (Micropterus salmoides). PLoS One 8, e59093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewski BK, Constanzer ML, Chavez-Eng CM 2003. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem 75, 3019–3030. [DOI] [PubMed] [Google Scholar]
- Mehinto AC, Kroll KJ, Jayasinghe BS, Lavelle CM, VanDervort D, Adeyemo OK, Bay SM, Maruya KA, Denslow ND, 2018. Linking in vitro estrogenicity to adverse effects in the inland silverside (Menidia beryllina). Environ. Toxicol. Chem 37, 884–892. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Grebe SK, OKane DJ, Singh RJ, 2004. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin. Chem 50, 373–384. [DOI] [PubMed] [Google Scholar]
- Parrott JL, Alaee M, Wang D, Sverko E, 2012. Fathead minnow (Pimephales promelas) embryo to adult exposure to decamethylcyclopentasiloxane (D5). Chemosphere 93, 813–818. [DOI] [PubMed] [Google Scholar]
- Penning TM, Lee SH, Jin Y, Gutierrez A, Blair IA, 2010. Liquid chromatography-mass spectrometry (LC-MS) of steroid hormone metabolites and its applications. J. Steroid Biochem. Mol. Biol 121, 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaud L, Monteau F, Deceuninck Y, Bichon E, Andre F, Bizec BL, 2007. Development and validation of a multi-residue method for the detection of a wide range of hormonal anabolic compounds in hair using gas chromatography-tandem mass spectrometry. Anal. Chim. Acta 586, 93–104. [DOI] [PubMed] [Google Scholar]
- Randall DJ, 1970. The Circulatory System in: Hoar WS, Randall DJ (Eds.), Fish Physiology, The nervous system, circulation and respiration. Academic Press, pp. 133–172. [Google Scholar]
- Schultz IR, Nagler JJ, Swanson P, Wunschel D, Skillman AD, Burnett V, Smith D, Barry R, 2013. Toxicokinetic, toxicodynamic, and toxicoproteomic aspects of short-term exposure to trenbolone in female fish. Toxicol. Sci 136, 413–429. [DOI] [PubMed] [Google Scholar]
- Szarka S, Nguyen V, Prokai L, Prokai-Tatrai K, 2013. Separation of dansylated 17β-estradiol, 17α-estradiol, and estrone on a single HPLC column for simultaneous quantitation by LC- MS/MS. Anal Bioanal Chem 405, 3399–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai SS-C, Welch MJ 2004. Development and evaluation of a candidate reference method for the determination of total cortisol in human serum using isotope dilution liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Anal. Chem 76, 1008–1014. [DOI] [PubMed] [Google Scholar]
- Van Der Gugten JG, Dubland J, Liu HF, Wang A, Joseph C, Holmes DT, 2012. Determination of serum aldosterone by liquid chromatography and tandem mass spectrometry: a liquid-liquid extraction method for the ABSCIEX API-5000 mass spectrometry system. J. Clin. Pathol 65, 457–462. [DOI] [PubMed] [Google Scholar]
- Vitku J, Chlupacova T, Sosvorova L, Hampl R, Hill M, Heracek J, Bicikova M, Starka L, 2015. Development and validation of LC-MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta 140, 62–67. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou X, Zhang Y, Chen H, Li G, Xu Y, Zhao Q, Song W, Jin H, Ding L, 2012. Dynamic microwave-assisted extraction coupled with salting-out liquid-liquid extraction for determination of steroid hormones in fish tissues. J. Agric. Food Chem 60, 10343–10351. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mesaros C, Blair IA, 2016. Ultra-high sensitivity analysis of estrogens for special populations in serum and plasma by liquid chromatography-mass spectrometry: Assay considerations and suggested practices. J. Steroid Biochem. Mol. Biol 162, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb MAH, Feist GW, Foster EP, Schreck CB, Fitzpatrick MS 2002. Potential classification of sex and stage of gonadal maturity of wild white sturgeon using blood plasma indicators. Trans. Am. Fish. Soc 131, 132–142. [Google Scholar]
- Yuan TF, Le J, Cui Y, Peng R, Wang ST, Li Y, 2019. An LC MS/MS analysis for seven sex hormones in serum. J. Pharm. Biomed. Anal 162, 34–40. [DOI] [PubMed] [Google Scholar]
- Zang L, Shimada Y, Nishimura Y, Tanaka T, Nishimura N 2015. Repeated Blood Collection for Blood Tests in Adult Zebrafish. J. Vis. Exp 102, e53272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


