Abstract
For metastatic bladder cancer patients, systemic cisplatin (CDDP)-based combination chemotherapy is the first-line choice of treatment. Although up to 70% of advanced bladder cancer patients initially show good tumor response to this form of combination chemotherapy, over 90% of good responders relapse and eventually die of the disease. According to the cancer stem cell theory, this phenomenon is attributable to the re-growth of bladder cancer-initiating cells (BCICs) that have survived chemotherapy. In this study, the authors have isolated BCICs from cultured human bladder cancer cells to analyze their sensitivity to CDDP and to investigate whether heat-shock protein 90 (Hsp90) inhibitors potentiate the cytotoxicity of CDDP on BCICs. First, the authors have confirmed that a CD44+ subpopulation of 5637 cells met the requirements to be considered tumor-initiating cells. These BCICs were more resistant to CDDP and exhibited more activity in the Akt and ERK oncogenic signaling pathways when compared with their CD44− counterparts. The Hsp90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG), which simultaneously inactivated both Akt and ERK signaling at noncytocidal concentrations, synergistically potentiated the cytotoxicity of CDDP against BCICs by enhancing CDDP-induced apoptosis in vitro. The potentiating effect of 17-DMAG was more effective than a combination of the two inhibitors specific for the Akt and ERK pathways. Finally, the authors have confirmed that, though human BCIC xenografts exhibited resistance to a single administration of CDDP and the Hsp90 inhibitor 17-(allylamino)-17-demethoxygeldanamycin (17-AAG), 17-AAG sensitized them to CDDP in a mouse model. These data encourage clinical trials of Hsp90 inhibitors as they may improve therapeutic outcomes of CDDP-based combination chemotherapy against advanced bladder cancer.
Keywords: bladder cancer, tumor-initiating cell, Hsp90 inhibitors, cisplatin resistance
Introduction
Bladder cancer is the fifth most common cancer in the United States. The estimated incidence and mortality in 2010 were 70,530 and 14,680 cases, respectively.1 One-third of patients with bladder cancer are diagnosed with muscle-invasive bladder cancer, and up to 50% of these patients develop distant metastases.2 Once a patient develops distant or nodal metastases, systemic cisplatin (CDDP)-based combination chemotherapy is given as the treatment of choice. Although up to 70% of patients show an initially good tumor response to systemic chemotherapy, more than 90% of patients develop recurrences and eventually die from the disease.3 From the viewpoint of cancer stem cell biology, this phenomenon can be explained as follows: although systemic chemotherapy kills the majority of bladder cancer cells leading to the clinical result of tumor shrinkage, the most important target, a small population of chemo-resistant cancer cells that possess tumorigenic capacity, is spared, thereby, allowing tumor re-growth.4
Recently, emerging evidence supports the existence of a cellular hierarchy within epithelial tumors. At the top of this hierarchy is a population of tumor-initiating cells (T-ICs) or cancer stem cells. According to the American Association of Cancer Research Workshop, T-ICs are defined as “cells within a tumor that possess the capacity for self-renewal and that can cause the heterogeneous lineages of cancer cells that constitute the tumor.”5 Several functional studies have identified additional characteristics of T-ICs including sustaining growth potential,5,6 spherical colony formation in vitro7 and inherent chemo- and radio-resistance.8,9 T-ICs of the epithelial lineage often show mesenchymal features.10,11
The first evidence for T-ICs came from acute myeloid leukemia,12 in which, a rare subset comprising 0.01–1% of the total population induced leukemia, when transplanted into immunodeficient mice. In solid tumors of various organs,11,13–16 a subset of tumor cells expressing surface markers such as CD133 and CD44 or those in a side population have been reported to meet the requirements for classification as T-ICs.7,11 In bladder cancer specifically, putative T-ICs have recently been isolated from clinical tumor samples based on their expression of CD44 and CD47,17 but neither profiles of resistance to anti-cancer therapy in bladder cancer-initiating cells (BCICs) nor the mechanisms underlying the acquisition of such resistance nor BCIC-targeting strategies have been fully investigated.
Heat-shock protein 90 (Hsp90) is a molecular chaperone required for the stability and function of numerous oncoproteins essential for the acquisition and maintenance of certain cancer hallmarks including evasion of apoptosis and self-renewal.18,19 Hsp90 inhibitors, now being evaluated in a number of clinical trials,20 destabilize Hsp90 clients by dissociating Hsp90–Hsp90 client complexes and thereby promoting antitumor activity.21,22 Hsp90 inhibitors can simultaneously inhibit multiple oncogenic pathways on which cancer cells depend for their development of therapeutic resistance and self-renewal. Recent reports suggest a potential role of Hsp90 in T-ICs: overexpression of the Hsp90 client oncoprotein erbB2 increases the T-IC population among breast cancer cells both in vitro and in vivo23 and Hsp90 inhibitors sensitize T-ICs of the breast to DNA-damaging agents including CDDP.24
In this study, the authors have first isolated BCICs from human bladder cancer cell lines based on their cell surface CD44 expression status, and demonstrated that they are more resistant to CDDP than their non-BCIC counterparts are. Next, the authors have investigated the potential role of Hsp90 inhibitors in overcoming the CDDP-resistance of BCICs.
Materials and Methods
Cell culture
Three human bladder cancer cell lines were used: JTC-30 (low-grade papillary, epithelial morphology), 5637 (low-grade, epithelial morphology) and T24 (high-grade, mesenchymal morphology). T24 cells were obtained from Japan Health Sciences Foundation on December 2, 2008. 5637 cells were obtained from the American Type Culture Collection on December 20, 2006. JTC-30 cells were established in our laboratory.25 The identities of 5637 and JTC-30 were confirmed by STR-profiling using the Cell ID System (Promega K.K., Tokyo, Japan). JTC-30 and T24 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C and 5% CO2. 5637 cells were maintained in RPMI 1640 supplemented with 10% FBS, 1% sodium pyruvate, 25 mmol/L HEPES and 1% penicillin/streptomycin at 37°C and 5% CO2.
Antibodies and reagents
Antibodies for p44/42 extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK), phospho-Thr202/Tyr204 p44/42 ERK/MAPK, Akt, phospho-Ser473 Akt, poly (ADP-ribose) polymerase (PARP), β-actin (Cell Signaling Technology, Danvers, MA) and erbB2 (CB11; Progen Biotechnik, Heidelberg, Germany) were used for immunoblotting. Anti-human CD44 mouse monoclonal antibody (BD Transduction Laboratories, Franklin Lakes, NJ) was used for immunohistochemistry and immunoblotting. Fluorescein isothiocyanate (FITC)-labeled mouse anti-human CD44 (BD Transduction Laboratories) and phycoerythrin (PE)-labeled mouse anti-human CD133/2 (Miltenyi Biotec, Bergisch Gladbach, Germany) were used for analysis of cell surface markers. The MAPK/ERK kinase (MEK) inhibitor PD98059 and the phosphatidylinositol-3-kinase (PI3K) inhibitor LY 294002 were purchased from Cell Signaling Technology. CDDP and DMSO were purchased from Sigma-Aldrich (St. Louis, MO). The Hsp90 inhibitors 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) and 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) were obtained from the NIH and from Kosan Biosciences (Hayward, CA).
Flow cytometry analysis and fluorescence-activated cell sorting
For surface marker analysis by flow cytometry, 70–80% confluent cells in 150-mm cell dishes (0.5–1 × 107 cells per dish) were washed once with PBS, then dissociated from dishes using Trypsin–EDTA and centrifuged. Cell pellets were re-suspended in 200 μL of phosphate-buffered saline supplemented with 0.5% bovine serum albumin and incubated at 4°C in the dark for 10 min with 30 μL of FITC-labeled anti-CD44 antibody, or re-suspended in 80 μL of the buffer with 20 μL of FcR Blocking Reagent (Miltenyi Biotec) and incubated at 4°C in the dark for 10 min with 10 μL of PE-labeled anti-CD133/2 antibody. Cells binding to the antibodies were re-suspended in 1,000 μL of serum-free RPMI and were sorted using a MoFlo cell sorter (Beckman Coulter, Brea, CA). Single cells were obtained by gating out cellular aggregates and by excluding dead cells. The purity of sorted cells was estimated to be >94% through re-analysis of a small sample of the collected cells. The results were analyzed using Summit software v4.3 (Dako Colorado, Fort Collins, CO).
Spheroid colony formation assay
A single fluorescence-activated cell sorting (FACS)-sorted human bladder cancer cell was incubated with 100 μL of RPMI-1640 medium supplemented with 1% sodium pyruvate, human recombinant epidermal growth factor (EGF, R&D Systems, Minneapolis, MN) at 20 ng/mL and human recombinant basic fibroblast growth factor (bFGF, R&D Systems) at 10 ng/mL in a well of ultra-low attachment 96-well plates (Corning Life Sciences, Corning, NY) in triplicate. EGF and bFGF were added every second day for 2 weeks. After 4 weeks, each well was examined under a light microscope and total well numbers with spheroid colonies were counted. Images of the spheroid colonies were recorded through an inverted phase microscope (Olympus IX70, Tokyo, Japan).
Immunohistochemistry
Immunostaining was performed as previously described.26 Briefly, 5-mm paraffin-embedded sections of tissue specimens were deparaffinized in xylene and re-hydrated. Slides were then incubated with 0.3% H2O2 in methanol to inhibit endogenous peroxidase and microwaved at 550 W for 15 min in 10 mM citrate buffer (pH 6.0). After blocking nonspecific binding with 10% goat serum, the slides were incubated with a primary antibody against human CD44 (dilution 1:50) overnight at 4°C. After incubation with a horseradish peroxidase-labeled secondary antibody (HISTOFINE Simple Stain MAX PO, Nichirei, Tokyo, Japan) for 30 min at room temperature, color was developed with 3,3-diaminobenzidine. The sections were counterstained with hematoxylin.
Cell proliferation and cytotoxicity assay
The cell proliferative potential of bladder cancer cells and their viability after exposure to CDDP, 17-DMAG, 17-AAG, PD98059 and LY 294002 were determined using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit (Promega K.K.) according to the manufacturer’s instructions. Cells (1–3 × 103) were suspended in 100 μL of medium with 10% FBS and plated in 96-well plates. Following exposure to reagents, these cells were incubated with serum-free medium. Next, cell viability was colorimetrically measured through a 3-(4,5-dimethylthiazol-2-yl)-5- (3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. After 1 hr of incubation, the absorbance at 490 nm was recorded using a Rainbow Thermo microtiter plate reader (TECAN, Männedrof, Switzerland).
Colony formation and clonogenic cell survival assay
For the colony formation assay, 1 × 102 cells were plated in 100-mm dishes. Following a 14-day incubation, colonies were fixed with ethanol and stained with Giemsa. The number of colonies composed of at least 50 cells was counted. The cytotoxic effects of various in vitro treatments were determined through a clonogenic assay. Briefly, cells at 70–80% confluence were exposed to reagents at specified concentrations. The cells were trypsinized, and equal numbers of cells were seeded into 100-mm dishes at multiple concentrations in fresh complete medium in duplicate. Following a 14-day incubation, the number of colonies that had been stained was determined as described above. Data were averaged, normalized against average survival rates of untreated samples and analyzed using CalcuSyn software (Biosoft, Cambridge, UK) based on the multiple drug-effect equation by Chou and Talalay.27 The combination index (CI) was calculated by the software to establish whether the drug interaction was synergistic or not. A CI of 1 indicates an additive effect whereas less than 1 indicates a synergistic effect.
Immunoblotting
Cells were lysed by scraping in TNESV lysis buffer (50 mmol/L Tris–HCl (pH 7.4), 1% NP40, 1 mmol/L EDTA, 100 mmol/L NaCl, 2 mmol/L Na3VO4) supplemented with complete proteinase inhibitors (Roche Applied Science, Basel, Switzerland). Following clarification by centrifugation, protein concentration was measured by means of the bicinchoninic acid assay (Pierce, Rockford, IL). Cell lysates were resolved by means of 5–15% SDS-PAGE, transferred to a nitrocellulose membrane and probed with antibodies. Protein expression was visualized through an enhanced chemiluminescence protein detection system (Pierce) using a Fujifilm LAS-4000 imager (Fujifilm, Tokyo, Japan).
Xenograft model
All animal studies were conducted in accordance with the Animal Welfare Regulations of our institution. To establish a FACS-sorted 5637 tumor xenograft model, a 1:1 mixture (by volume) of RPMI 1640 with 10% FBS containing 1 × 103 to 1 × 105 5637 cells and Matrigel basement membrane matrix (BD Biosciences) was inoculated subcutaneously into the dorsum of 4–6 week-old male ICR-severe combined immunodeficient (SCID) mice (CLEA Japan, Tokyo, Japan). To obtain tumor growth curves, tumor volumes were measured twice weekly with calipers and calculated according to the standard formula (length × width × height)/2. Tumor latency and incidence were also recorded over the 6 months following transplantation, but tumors were not allowed to grow beyond 4,000 mm3. For serial tumor transplantation assay, tumors were removed, minced into approximately 1-mm pieces with sterile scalpel blades and enzymatically dissociated by means of a 1-hr incubation with Dispase II (Sanko Junyaku, Tokyo, Japan) at 37°C in single-cell suspensions. Cells were passed through a 40-μm mesh screen and then incubated in complete medium at 37°C and 5% CO2. After propagation, 1 × 104 cells were inoculated into two other recipient mice. For chemo-resistance experiments in vivo, mice received the following treatments 7 days after inoculation with 1 × 106 CD44+ cells: (a) vehicle (DMSO) alone (n = 5), (b) 17-AAG alone (i.p., at 100 mg/kg, n = 5), (c) CDDP alone (i.p., at 6 mg/kg, n = 5) and (d) 17-AAG plus CDDP (n = 5).
Statistical analysis
Differences between groups were assessed using the Chi-square test for categorical data or the Wilcoxon test for continuous variables. All statistical analyses and partition analyses were performed using JMP 7.0 statistical software (SAS Institute, Cary, NC). Differences were considered significant at p < 0.05.
Results
Spheroid colony formation capacity of CD44+ bladder cancer cells
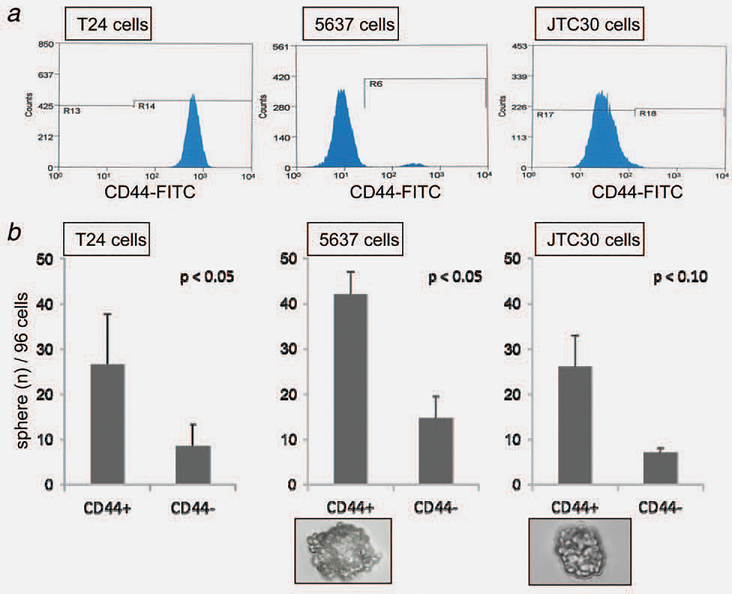
First, the expression patterns of CD44 and CD133, the candidate cell surface markers for BCICs, were analyzed in three human bladder cancer cell lines, T24, 5637 and JTC30 using FACS. All cell lines expressed CD44 to varying degrees; representative results are shown in Figure 1a. T24 cells, derived from high-grade and invasive disease, expressed CD44 to a high degree (98–100%), while 5637 and JTC30 cells, both derived from low-grade bladder cancer, expressed it much less strongly (2.5 and 0.6–1.3%, respectively). In all three cell lines, CD133 was expressed quite weakly (0.03–0.11%, data not shown).
Figure 1.
Distributions of cells from three bladder cancer cell lines expressing CD44 analyzed by fluorescence-activated cell sorter (FACS) and their spheroid colony formation capacity according to CD44 expression status. (a) Distribution of CD44+ cells by FACS analysis for T24 (left), 5637 (middle) and JTC30 cells (right). (b) CD44+ subpopulations of bladder cancer cells exhibit higher spheroid colony formation capacity than their CD44− counterparts do. A single cell was isolated through FACS sorting, identified as CD44+, and incubated with serum-free medium containing human epidermal growth factor (20 ng/mL) and basic fibroblast growth factor (10 ng/mL) in a well of ultra-low-attachment 96-well plates. Average percentages of colony-forming cells after 4 weeks of culture were calculated for T24 (left), 5637 (middle) and JTC30 cells (right). Each experiment was conducted in triplicate. Bars, SE. Photomicrograph of spheroid colony formation from a single CD44+ 5637 cell (lower middle) and a single JTC30 cell (lower right) in serum-free media ×400.
The growth of spherical colonies is considered indicative of self-renewal capacity and would be consistent with a T-IC phenotype.28 Indeed, spheroid colony formation has been used as a means of identifying T-ICs in vitro.5,6 To investigate whether CD44 and CD133 were potential BCIC markers, spheroid colony formation assay was conducted. In all three of the bladder cancer cell lines, the CD44+ cell fraction generated more spheroid colonies than the CD44− cell fraction did (Fig. 1b). The CD133+ cell fraction was so small in all cell lines that spheroid colony formation capacity could not be evaluated based on CD133 expression status. These findings, at least, indicate that more BCICs exist in the CD44+ fraction than in its CD44− counterpart. Since sufficient amounts of both CD44+ and CD44− cells could be consistently obtained only from 5637 cells and T24 cell line has no tumorigenicity when injected subcutaneously into immunocompromised mice,29 the remaining experiments were conducted using 5637 cell line.
Tumorigenicity of CD44+ bladder cancer cells
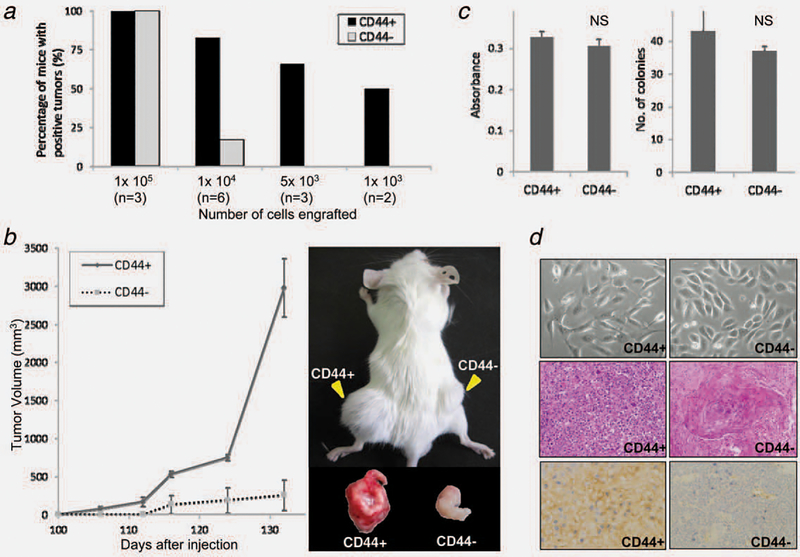
To examine the in vivo tumorigenicity of 5637 cells based on their CD44 expression status, a limiting dilution transplantation assay was performed. As shown in Figure 2a, when 1 × 105 cells were inoculated, both CD44+ and CD44− cells showed 100% tumorigenicity (3/3 SCID mice for both). At 1 × 104 cells, tumorigenicity was 83% (5/6) for CD44+ cells but only 17% (1/ 6) for CD44− cells. At 5 × 103 cells or fewer, CD44− cells did not form tumors within 24 weeks (0/3 and 0/2 at 5 × 103 and 1 × 103 cells, respectively). In contrast, CD44+ cells were still tumorigenic in low numbers; tumor formation was observed in 2/3 (67%) and 1/2 mice (50%) at 5 × 103 and 1 × 103 cells, respectively. These indicate that the tumorigenic potential of CD44+ bladder cancer cells is higher than that of CD44− cells by 10- to 100-fold. In addition, CD44+ tumors grew more rapidly than CD44− tumors did when 1 × 105 cells were inoculated (Fig. 2b), despite the comparable in vitro cell proliferative and colony formation capacity of CD44+ and CD44− cells (Fig. 2c).
Figure 2.
Tumorigenicity, proliferative capacity and morphological characteristics of 5637 cells according to CD44 expression status. (a) Higher tumorigenic potential of CD44+ 5637 cells when compared with their CD44− counterparts as demonstrated by a limiting dilution transplantation assay. Percentages of tumor formation per inoculation at 1 × 103, 5 × 103, 1 × 104 and 1 × 105 cells in SCID mice were recorded throughout the 24 weeks following inoculation. Data are expressed as % calculated as number of tumors formed/number of inoculations according to CD44 expression status. (b) Higher proliferative capacity of CD44+ 5637 cells for tumor xenografts compared with their CD44− counterparts. Average tumor volumes were calculated at the indicated time points after inoculation of 1 × 105 CD44+ or CD44− 5637 cells into three SCID mice (left). Bars, SE. A representative photogram depicting tumor formation in a SCID mouse at the injection sites 3 months after inoculation of 1 × 104 CD44+ and CD44− cells (right). (c) Similar cell proliferative and colony formation capacity of 5637 cells irrespective of CD44 expression status in vitro. Cell proliferative capacity according to CD44 expression status was analyzed by MTS assay (left). A thousand cells were incubated with complete medium for 72 hr before the assay. Colony formation capacity was analyzed according to CD44 expression status by clonogenic assay (right). One hundred CD44+ or CD44− cells were seeded into 100-mm tissue culture dishes. Following a 14-day incubation, average number of colonies was calculated. Individual assays were conducted in duplicate. Bars, SE. NS, not significant. (d) Morphological features of 5637 cells in vitro and in vivo according to CD44 expression status. Cultured cells that are positive for CD44 show stromal features, appearing as isolated spindle-shaped cells, while those that are negative for CD44 have epithelial features, appearing as approximately round cells that form small clusters (upper). Histologically, CD44+ tumor xenografts were poorly differentiated, in clear contrast to CD44− tumor xenografts showing remarkable keratinization, a feature of terminal squamous differentiation (middle; H&E staining, reduced from ×400). Tumor xenografts of CD44+ cells showed sustained CD44 expression while those of CD44− cells were negative for it (lower; reduced from ×400).
Self-renewal capacity of CD44+ bladder cancer cells
To examine the self-renewal properties of CD44+ 5637 cells in vivo, a serial transplantation assay was conducted. Tumor formation was observed 3 weeks after inoculation in secondary recipient mice. The authors have repeated the transplantation assay up to the third generation and confirmed the sustained tumorigenicity of CD44+ cells in tertiary recipients. In contrast, CD44− cells did not generate tumors in either of two secondary recipients, when 1 × 104 cells were inoculated.
Morphological features of CD44+ bladder cancer cells
Cultured parental and CD44− 5637 and JTC30 cells exhibited some epithelial features, including a relatively round shape and the tendency to form small clusters, whereas CD44+ cells exhibited mesenchymal features, most commonly taking the form of isolated spindle-shaped cells (Fig. 2d, upper). T24 cells, which abundantly express CD44, originally have mesenchymal features.30 Histologically, CD44+ 5637 cell-derived tumor xenografts were poorly differentiated, in clear contrast to CD44− tumor xenografts showing remarkable keratinization, a feature of terminal squamous differentiation (Fig. 2d, middle). CD44+ tumor xenografts sustained CD44 expression, while CD44− tumors tested negative for it (Fig. 2d, lower). Given their high tumorigenic potential, self-renewal capacity and mesenchymal features, CD44+ bladder cancer cells meet the requirements to be considered T-ICs.10,11
CDDP-resistance of bladder cancer-initiating cells
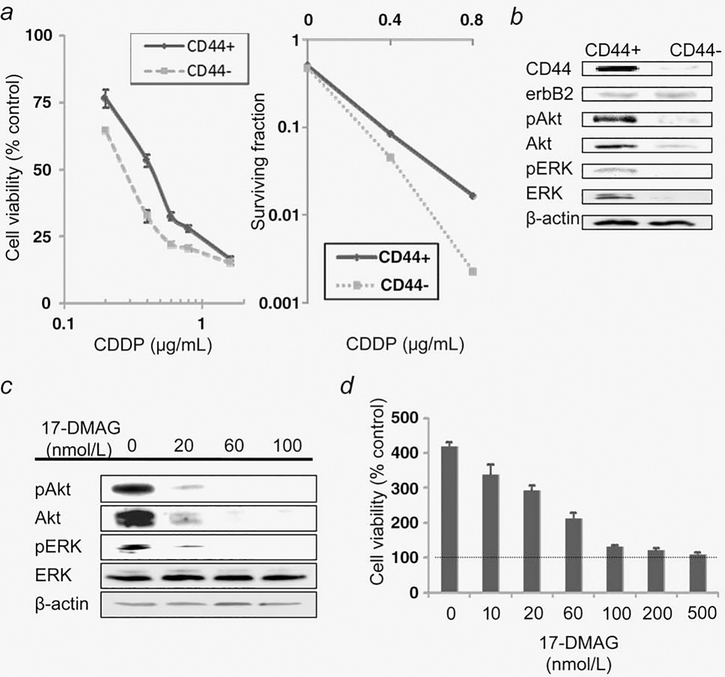
CDDP is one of the most commonly used and most effective chemotherapeutic agents against bladder cancer. The authors have compared sensitivity to CDDP between CD44+ and CD44− 5637 cells. Cells were treated with various concentrations of CDDP for 24 hr. CD44+ cells, with an estimated 50% inhibitory concentration (IC50) of 0.43 μg/mL, were more resistant to CDDP than CD44 cells, with an estimated IC50 of 0.25 μg/mL (Fig. 3a, left panel). In a clonogenic cell-survival assay, CD44+ cells also proved more resistant to CDDP than CD44− cells (Fig. 3a, right panel).
Figure 3.
BCICs are more resistant to CDDP and have higher activity of Akt and ERK than their non-BCIC counterparts in vitro. (a) Cell viability according to CD44 expression status was analyzed by MTS assay for 1 × 103 5637 cells simultaneously treated with various concentrations of CDDP for 24 hr followed by treatment with CDDP-free fresh medium for 48 hr (left). Bars, SE. Colony-forming efficacy according to CD44 expression status was analyzed by clonogenic assay for 5637 cells treated with CDDP at 0.4 and 0.8 μg/mL for 24 hr (right). (b) A CD44+ subpopulation expresses higher levels of Akt, ERK and their phosphorylated forms than its CD44− counterpart expresses. Equal amounts of protein extracted from CD44+ or CD44− subsets of 5637 cells, which were incubated and propagated for 8 hr after isolation by FACS, were loaded to measure expression levels of indicated proteins by immunoblot. (c) Dose-dependent inactivation of Akt and ERK signaling pathways by 17-DMAG at a lower concentration range. A CD44+ subset of 5637 cells treated with 17-DMAG at the indicated concentrations for 48 hr was analyzed for expression of Akt, ERK, their phosphorylated forms and β-actin. (d) 72-hr treatment with 17-DMAG at 20 nmol/L does not exert cytocidal effects on CD44+ cells. After 72-hr exposure to 17-DMAG at indicated concentrations or vehicle, cell viability was analyzed by MTS assay. Control indicates cell viability at time 0 of treatment. Bars, SE.
To overcome the CDDP-resistance of BCICs, the authors have investigated the potential role of Hsp90 inhibitors in sensitizing BCICs against CDDP. First, the authors have examined the expression patterns of certain oncogenic proteins to which the chemoresistance of BCICs could be attributed, including Akt, ERK, their phosphorylated forms and erbB2.31–33 As shown in Figure 3b, expression levels of Akt, ERK and their phosphorylated forms were higher in CD44+ BCICs than in CD44− cells. Next, the authors have determined the minimal concentrations at which the Hsp90 inhibitor 17-DMAG destabilizes Hsp90 client proteins and inhibits oncogenic signaling but does not exert a significant cytotoxic effect in CD44+ cells. 17-DMAG at 20 nmol/L destabilized Akt and erbB2 and attenuated Akt and ERK activity by more than 90% (Fig. 3c). As shown in Fig. 3d, 72-hr treatment with 17-DMAG at 20 nmol/L was cytostatic but not cytocidal. Therefore, 17-DMAG at 20 nmol/L was applied in subsequent experiments.
Sensitization of bladder cancer-initiating cells to CDDP by Hsp90 inhibitors
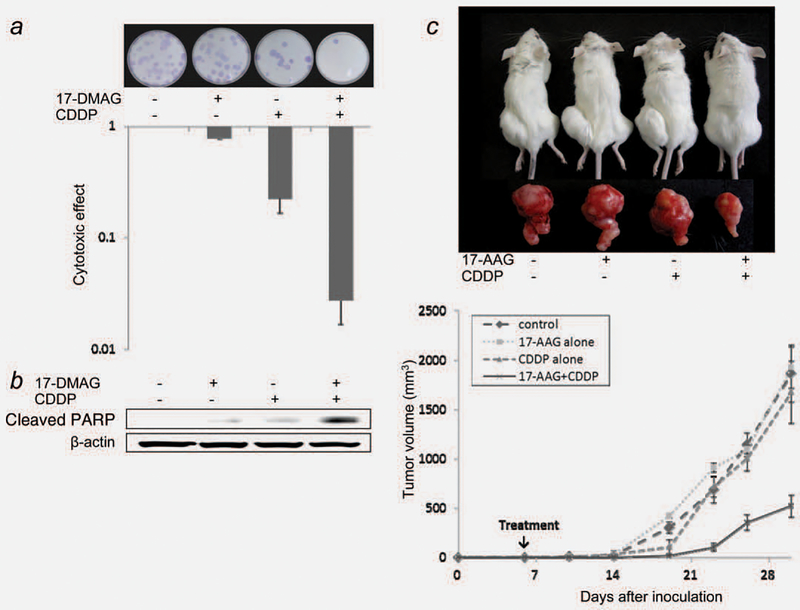
To examine whether Hsp90 inhibitors at noncytocidal concentrations sensitize BCICs to CDDP in vitro, a clonogenic assay was conducted in CD44+ 5637 cells. A CDDP concentration of 0.4 μg/mL was used for in vitro experiments since the serum concentration of CDDP stabilizes at approximately 0.4 μg/mL, when approximately 20 mg is given to patients intravenously.34 As shown in Figure 4a, 17-DMAG alone was less toxic to BCICs than CDDP was, and a 24-hr pretreatment with 17-DMAG synergized the cytotoxic effect of CDDP with a CI of 0.59.
Figure 4.
Hsp90 inhibitors potentiate the anti-tumor effects of CDDP on BCICs. (a) 17-DMAG synergistically sensitizes BCICs to CDDP in vitro. A CD44+ subset of 5637 cells was treated with or without 17-DMAG (20 nmol/L) for 24 hr followed by co-incubation with CDDP (0.4 μg/mL) or vehicle for an additional 24 hr. One hundred cells thus treated were seeded into 10-mm dishes, then incubated in complete medium for 14 days. Colonies formed were visualized by means of Giemsa staining (upper). The fraction surviving each treatment was calculated (lower). Bars, SE. (b) 17-DMAG at a noncytocidal concentration potentiates CDDP-induced apoptosis in BCICs. A CD44+ subset of 5637 cells treated as described above were subjected to immunoblot for measurement of cleaved PARP. (c) 17-AAG sensitizes BCIC xenografts, which are otherwise CDDP-resistant, to CDDP in a mouse model. Mice were treated with vehicle alone, 17-AAG alone (6 mg/kg, i.p.), CDDP alone (100 mg/kg, i.p.) or a combination of 17-AAG and CDDP (n = 5 for each treatment) 7 days after inoculation with 1 × 106 CD44+ 5637 cells. Representative photographs of mice in each treatment group on day 30 after tumor inoculation (upper). Average tumor volumes at the indicated time points for each treatment were plotted (lower). Bars, SE.
One potential mechanism that may be involved in the synergistic effect observed in BCICs is the activation of apoptotic pathways. To test this hypothesis, cleaved PARP, a marker of apoptosis, was measured in CD44+ cells treated with CDDP in the presence or absence of 17-DMAG (Fig. 4b). Treatment with 17-DMAG or CDDP alone induced PARP cleavage weakly, while the combination induced it strongly, indicating that Hsp90 inhibitors potentiate CDDP-induced activation of apoptotic pathways.
Since Hsp90 inhibitors simultaneously block multiple oncogenic pathways,18,19 the authors have hypothesized that Hsp90 inhibitors potentiate the cytotoxic effects of CDDP on BCICs more effectively than does the inhibition of one or both of the major oncogenic pathways, Akt and ERK. To test this hypothesis, the authors have evaluated the CDDP-sensitizing effects of the PI3K inhibitor LY294002 and the MEK inhibitor PD98059 applied singly or in combination to BCICs in vitro. Pretreatment with LY294002 and PD98059 at 5 μmol/L for 1 hr inhibited the phosphorylation of Akt and ERK, respectively, by approximately 80% (Supporting Information Figure S1a). Since even a 48-hr treatment with these agents at 5 μmol/L was not cytocidal within a 96-hr observation period (Supporting Information Figure S1b), treatment conditions for the clonogenic assay included a 24-hr pretreatment with vehicle, LY294002 alone, PD98059 alone, or LY294002 and PD98059 together, followed by a 24-hr co-incubation with vehicle or CDDP. In contrast to the synergistic effect of 17-DMAG, either LY294002 or PD98059 alone did not exhibit an additive effect on CDDP cytotoxicity but their combination did (Supporting Information Figure S1c). These results reveal that, in terms of sensitization of BCICs to CDDP, the effect of the simultaneous inhibition of multiple oncogenic pathways by Hsp90 inhibitors surpasses that of dual inhibition of the Akt and ERK pathways, both of which are activated in BCICs.
Finally, the authors have investigated the role of Hsp90 inhibitors in sensitizing BCICs against CDDP in vivo. 17-AAG has biological profiles similar to 17-DMAG in terms of Hsp90 inhibition.35 As shown in Figure 4c, CD44+ cell-derived tumors were completely resistant to a single administration of CDDP or 17-AAG throughout the observation period. A combination of 17-AAG and CDDP suppressed tumor growth significantly more powerfully than either drug alone did. Thus, Hsp90 inhibitors potentially enhance the anti-tumor effect of CDDP on CDDP-resistant BCICs in a mouse model.
Discussion
In this study, the authors have isolated BCICs from human bladder cancer 5637 cells based on their CD44 expression status and demonstrated that CD44+ BCICs have greater CDDP-resistance and more abundant expression of oncogenic signaling proteins than CD44− cells do. Hsp90 inhibitors at noncytocidal concentrations synergistically potentiated the cytotoxic effects of CDDP on BCICs in vitro and successfully sensitized CDDP-resistant BCIC-derived tumor xenografts to CDDP. These data encourage clinical trials of Hsp90 inhibitors as possible tools to improve therapeutic outcomes of CDDP-based combination chemotherapy against advanced bladder cancer.
The mechanism responsible for the drug resistance of T-ICs has not been fully elucidated. Some T-ICs express higher levels of drug resistance-related proteins such as ATP-binding cassette half-transporter proteins and multidrug resistance protein 1 transporters.36 The authors have found that BCICs express higher levels of Akt, ERK and their activated forms than non-BCICs do. Although the mechanisms by which BCICs abundantly express these oncogenic proteins remain to be elucidated, the current in vitro data suggest that the impact of Hsp90 inhibitors on CDDP resistance of BCICs is likely to be multimodal since more specific inhibition of either Akt or ERK had minimal impact on the resistance.
Hsp90 client proteins include key components of multiple oncogenic pathways relevant to growth and survival other than the Akt and ERK pathways,18 such as hypoxia-inducible factor-1α (HIF-1α), erbB2 and inhibitor of κB kinase β, which mediates nuclear factor (NF)-κB activation. HIF-1α, which is commonly overexpressed in aggressive tumors, plays roles in tumor progression and resistance to chemotherapy.37 The authors have previously reported that low-dose Hsp90 inhibitors attenuate invasive and angiogenic potentials of bladder cancer cells by interfering with the HIF-1 pathway.38 The authors have recently reported that overexpression of erbB2 and activated NF-κB is relevant to chemoradiotherapy resistance of bladder cancer in clinical settings and that low-dose Hsp90 inhibitors potentiate the therapeutic effects by simultaneously blocking the erbB2 and NF-κB pathways.39
To overcome the therapeutic resistance of T-ICs by simultaneously abrogating multiple anti-apoptotic pathways, a combination of Hsp90 inhibitors with cytotoxic therapies is an attractive approach. Wright et al.24 have found that the use of 17-DMAG simultaneously with or after chemotherapeutic agents sensitizes BRCA1 cancer stem cells to CDDP, suggesting the effectiveness of Hsp90 inhibition not only in targeting the bulk of the tumor but also in minimizing recurrences originating from T-ICs. The current study has demonstrated that Hsp90 inhibitors sensitize BCICs to CDDP. In the attempt to clinically develop a novel combination therapy involving Hsp90 inhibitors and CDDP, it is essential to evaluate the toxic profiles of each component of the therapy in normal tissues. The authors have shown that Hsp90 inhibitors sensitize bladder cancer cells but not primarily cultured normal urothelial cells to CDDP,39 suggesting the tumor-selectivity of this combination therapy.
The complete eradication of T-ICs is necessary to “cure” advanced cancer patients. Thus, a preclinical model using T-ICs would make drug discovery and the development of novel anti-cancer strategies more efficient. In this respect, it is important to identify additional markers for isolating T-ICs of higher purity.
Presently, various solid tumors share CD44 as a T-IC marker.11,14,16 The current study clearly demonstrates that the CD44+ subpopulations isolated from our three human bladder cancer cell lines meet the requirements to be considered T-ICs, confirming the previous study by Chan et al. in which BCICs were isolated from clinical bladder cancer tissues.17 The CD44+ subpopulation of cancer cells is unlikely to consist of pure BCICs, however; for example, pancreatic cancer cells that are triply positive for CD44/CD24/ epithelial-specific antigen have twice the tumorigenic potential of the CD44+ subpopulation.16 The isolation of BCICs of higher purity accomplished through recognition of a combination of several markers would contribute to more efficient development of novel and curative strategies against advanced bladder cancer.
In the current study, BCICs showed much higher proliferative potential than non-BCICs did in vivo, despite the two populations' comparable in vitro cell proliferative capacities. CD44 is a cell surface receptor for hyaluronan (HA).40 Upon HA binding to CD44 expressed on cancer cells, the complex interacts with transmembrane receptor kinases such as ErbB2, EGF receptor and transforming growth factor-β,40,41 which mediates activation of Ras/MAPK and Ras/phosphatidylinositol-3-kinase/Akt pathways and eventually promotes proliferation, survival, motility, invasiveness and chemoresistance. Because of the presence of HA in in vivo experiment42,43 and accordingly potential activation of the above-mentioned pathways, higher proliferative activity of BCICs in vivo is conceivable. Indeed, the similar findings were reported for T-ICs derived from gastric cancer and brain tumor.28,44
After treatment with Hsp90 inhibitors, another mechanism conferring treatment resistance may emerge: once heat shock factor (HSF)-1 is released from an Hsp90-containing heterocomplex by abrogation of Hsp90 function,45 HSF-1 translocates to the nucleus, binds to heat shock elements and transactivates Hsp90, 70 and 27.46 Since Hsp70 and 27 are associated with treatment resistance,47,48 Hsp90 inhibition might potentiate CDDP resistance, in part, by increasing expression of these chaperone proteins. In this respect, simultaneous inhibition of Hsp70 and 27 might enhance the sensitizing effects of Hsp90 inhibitors on BCICs to CDDP. Reportedly, an antisense oligonucleotide OGX-427 targeting Hsp27 sensitizes bladder cancer cells to paclitaxel in vitro.49
The limitation of 5637 cell line as a model for BCICs might be that this cell line is derived from grade II bladder cancer and part of the current results may not represent aggressive bladder cancer in the clinical setting, which typically consists of grade III bladder cancer. In high-grade bladder cancer cells, Hsp90 inhibitors would be also expected to overcome CDDP resistance of BCICs. A couple of groups including us showed that Hsp90 inhibitors efficiently attenuate oncogenic activities of T24 cells even at low and noncytotoxic concentrations.38,50 The authors have confirmed that Hsp90 inhibitors sensitize CDDP-resistant T24 cells to CDDP.39
In conclusion, BCICs isolated based on their CD44 expression status were more resistant to CDDP compared with their CD44− counterparts, but Hsp90 inhibitors efficiently sensitized them to CDDP. These results encourage clinical trials of Hsp90 inhibitors as adjuncts to CDDP-based systemic chemotherapy, as they may improve therapeutic outcomes of advanced bladder cancer patients.
Supplementary Material
Acknowledgements
This work was supported, in part, by grants-in-aids for Scientific research 23791744 (Manabu Tatokoro), 23501266 (Fumitaka Koga), from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- 17-AAG
17-(allylamino)-17-demethoxygeldanamycin
- 17-DMAG
17-(dimethylaminoethylamino)-17-demethoxygeldanamycin
- BCICs
bladder cancer-initiating cells
- bFGF
basic fibroblast growth factor
- CDDP
cisplatin
- CI
Combination Index
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- HA
hyaluronan
- HSF
heat shock factor
- Hsp90
heat-shock protein 90
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- PARP
poly (ADP-ribose) polymerase
- PE
phycoerythrin
- PI3K
phosphatidylinositol-3-kinase
- SCID
severe-combined immunodeficient
- T-IC
tumor-initiating cell
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2.Bassi P, Ferrante GD, Piazza N, Spinadin R, Carando R, Pappagallo G, Pagano F. Prognostic factors of outcome after radical cystectomy for bladder cancer: a retrospective study of a homogeneous patient cohort. J Urol 1999;161: 1494–7. [PubMed] [Google Scholar]
- 3.Saxman SB, Propert KJ, Einhorn LH, Crawford ED, Tannock I, Raghavan D, Loehrer PJ, Sr Trump D. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9. [DOI] [PubMed] [Google Scholar]
- 4.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5:275–84. [DOI] [PubMed] [Google Scholar]
- 5.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006; 66:9339–44. [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells – old concepts, new insights. Cell Death Differ 2008;15:947–58. [DOI] [PubMed] [Google Scholar]
- 7.Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J 2007;21: 3777–85. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008;100:672–9. [DOI] [PubMed] [Google Scholar]
- 9.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756–60. [DOI] [PubMed] [Google Scholar]
- 10.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, Radisky DC, Ferrone S, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 2009;69:2887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A 2007; 104:10158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367: 645–8. [DOI] [PubMed] [Google Scholar]
- 13.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature 2004;432:396–401. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106–10. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030–7. [DOI] [PubMed] [Google Scholar]
- 17.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J,Chang HY Jr., van de Rijn M, Shortliffe L, Weissman IL. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A 2009; 106:14016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutic agents. Expert Opin Emerg Drugs 2002;7:277–88. [DOI] [PubMed] [Google Scholar]
- 19.Koga F, Kihara K, Neckers L. Inhibition of cancer invasion and metastasis by targeting the molecular chaperone heat-shock protein 90. Anticancer Res 2009;29:797–807. [PubMed] [Google Scholar]
- 20.Wang Y, Trepel JB, Neckers LM, Giaccone G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs 2010;11:1466–76. [PubMed] [Google Scholar]
- 21.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A 1994; 91:8324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 2003;3:213–7. [DOI] [PubMed] [Google Scholar]
- 23.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008;27:6120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res 2008;10:R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakuya T, Yamada T, Yokokawa M, Ueda T. Establishment of cell strains from human urothelial carcinoma and their morphological characterization. In Vitro 1983;19:591–9. [DOI] [PubMed] [Google Scholar]
- 26.Koga F, Kawakami S, Fujii Y, Saito K, Ohtsuka Y, Iwai A, Ando N, Takizawa T, Kageyama Y, Kihara K. Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin Cancer Res 2003;9:5501–7. [PubMed] [Google Scholar]
- 27.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 28.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009;27:1006–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bubenik J, Baresova M, Viklicky V, Jakoubkova J, Sainerova H, Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer 1973;11: 765–73. [DOI] [PubMed] [Google Scholar]
- 30.Fukushima H, Koga F, Kawakami S, Fujii Y, Yoshida S, Ratovitski E, Trink B, Kihara K. Loss of DeltaNp63alpha promotes invasion of urothelial carcinomas via N-cadherin/Src homology and collagen/extracellular signal-regulated kinase pathway. Cancer Res 2009;69: 9263–70. [DOI] [PubMed] [Google Scholar]
- 31.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, Schmidt M, Mills GB, Mendelsohn J, Fan Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003;22:3205–12. [DOI] [PubMed] [Google Scholar]
- 32.Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, Siddik ZH, Mills GB, Claret FX. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem 2003;278: 19245–56. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Yang W, Lan KH, Sellappan S, Klos K, Hortobagyi G, Hung MC, Yu D. Enhanced sensitization to taxol-induced apoptosis by herceptin pretreatment in ErbB2-overexpressing breast cancer cells. Cancer Res 2002;62:5703–10. [PubMed] [Google Scholar]
- 34.Sawada M, Okudaira Y, Matsui Y, Nishiura H, Yanagida T. Pharmacokinetics of cis-platinum diammine dichloride. Gan To Kagaku Ryoho 1982;9:55–65. [PubMed] [Google Scholar]
- 35.Smith V, Sausville EA, Camalier RF, Fiebig HH, Burger AM. Comparison of 17-dimethylaminoethylamino-17-demethoxy-gelda-namycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models. Cancer Chemother Pharmacol 2005;56:126–37. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone RW, Cretney E, Smyth MJ. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood 1999;93:1075–85. [PubMed] [Google Scholar]
- 37.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–32. [DOI] [PubMed] [Google Scholar]
- 38.Koga F, Tsutsumi S, Neckers LM. Low dose geldanamycin inhibits hepatocyte growth factor and hypoxia-stimulated invasion of cancer cells. Cell Cycle 2007;6:1393–402. [DOI] [PubMed] [Google Scholar]
- 39.Koga F, Yoshida S, Tatokoro M, Kawakami S, Fujii Y, Kumagai J, Neckers L, Kihara K. Potential role of heat shock protein 90 inhibitors to overcome chemoradiotherapy resistance associated with HER-2 and NF-kB overexpression in muscle-invasive bladder cancer. Eur Urol Suppl 2011;10: 167. [Google Scholar]
- 40.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004;4:528–39. [DOI] [PubMed] [Google Scholar]
- 41.Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras coactivation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem 2006;281:14026–40. [DOI] [PubMed] [Google Scholar]
- 42.Simpson MA, Wilson CM, McCarthy JB. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. Am J Pathol 2002;161:849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josefsson A, Adamo H, Hammarsten P, Granfors T, Stattin P, Egevad L, Engstrom Laurent A, Wikstrom P, Bergh A. Prostate cancer increases hyaluronan in surrounding nonmalignant stroma and this response is associated with tumor growth and an unfavorable outcome. Am J Patho 2011; 179:1961–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells 2008;26:3027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCollum AK, Lukasiewicz KB, Teneyck CJ, Lingle WL, Toft DO, Erlichman C. Cisplatin abrogates the geldanamycin-induced heat shock response. Mol Cancer Ther 2008;7: 3256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005;5: 761–72. [DOI] [PubMed] [Google Scholar]
- 47.Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene 2003;22:9041–7. [DOI] [PubMed] [Google Scholar]
- 48.Zoubeidi A, Chi K, Gleave M. Targeting the cytoprotective chaperone, clusterin, for treatment of advanced cancer. Clin Cancer Res 2010;16: 1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamada M, So A, Muramaki M, Rocchi P, Beraldi E, Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol Cancer Ther 2007;6:299–308. [DOI] [PubMed] [Google Scholar]
- 50.Karkoulis PK, Stravopodis DJ, Margaritis LH, Voutsinas GE. 17-Allylamino-17-demethoxygeldanamycin induces downregulation of critical Hsp90 protein clients and results in cell cycle arrest and apoptosis of human urinary bladder cancer cells. BMC Cancer 2010; 10:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.