Abstract
Objective
The aim of the study was to summarize the latest evidence for patient bathing with a 2% to 4% chlorhexidine gluconate solution to reduce multidrug-resistant organism (MDRO) transmission and infection.
Methods
We searched 3 databases (CINAHL, MEDLINE, and Cochrane) for a combination of the key words “chlorhexidine bathing” and MeSH terms “cross-infection prevention,” “drug resistance, multiple, bacterial,” and “drug resistance, microbial.” Articles from January 1, 2008, to December 31, 2018, were included, as well as any key articles published after December 31.
Results
Our findings focused on health care–associated infections (HAIs) and 3 categories of MDROs: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and carbapenem-resistant Enterobacteriaceae (CRE). Chlorhexidine bathing reduces MRSA acquisition and carriage, but not all studies found significant reductions in MRSA infections. Several studies found that chlorhexidine bathing reduced VRE acquisition and carriage, and one study showed lower VRE infections in the bathing group. Two studies found that bathing reduced CRE carriage (no studies examined CRE infections). Two very large studies (more than 140,000 total patients) found bathing significantly reduced HAIs, but these reductions may be smaller when HAIs are already well controlled by other means.
Conclusions
There is a high level of evidence supporting chlorhexidine bathing to reduce MDRO acquisition; less evidence is available on reducing infections. Chlorhexidine bathing is low cost to implement, and adverse events are rare and resolve when chlorhexidine use is stopped. There is evidence of chlorhexidine resistance, but not at concentrations in typical use. Further research is needed on chlorhexidine bathing’s impact on outcomes, such as mortality and length of stay.
Key Words: chlorhexidine, multidrug-resistant organisms, drug resistance, infection prevention, infection control
Multidrug-resistant organisms (MDROs) are microorganisms, mainly bacteria, that are resistant to 1 or more antimicrobial agents.1 The World Health Organization recognizes MDROs as a growing threat.2 Multidrug-resistant organisms are of particular concern for vulnerable patients, such as those who have received organ transplantation, those with cancer, preterm infants, and immune-suppressed patients.2 With limited effective antimicrobials,3 MDROs are responsible for approximately 23,000 deaths annually in the United States.1 The Centers for Disease Control and Prevention (2018) states that 11% of individuals screened in healthcare facilities are asymptomatic carriers for a transmissible, “hard-to-treat” microorganism.4
Chlorhexidine solutions are used as topical disinfectants and as part of recommended strategies for MDRO control.5,6 Chlorhexidine solutions are commercially available in concentrations from 0.5% to 4%, with bathing solutions (such as prepacked cloths or liquid soap) generally ranging from 2% to 4%.7 This review article summarizes the recent evidence for chlorhexidine bathing to reduce MDRO transmission and infection.
METHODS
We searched 3 databases (CINAHL, MEDLINE, and Cochrane) for a combination of the key words “chlorhexidine bathing” and MeSH terms related to “cross-infection prevention,” “drug resistance, multiple, bacterial,” and “drug resistance, microbial.” Articles from 2008 through December 31, 2018, were included. Any relevant articles published after the original search are included in Figure 1, the search methods flow chart, as additional sources.
FIGURE 1.
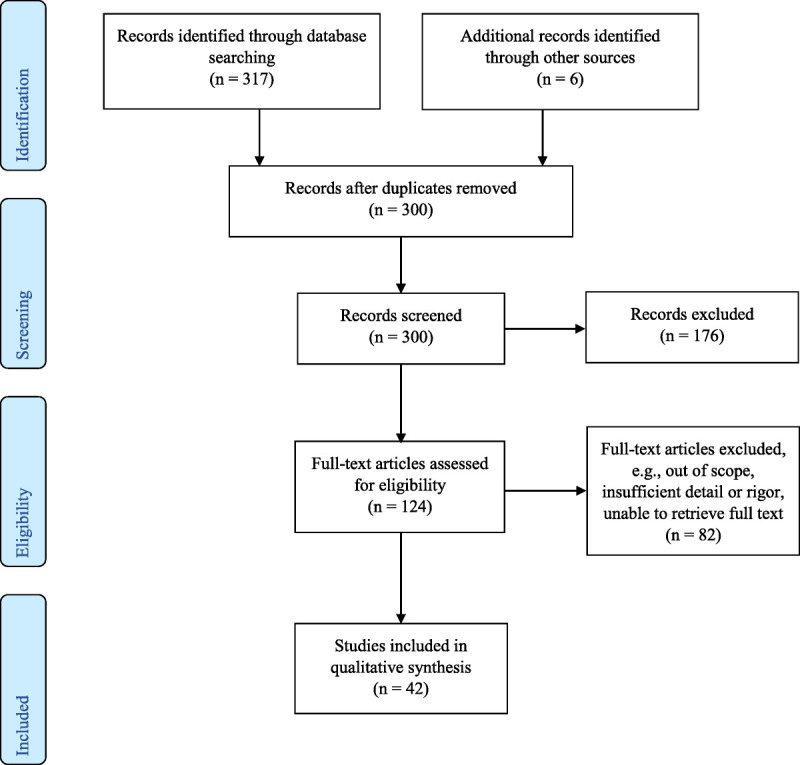
Chlorhexidine bathing study selection for review.
The initial search yielded 317 results; after duplicates were removed, 300 (including 6 articles from other sources) were screened for inclusion and 124 full-text articles were retrieved. Of those, 42 were selected for inclusion. Articles were excluded if they did not mention skin or oropharyngeal application of chlorhexidine or use of chlorhexidine outside of health care environments. Chlorhexidine oral care was included in this review, as were in vitro studies of chlorhexidine resistance. For systematic reviews or meta-analyses, the project team accepted the authors’ assessments of study quality and overall strength of evidence.
In this review, we define “chlorhexidine bathing” as no-rinse application of chlorhexidine to the skin or oropharyngeal surfaces, for the purposes of decolonization and infection prevention. Oropharyngeal surfaces are a reservoir for MDROs in mechanically ventilated patients, and thus, we include oral care as part of a chlorhexidine bathing routine.6
RESULTS
With a wide variety of outcomes across all studies, we chose to focus on results for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), carbapenem-resistant Enterobacteriaceae (CRE), and health care–associated infections (HAIs). Methicillin-resistant S. aureus (MRSA) continues to make up half of S. aureus–related health care–associated infections.8 Similarly, 30% of health care–associated Enterococcus infections are vancomycin-resistant and are increasingly resistant to alternative antibiotic treatments.9 Carbapenem-resistant Enterobacteriaceae is considered an urgent public health threat by the Centers for Disease Control and Prevention because of its difficulty to treat.8 In addition, CRE and carbapenem-resistant genes are increasingly widespread: some subsets are endemic in healthcare facilities in parts of the United States, and there is evidence of carbapenem resistance in the community.10 The results of the review are summarized hereinafter, with additional detail on each study in Tables 1–5. Where possible, we specify whether all infections or MDRO-only infections are reported.
TABLE 1.
Summary of MRSA Results

TABLE 5.
Summary of Other Results

TABLE 2.
Summary of VRE Results

TABLE 3.
Summary of CRE Results

TABLE 4.
Summary of HAI Results

Methicillin-Resistant Staphylococcus aureus
Evidence suggests that chlorhexidine bathing in the hospital setting reduces MRSA acquisition and carriage, but this may not always result in fewer MRSA-related infections. Three systematic reviews and 3 studies (2 experimental, 1 quasi-experimental) found evidence that chlorhexidine bathing reduces MRSA acquisition and carriage, although one review did include studies where no reduction was found.7,11,12,14,15,17 A prospective cohort study by Ruiz et al16 (2017) found no reduction in MRSA colonization rates but did find a significant reduction in total MDRO colonization.
Interpreting the impact of chlorhexidine bathing on infection rates is complicated by its use in multicomponent decolonization protocols (including nasal mupirocin and oral antibiotics). For MRSA, it may be more appropriate to study how chlorhexidine bathing can reduce resource-intensive practices, such as patient isolation.6,30 Peterson et al’s15 cluster-randomized study in long-term care facilities demonstrated that a thorough decolonization protocol including chlorhexidine bathing can reduce MRSA colonization without patient isolation.
Vancomycin-Resistant Enterococcus
Similar to MRSA, studies reported on various VRE prevention outcomes: acquisition, colonization, carriage, and infection. Three systematic reviews found that chlorhexidine can reduce VRE carriage in hospital patients.7,12,17 One rigorous multicenter study and 2 quasi-experimental studies found that chlorhexidine bathing reduces VRE acquisition.11,14,18 One of the quasi-experimental studies also found a reduction in VRE-related infections when daily bathing was combined with skin antisepsis for central venous catheter insertion and before surgery or biopsies.18
Carbapenem-Resistant Enterobacteriaceae
Few studies directly addressed chlorhexidine bathing for CRE. Of those that did, 2 observational cohort studies found that chlorhexidine bathing can reduce CRE colonization and potentially CRE infection.16,19
Other Pathogens
No systematic reviews recommended or discouraged chlorhexidine bathing for preventing/reducing general multidrug-resistant gram-negative bacteria (MDR-GNB) colonization.12,17,29 One review found only temporary decolonization of MDR-GNB using chlorhexidine, and 1 randomized, open-label controlled trial found no reduction or delay in MDR-GNB acquisition.20,29 Kengen et al’s24 retrospective time study (2018) found no difference in MDRO acquisition with chlorhexidine bathing as compared with soap and water, whereas Ruiz et al16 (2017) saw a reduction in MDRO acquisition, including MDR-GNB.16,24 Musuuza et al’s14 pre-post study (2017) found reduced MDR-GNB colonization with chlorhexidine bathing, but Mendes et al’s18 quasi-experimental observational study did not. Maxwell et al27 (2017) found no difference between chlorhexidine and soap bathing for lowering all-MDRO infection rates.
Health Care–Associated Infections
Many studies examined the effect of chlorhexidine bathing on catheter-associated urinary tract infection (CAUTI), ventilator-associated pneumonia (VAP), and central line–associated blood stream infection (CLABSI). One review and several studies, including 2 large studies with more than 400,000 patients, found evidence that chlorhexidine bathing can reduce device-associated HAIs.7,13,23 Specifically, Abboud et al’s19 observational cohort study found chlorhexidine bathing reduced HAIs in CRE-colonized patients. Among ICU patients, 2 studies11,23 found significant reductions in CLABSIs, although the reduction in MRSA-related BSIs in Huang et al23 (2013) study was not statistically significant. However, in Huang et al’s13 later (2019) study of noncritical care patients, the authors found significant reductions in all-cause BSIs for the subgroup of patients with medical devices.
Although some studies did not show an effect on HAIs, these were considerably smaller than the 2 studies by Huang et al.13,23 A 2015 rigorous cluster-randomized trial by Noto et al25 found no impact on CLABSI, CAUTI, VAP, or Clostridioides difficile infection rates among the 9340 patients in the study. Ruiz et al16 observed reduced MDRO colonization in their single-site study, but this did not lead to a reduction in HAIs. In addition, they noted that longer ICU stays were associated with higher overall incidence of HAIs, regardless of chlorhexidine bathing.16
Two studies compared chlorhexidine bathing to bathing with soap and water and found no improvement in HAI rates, especially when the HAIs are caused by MDR-GNB.20,24 Camus et al21 (2014) were able to reduce HAIs from MDR-GNB by adding mupirocin application to chlorhexidine bathing (for all patients) and polymyxin/tobramycin/amphotericin B in the oropharynx and gastric tubes of intubated patients. However, this study was not designed to control for the impact of these additional steps, and more research is needed on whether these may be sufficient (in the presence or absence of chlorhexidine bathing).
Two studies found chlorhexidine bathing only effective for some HAIs. Dusznyska et al’s22 observation study (2017) also found no reduction in intubation-related pneumonia, nor in UTIs, although overall infections and catheter-related infections were significantly lower. A randomized trial of oropharyngeal decontamination using chlorhexidine found no effect on reduced BSIs from MDR-GNB in mechanically ventilated patients.26
Although chlorhexidine is routinely used as a preoperative antisepsis, Abboud et al19 (2016) found no supporting literature that chlorhexidine bathing reduces SSIs despite observing a reduction in SSIs among CRE-colonized patients in their study. Another review found mixed evidence on the efficacy of chlorhexidine bathing for preventing SSIs.7 Two meta-analyses from the Cochrane Database of Systematic Reviews (Dumville et al,31 2013; Webster and Osborne,32 2015) found no definitive evidence that chlorhexidine antisepsis or patient showering/bathing (including bathing with rinsing) before surgery reduced SSI rates.
Finally, Urbanic et al33 (2018) raise an important consideration: HAIs can be infrequent events, and the number needed to treat with chlorhexidine bathing to significantly reduce infections may have been, in some cases, larger than the study population. This also suggests that chlorhexidine bathing has limited benefit for HAI reduction in settings where HAIs are already well controlled by other means.
Chlorhexidine Resistance
Resistance to chlorhexidine is detected by observing higher minimum inhibitory concentrations (MICs) and higher minimum bactericidal concentrations. Two in vitro studies found chlorhexidine resistance more common in settings with routine chlorhexidine bathing.34,35 One retrospective cohort study found no conclusive trends in the prevalence of chlorhexidine-resistant MDROs after implementing bathing, but the authors hypothesize that some increases may be due to readmitted patients with persistent colonization.36
McNeil et al’s37 study of S. aureus in a pediatric hospital (2014) showed that organisms with chlorhexidine resistance genes had MICs twice as high and minimum bactericidal concentrations 8 to 16 times as high as more susceptible organisms (P < 0.005). However, one in vitro study of ICU isolates collected after a chlorhexidine bathing initiative found that resistance genes were linked to higher MICs in 1 MRSA strain but not another.38 Similarly, Musuuza et al’s14 pre-post study did not show increased MICs in MRSA and fluoroquinolone-resistant GNB after a daily bathing intervention in their hospital. Although not genetically resistant, oral MRSA biofilms studied in vitro show considerable resistance to chlorhexidine mouthwashes, which may account for failure of oral washing to prevent VAP and for frequent oral MRSA recolonization.39
The clinical impact of chlorhexidine resistance genes is unclear. One in vitro study of hospital MRSA isolates found that resistant strains showed more resistance to chlorhexidine than methicillin-susceptible strains.40 Similarly, Alotaibi et al41 (2017) found more chlorhexidine resistance in VRE than in vancomycin-susceptible Enteroccoci. Hayashi et al42 (2016) found that Acinetobacter baumanii epidemic strains from hospital isolates showed increased resistance to chlorhexidine in vitro, but not at concentrations generally used for disinfection.
Two studies found evidence that chlorhexidine bathing can favor general resistance. Abboud et al19 found that an increase in colonization with Pseudomonas aeruginosa and A. baumanii after chlorhexidine bathing was implemented in an ICU. However, Camus et al43 (2016) found no increase in MDR-GNB rates after implementation of oral chlorhexidine bathing for ventilated patients, but it is unclear what affect the additional components of that intervention (mupirocin ointment and antibiotics) had on MDR-GNB rates. Finally, 2 studies found that chlorhexidine-resistant genes were also associated mupirocin resistance in isolates.37,44
DISCUSSION
This review found evidence that chlorhexidine bathing can reduce MDRO acquisition and carriage, but not necessarily infection. A recent (2019) Cochrane review concluded that more evidence is needed on whether this reduces infections, mortality, and length of stay in ICUs.45 At the concentrations typically used for bathing (2%–4%), chlorhexidine is still effectively microbicidal; however, overdiluted solutions may fail to kill organisms, especially when biofilms develop.46–48
In addition to efficacy against CRE and emerging chlorhexidine resistance, additional research on chlorhexidine bathing could include:
-
▪
studies on frequency and duration of bathing;
-
▪
studies that examine the efficacy chlorhexidine in reducing infections due to existing colonization (“self-infection”) as well as infections caused by MDRO shedding;
-
▪
evaluations of chlorhexidine bathing’s role in multicomponent programs (also suggested in commentary by Horner et al,49 2013); and
-
▪
continued research on chlorhexidine resistance and related clinical outcomes, especially for biofilms (suggested by Grascha,50 2014) and Gram-negative bacteria (suggested by Strich and Palmore,51 2017).
LIMITATIONS
This study only included publications for which English-language versions were available. Few studies specifically examined CRE; instead, many more studies examined MDR-GNB (including Enterobacteriaceae species). Although the use of the key word “chlorhexidine bathing” was consistent with the key words used in the included articles, this may have excluded studies that meet our operational definition of “chlorhexidine bathing” without using that term.
CONCLUSIONS
Chlorhexidine bathing is effective at reducing acquisition and decolonization, particularly by MDR gram-positive bacteria; more evidence is needed to show whether this ultimately reduces infection, length of stay, and mortality. As an intervention, chlorhexidine bathing is low cost to implement with few adverse events (skin sensitivity, which resolves after stopping bathing), but compliance can wane over time. Low levels of chlorhexidine resistance have been observed in vitro but at concentrations far below those recommended for bathing. Although there are no clinical impacts described in the literature to date, resistance should continue to be monitored.
Footnotes
The authors disclose no conflict of interest.
This work was funded by the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services (Contracts HHSP233201500013I and HHSP23337002T).
REFERENCES
- 1.Centers for Disease Control and Prevention Multidrug-resistant organisms (MDRO) Management [CDC Infection Control web site]. 2006. Available at: https://www.cdc.gov/infectioncontrol/guidelines/mdro/index.html#. Accessed November 22, 2019.
- 2.World Health Organization A global health guardian: Climate change, air pollution and antimicrobial resistance. In: WHO, ten years in public health, 2007–2017. [WHO 10-year review web site]. 2017:136–142. Available at: https://www.who.int/publications/10-year-review/chapter-guardian.pdf?ua=1. Accessed November 22, 2019.
- 3.Trick WE Lin MY Cheng-Leidig R, et al. Electronic public health registry of extensively drug-resistant organisms, Illinois, USA. Emerg Infect Dis. 2015;21:1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention CDC Vital Signs: containing unusual resistance [CDC Vital Signs web site]. 2018. Available at: https://www.cdc.gov/vitalsigns/pdf/2018-04-vitalsigns.pdf. Accessed November 22, 2019.
- 5.Edmiston CE Bruden B Rucinski MC, et al. Reducing the risk of surgical site infections: does chlorhexidine gluconate provide a risk reduction benefit? Am J Infect Control. 2013;41:S49–S55. [DOI] [PubMed] [Google Scholar]
- 6.Yokoe DS Anderson DJ Berenholtz SM, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol. 2014;35:S21–CS31. [DOI] [PubMed] [Google Scholar]
- 7.Denny J, Munro CL. Chlorhexidine bathing effects on health-care-associated infections. Biol Res Nurs. 2017;19:123–126. [DOI] [PubMed] [Google Scholar]
- 8.Magill SS O’Leary E Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379:1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Antibiotic resistance threats in the United States, 2019 [CDC Antibiotic / Antimicrobial Resistance (AR / AMR) web site]. Atlanta, GA: U.S. Department of Health and Human Services; 2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed November 21, 2019. [Google Scholar]
- 10.National Institutes of Health National Institute of Allergy and Infectious Diseases (NIAID). NIAID’s Antibacterial Resistance Program: Current Status and Future Directions [NIH Antimicrobial (Drug) Resistance web site]. 2014. Available at: https://www.niaid.nih.gov/sites/default/files/arstrategicplan2014.pdf. Accessed March 9, 2018. [Google Scholar]
- 11.Climo MW Yokoe DS Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derde LP, Dautzenberg MJD, Bonten MJM. Chlorhexidine body washing to control antimicrobial-resistant bacteria in intensive care units: a systematic review. Intensive Care Med. 2012;38:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang SS Septimus E Kleinman K, et al. Chlorhexidine versus routine bathing to prevent multidrug-resistant organisms and all-cause bloodstream infections in general medical and surgical units (ABATE Infection trial): a cluster-randomised trial. Lancet. 2019;393:1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musuuza JS Sethi AK Roberts TJ, et al. Implementation of daily chlorhexidine bathing to reduce colonization by multidrug-resistant organisms in a critical care unit. Am J Infect Control. 2017;45:1011–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson LR Boehm S Beaumont JL, et al. Reduction of methicillin-resistant Staphylococcus aureus (MRSA) infection in long term care is possible while maintaining patient socialization: a prospective randomized clinical trial. Am J Infect Control. 2016;44:1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz J Ramirez P Villarreal E, et al. Daily bathing strategies and cross-transmission of multidrug-resistant organisms: impact of chlorhexidine-impregnated wipes in a multidrug-resistant gram-negative bacteria endemic intensive care unit. Am J Infect Control. 2017;45:1069–1073. [DOI] [PubMed] [Google Scholar]
- 17.Silder JA Battegay M Tschudin-Sutter S, et al. Enterococci, Clostridium difficile and ESBL-producing bacteria: epidemiology, clinical impact and prevention in ICU patients. Swiss Med Wkly. 2014;144:w14009. [DOI] [PubMed] [Google Scholar]
- 18.Mendes ET Ranzani OT Marcha AP, et al. Chlorhexidine bathing for the prevention of colonization and infection with multidrug-resistant microorganisms in a hematopoietic stem cell transplantation unit over a 9-year period: impact on chlorhexidine susceptibility. Medicine. 2016;95:e5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abboud CS de Souza EE Zandonadi EC, et al. Carbapenem-resistant Enterobacteriaceae on a cardiac surgery intensive care unit: successful measures for infection control. J Hosp Infect. 2016;94:60–64. [DOI] [PubMed] [Google Scholar]
- 20.Boonyasiri A Thaisiam P Permpikul C, et al. Effectiveness of chlorhexidine wipes for the prevention of multidrug-resistant bacterial colonization and hospital-acquired infections in intensive care unit patients: a randomized trial in Thailand. Infect Control Hosp Epidemiol. 2016;37:245–253. [DOI] [PubMed] [Google Scholar]
- 21.Camus C Salomon S Bouchigny C, et al. Short-term decline in all-cause acquired infections with the routine use of a decontamination regimen combining topical polymyxin, tobramycin, and amphotericin b with mupirocin and chlorhexidine in the ICU: a single- center experience. Crit Care Med. 2014;42:1121–1130. [DOI] [PubMed] [Google Scholar]
- 22.Dusznyska W Adamik B Lentka-Bera K, et al. Effect of universal chlorhexidine decolonisation on the infection rate in intensive care patients. Anaesthesiol Intensive Ther. 2017;49:28–33. [DOI] [PubMed] [Google Scholar]
- 23.Huang SS Septimus E Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kengen R Thoonen E Daveson K, et al. Chlorhexidine washing in intensive care does not reduce bloodstream infections, blood culture contamination and drug-resistant microorganism acquisition: an interrupted time series analysis. Crit Care Resusc. 2018;20:231–240. [PubMed] [Google Scholar]
- 25.Noto MJ Domenico HJ Byrne DW, et al. Chlorhexidine bathing and healthcare-associated infections: a randomized clinical trial. JAMA. 2015;313:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittekamp BH Plantinga NL Cooper BS, et al. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA. 2018;320:2087–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxwell RA Croft CA Creech CB, et al. Methicillin-resistant Staphylococcus aureus in a trauma population: does decolonization prevent infection? Am Surg. 2017;83:1407–1412. [PubMed] [Google Scholar]
- 28.Pedriera MLG Kusahara DM Brunow de Carvalho W, et al. Oral care interventions and oropharyngeal colonization in children receiving mechanical ventilation. Am J Crit Care. 2009;18:319–328. [DOI] [PubMed] [Google Scholar]
- 29.Tacconelli E Cataldo MA Dancer SJ, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20:1–55. [DOI] [PubMed] [Google Scholar]
- 30.Calfee DP Salgado CD Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:772–796. [DOI] [PubMed] [Google Scholar]
- 31.Dumville JC McFarlane E Edwards P, et al. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2013;3:CD003949. [DOI] [PubMed] [Google Scholar]
- 32.Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2015;2:CD004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbanic KF Mårtensson J Glassford N, et al. Impact of unit-wide chlorhexidine bathing in intensive care on bloodstream infection and drug-resistant organism acquisition. Crit Care Resusc. 2018;20:109–116. [PubMed] [Google Scholar]
- 34.Hijazi K Mukhopadhya I Abbot F, et al. Susceptibility to chlorhexidine in multidrug resistant clinical isolates of Staphylococcus epidermidis from bloodstream infections. Int J Antimicrob Agents. 2016;48:86–90. [DOI] [PubMed] [Google Scholar]
- 35.Suwantarat N Carrol KC Tekle T, et al. High prevalence of reduced chlorhexidine susceptibility in organisms causing central line–associated bloodstream infections. Infect Control Hosp Epidemiol. 2014;35:1183–1186. [DOI] [PubMed] [Google Scholar]
- 36.Warren DK Prager M Munigala S, et al. Prevalence of qacA/B genes and mupirocin resistance among methicillin-resistant Staphylococcus aureus (MRSA) isolates in the setting of chlorhexidine bathing without mupirocin. Infect Control Hosp Epidemiol. 2016;37:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeil JC Hulten KG Kaplan S, et al. Decreased susceptibilities to retapamulin, mupirocin, and chlorhexidine among staphylococcus aureus isolates causing skin and soft tissue infections in otherwise healthy children. Antimicrob Agents Chemother. 2014;58:2878–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otter JA Patel A Cliff PR, et al. Selection for qacA carriage in CC22, but not CC30, methicillin-resistant Staphylococcus aureus bloodstream infection isolates during a successful institutional infection control programme. J Antimicrob Chemother. 2014;68:992–999. [DOI] [PubMed] [Google Scholar]
- 39.Smith K Robertson DP Lappin DF, et al. Commercial mouthwashes are ineffective against oral MRSA biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:624–629. [DOI] [PubMed] [Google Scholar]
- 40.Marolf CT Alter R Lyden E, et al. Susceptibility of nosocomial Staphylococcus aureus to chlorhexidine after implementation of a hospital-wide antiseptic bathing regimen. Infect Control Hosp Epidemiol. 2017;38:873–875. [DOI] [PubMed] [Google Scholar]
- 41.Alotaibi SMI Ayibieka A Pedersen AF, et al. Susceptibility of vancomycin-resistant and -sensitive enterococcus faecium obtained from Danish hospitals to benzalkonium chloride, chlorhexidine and hydrogen peroxide biocides. J Med Microbiol. 2017;66:1744–1751. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi M Kawamura K Matsui M, et al. Reduction in chlorhexidine efficacy against multi-drug-resistant Acinetobacter baumannii international clone II. J Hosp Infect. 2017;95:318–323. [DOI] [PubMed] [Google Scholar]
- 43.Camus C Sauvadet E Tavenard A, et al. Decline of multidrug-resistant gram-negative infections with the routine use of a multiple decontamination regimen in ICU. J Infect. 2016;73:200–209. [DOI] [PubMed] [Google Scholar]
- 44.Cho OH, Park KH, Song JY. Prevalence and microbiological characteristics of qacA/B-positive methicillin-resistant Staphylococcus aureus isolates in a surgical intensive care unit. Microb Drug Resist. 2018;24:283–289. [DOI] [PubMed] [Google Scholar]
- 45.Lewis SR Schofield-Robinson OJ Rhodes S, et al. Chlorhexidine bathing of the critically ill for the prevention of hospital-acquired infection. Cochrane Database Syst Rev. 2019;8:CD012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekizoğlu M Sağiroğlu M Kiliç E, et al. An investigation of the bactericidal activity of chlorhexidine digluconate against multidrug-resistant hospital isolates. Turk J Med Sci. 2016;46:903–909. [DOI] [PubMed] [Google Scholar]
- 47.Naparstek L Carmeli Y Chmelnitsky I, et al. Reduced susceptibility to chlorhexidine among extremely-drug-resistant strains of Klebsiella pneumoniae. J Hosp Infect. 2012;81:15–19. [DOI] [PubMed] [Google Scholar]
- 48.Taheri N Ardebili A Amouzandeh-Nobaveh A, et al. Frequency of antiseptic resistance among Staphylococcus aureus and coagulase-negative staphylococci isolated from a university hospital in central Iran. Oman Med J. 2016;31:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horner C, Mawer D, Wilcox M. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? J Antimicrob Chemother. 2012;67:2547–2559. [DOI] [PubMed] [Google Scholar]
- 50.Grascha P. Assessment of biocides in order to minimize the potential of bacterial resistance. Can J Infect Control. 2014;195–200. [Google Scholar]
- 51.Strich JR, Palmore TN. Preventing transmission of multidrug-resistant pathogens in the intensive care unit. Infect Dis Clin North Am. 2017;31:535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]


