Objectives:
The purpose of this study was to develop a reproducible preclinical Fusarium solani keratitis model, which would allow comparative testing of currently available antifungals (NATACYN [Alcon, Fort Worth, TX], voriconazole 1%, and amphotericin B 0.1%) as well as efficacy testing of new antifungals for translation into clinical practice in the future.
Methods:
The rabbit F. solani keratitis model was developed in New Zealand white rabbits using local and systemic immunosuppression. Infection was introduced by intrastromal injection of F. solani spores into one of the immunosuppressed rabbit eyes while the contralateral eye was a control. Progress of the infection was assessed by the clinical features, histopathology, and viable fungal counts. In this study, the efficacy of currently available antifungals (NATACYN [Alcon], voriconazole 1%, and amphotericin B 0.1%) was compared. Rabbits were randomly divided (n=4 in each group), and the respective antifungal was instilled topically 5 times/day for 7 days. Treatment effects were analyzed by evaluating the anterior segment with the help of slit-lamp, histopathological findings and viable fungal culture at the end of the experiment.
Results:
We report the development of a reproducible and progressive rabbit F. solani keratitis model as shown by the substantial viable fungal counts (3 log CFU), the presence of large patchy lesions and substantial hypopyon in the 12-day model correlated with specific histopathological analysis for fungus (extended F. solani hyphae from midcorneal stroma into the anterior chamber and traverse Descemet membrane with anterior chamber suppurative plaque). Voriconazole 1% and NATACYN revealed significant reduction of the fungal wound area (P=0.02 and 0.021), respectively, while amphotericin B 0.1% exhibited P value of 0.083 compared with their infected nontreated controls. Voriconazole 1% and amphotericin B 0.1% showed significant viable fungal count differences (P=0.004 and 0.01), respectively, whereas P value of NATACYN was 0.337 compared with control infected corneas.
Conclusion:
The reported rabbit fungal keratitis model can be used for screening new antifungals and evaluating currently available antifungals to facilitate better clinical outcomes. Voriconazole 1% showed the best efficacy among the three tested currently available antifungals by showing the significant differences in both wound size and viable fungal count comparisons in our F. solani rabbit keratitis model.
Key Words: Antifungals, Efficacy, Infection, Experimental animal models, Cornea
Corneal infections or diseases are the second major leading cause of blindness worldwide after cataract according to the WHO report.1 Approximately 1.5 to 2 million new cases of corneal blindness have been reported annually due to ocular trauma and corneal ulceration.1 Corneal keratitis, inflammation of the layers of the cornea, can be caused by bacterial, viral, protozoa, or fungal organisms, which invade the corneal layers, causing inflammation of corneal tissue, destruction of the layers, and, ultimately, blindness. Of these organisms, fungi remain as one of the most challenging pathogens for the ophthalmologists to diagnose and treat effectively.2 One report suggests that fungal keratitis can be more virulent, damaging to corneal tissue, and leading to corneal perforation compared with bacterial keratitis.3 Seventy species of fungal organisms are reported, and 2 major fungal organisms, filamentous fungi or yeast, are stated to cause fungal keratitis.4 Yeasts commonly inhabit the digestive or genital tract, skin, and environment5 while filamentous fungi are frequently found in the environment such as soil, water, and air in the form of spores.6 Yeast-related keratitis (mainly Candida species) is commonly found as the pathogen in 30% to 52% of fungal keratitis occurring in temperate climate countries such as Australia, Northern United States, and Europe.7,8 Filamentous fungi-related keratitis (mainly Fusarium and Aspergillus) commonly occur in tropical climates such as Southern United States, Mexico, Africa, China, South America, Central America, Middle East, India, and Southeast Asia.2,9 The greater risk of corneal destruction and visual morbidity, poor prognosis,10 and the past Fusarium outbreak11,12 has stimulated the need for developing a standard, reproducible rabbit model to compare and to develop better treatments. There have been several reports published in the literature regarding the Fusarium solani keratitis rabbit model using diverse methodologies. Most of the reported studies use the method of direct intrastromal inoculation of fungal spores into the naive rabbits' cornea.13–18 A few studies have used subconjunctival injection of steroids to suppress the local immunity before infection into the corneal stroma19–21 while others used systemic immunosuppression of dexamethasone or cortisone to suppress the systemic immunity.22,23 Hence, we have used an integrated approach to achieve a successful F. solani rabbit keratitis model and reported a useful, reproducible rabbit model of F. solani keratitis which we used to compare three commonly used commercially available antifungals.
MATERIALS AND METHODS
Ethical Statement
New Zealand white rabbits (combination of male and female) were purchased from the National University of Singapore. All animal research conducted in this study followed the SingHealth Institutional Animal Care and Use Committee guidelines, and all the animal experiments were in accordance with the recommendations of the Association for Research in Vision and Ophthalmology for the conduct of animal research. All rabbits were initially examined by slit-lamp with fluorescein application to ensure that the anterior segment was clinically normal.
F. solani Keratitis Model
Rabbits were immunosuppressed by subconjunctival injection of dexamethasone 0.8 mg once/day for 5 days only before infection. In addition, dexamethasone 0.1% was applied topically 5 times/day for 5 days before infection and for 5 days after infection. A standard systemic immunosuppression protocol used for rodents was applied with minor changes.24 In brief, cortisone acetate 100 mg/kg and cyclophosphamide 100 mg/kg were given subcutaneously at 2 days before infection and 3 days after infection. Rabbits received ceftazidime (50 mg/kg) subcutaneously every day from the beginning of the systemic immunosuppressive protocol. Sedation of rabbits was performed by injecting the combination of ketamine 35 mg/kg and xylazine 5 mg/kg intramuscularly, and local anesthesia was achieved by the topical application of lignocaine (5 mg/mL) before the infection procedure. F. solani ATCC 46492 was subcultured onto potato dextrose agar (PDA) at 30°C for 3 to 5 days. 0.1% Tween 80 in sterile phosphate-buffered saline (PBS) was used to wash the fungal growth, and the suspension was filtered through sterile gauze to remove hyphal elements. An initial inoculum ≈7×105 colony-forming units (CFU), 15 μL was administered by midstromal injection (estimated depth—200 μm) of a fungal spore suspension using BD insulin syringe with 31-G needle to the right eye while the left eye remained normal. Rabbits were followed for up to 12 days. Euthanasia was achieved by intracardiac injection of sodium pentobarbital 100 mg/kg in the sedated rabbits.
Disease Progression Follow-up by Slit-Lamp Microscopy
Infected rabbit corneas were monitored using a new-generation Zoom clinical Slit Lamp, NS-2D, Righton, Japan, at 1, 5, 8, and 12 days after infection (DPI). Minims fluorescein sodium eye drops (Bausch and Lomb, 2% wt/vol) was used to check for the presence of an ulcer with the aid of cobalt-blue filter equipped slit-lamp biomicroscopy at DPI-5 and 12. Corneal ulcer wound area was calculated by ImageJ 1.52k.
Efficacy Testing of Three Commercially Available Antifungals
NATACYN (natamycin ophthalmic suspension 5%, Alcon, Fort Worth, TX), voriconazole 1%, and amphotericin B 0.1% prepared in PBS (pH∼7) by Singapore General Hospital laboratory were used to treat the rabbit Fusarium keratitis model. Three independent experiments were performed: (1) natamycin 5% and control, (2) voriconazole 1% and control, and (3) amphotericin B 0.1% and control, whereas the control group was the F. solani–infected group with PBS treatment. Four animals (n=4) were used for each group. All the treatments started at DPI-5, and topical application of the respective antifungal was performed 5 times/day for 7 days. At the end of the experiment, rabbits were sacrificed humanely, the corneas (n=3) were dissected and homogenized in sterile PBS for fungal counts,25 and one cornea (n=1) was fixed in a mixture of 4% paraformaldehyde (PFA) (Sigma-Aldrich, St. Louis, MO) and 2.5% neutral-buffered formalin solution (Leica Surgipath; Leica Biosystems Richmond, Inc., Richmond, IL) for histopathological analysis. The homogenates were serially diluted in sterile PBS, duplicated, plated in PDA, and incubated at 30°C for 3 to 5 days. Results were recorded as the log10 number of CFU/cornea,26 and the difference in log CFU/cornea between the experimental and control groups was reported. Viable fungal counts and corneal wound sizes at 7 days post-treatment (DPT) were compared for their statistical difference between the treatment group and its respective control group. Data were analyzed by the Mann–Whitney U test (PASW statistic 18), and P value was set to be significant at 0.05.
Histopathological Assessments of Fusarium Keratitis and Antifungal Treatments
Fixed corneal specimens were sent to the Ophthalmic Pathology service at the Singapore National Eye Center/Singapore General Hospital in a mixture of 4% paraformaldehyde (PFA) (Sigma-Aldrich) and 2.5% neutral-buffered formalin solution (Leica Surgipath; Leica Biosystems Richmond, Inc.). They were processed and embedded in paraffin (Leica-Surgipath; Leica Biosystems Richmond, Inc.) according to standard clinical protocols. Four-micrometer sections were then cut, and sections dried in an oven at 37°C for at least 24 hr. To prepare the sections for histochemical stains, the sections were heated on a 60°C plate warmer, deparaffinized in xylene, and rehydrated in decreasing concentrations of ethanol. Based on previously published protocols, hematoxylin and eosin stain and Grocott–Gömöri methenamine silver (GMS) stain were performed.27,28 Sections were then read using standard light microscopy (Olympus BX-40) and imaged on a Philips Digital Pathology Solution platform.
RESULTS
Rabbit F. solani Keratitis Model
To define the model after preliminary work, the corneas of four animals (n=4) were inoculated. At DPI-1, small satellite lesions developed in the central cornea where the fungal spores were injected (Fig. 1A). The lesion extent increased in size as the presence of patchy lesions was observed and corneal haze was observed by slit-lamp microscopy at DPI-5 (Fig. 1B). In half of the experimental animals (n=2), hypopyon was present starting from DPI-5 (Fig. 1B). Progression of the infection continued to DPI-12 as noted by the presence of increased large patchy lesions and substantial hypopyon (Fig. 1D). Corneal ulcers were detected at the patchy lesions (wound size=4.72 mm2) starting at DPI-5 and ulcer increased to 9.14 mm2 at DPI-12 as shown by Minims fluorescein sodium eye drops (Fig. 1G). Central corneal ulcers were observed in all the experimental rabbits. The above findings suggested that a full-blown F. solani keratitis infection was in place at DPI-5, and DPI-5 was set as a starting time point for the treatment part of the experiment. Initially, an ulcer was localized at the fungal spore injection site. Corneal neovascularization was evident at DPI-12 at 2 and 9 o'clock positions in half of the experimental rabbits (Fig. 1E, F). There was no lid edema or secretion in all the experimental animals during the course of infection. Fungal retrieval count was 3.4 log CFU.
FIG. 1.
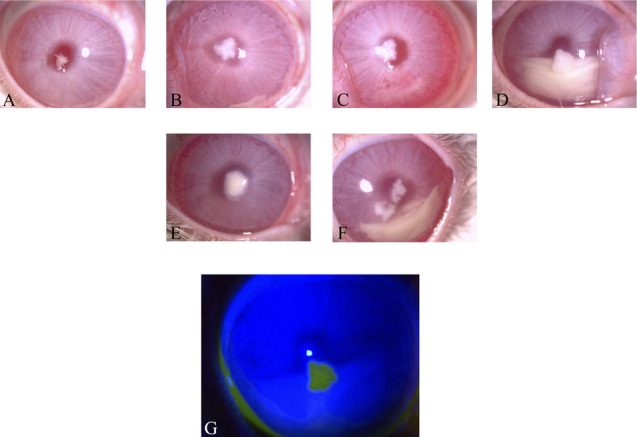
Slit-lamp microscopy images of rabbit F. solani keratitis model (A) DPI-1, (B) DPI-5, (C) DPI-8, and (D) DPI-12. Corneal neovascularization was occurred at 2 o'clock and 9 o'clock positions at DPI-12 (E and F). The corneal wound was visualized in the DPI-12 fluorescein-stained slit-lamp microscopy image (G). DPI, days postinfection. *P ≤ 0.05 was set to be statistically significant.
Histopathological Assessment
Inspecting slides after H&E staining revealed fungal elements composed of spores and hyphae (Fig. 2A). The hyphae were seen extending from the nidus of the infection in the midcorneal stroma into the anterior chamber and traverse Descemet membrane (Fig. 2A). The inflammatory response was seen within the corneal stroma with a marked suppurative anterior chamber endothelial plaque (Fig. 2A). GMS stain also showed fungal hyphae extending from the midstroma to the peripheral parts (Fig. 2B).
FIG. 2.
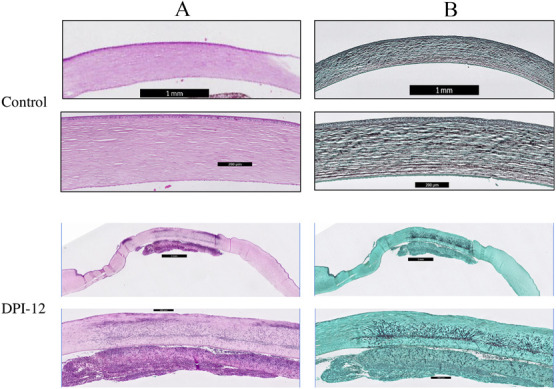
(A) Hematoxylin and eosin staining images of normal uninfected cornea after immunosuppressive regimes and DPI-12. Presence of fungal hyphae and spores was observed in the midcorneal stroma accompanying with the presence of suppurated anterior chamber endothelial plague. (B) Grocott–Gömöri methenamine silver staining images showing the confirmation of fungal hyphae presence in the midstroma of DPI-12 sample. DPI, days postinfection.
Efficacy of Three Commercially Available Antifungals
After establishing the fungal infection model, the next step was to determine its usefulness in evaluating current antifungals. Natamycin, commercially available as NATACYN, along with voriconazole 1% and amphotericin B underwent testing in the rabbit Fusarium keratitis model to determine their comparative efficacy for this infection.
Treatment was started at DPI-5 when the infected cornea was cloudy with a central corneal ulcer associated with intense conjunctival inflammatory responses. Effects of different treatment are summarized in Table 1.
TABLE 1.
Summary Table of Different Treatment Effects in Experimental F. solani Keratitis Rabbit Model
| Clinical Response | Corneal Fungal Wound Size Difference at DPT-7 | Histopathological Analysis | Log Reduction of Viable Fungal Counts at DPT-7 | |
| Efficacy testing of NATACYN (natamycin ophthalmic suspension 5%, Alcon) | Decrease in conjunctival inflammation (Fig. 3B) | 4.03 mm2 (Fig. 3B) | Slight decrease in fungal burden (Fig. 3B) | 0.77 (Fig. 4A) |
| Efficacy testing of voriconazole 1% | Marked inhibition of conjunctival inflammation (Fig. 3C) | 5.6 mm2 (Fig. 3C) | Significant reduction of fungal loads (Fig. 3C) | 0.99 (Fig. 4B) |
| Efficacy testing of amphotericin B 0.1% | Distinct decrease in conjunctival inflammation (Fig. 3D) | 3.43 mm2 (Fig. 3D) | Noticeable inhibition of fungal loads (Fig. 3D) | 0.75 (Fig. 4C) |
DISCUSSION
The management of fungal keratitis is challenging because of the time required for diagnosis which increases the risk of a bad prognosis.10 Corneal trauma,29 contact lens wear,7 topical steroid use,29 corneal surface disorders, dry eye,30 and laser-assisted in situ keratomileusis31 have been identified as risk factors for fungal keratitis. A recent article from the Asia Cornea Society stated that filamentous fungi, Fusarium species, and Aspergillus flavus, are the first and third most common microorganism causing keratitis respectively in an Asian multicenter study.32 Owing to the clinical difficulties with fungal keratitis and F. solani in particular, there have been a number of research reports describing experimental animal models for the study of the pathogenesis and efficacies of antifungals using rabbit model with different experimental methodologies (Table 2).13–23 The studies have shown mixed results for establishing a F. solani rabbit keratitis model (in terms of clinical features, viable CFU, and total experimental time reflective of sustained experimental infection duration [Table 2]13–23). F. solani keratitis is difficult to maintain for a long period without losing its pathologic features and viable fungal count retrieval. It was reported that local pretreatment with steroid was essential for achieving sustained F. solani keratitis infection in rabbits,20 and we have integrated both local and systemic immunosuppressive techniques while maintaining its pathologic features to develop the sustained and progressive F. solani rabbit keratitis for us to study the antifungals treatment effects over extended times Figure 1A–G. It was also observed that some of the animal models developed from previous reports yielded relatively few viable fungal counts which could lead to the false-negative reports (Table 2).13–23 In this report, we have developed a highly reproducible rabbit Fusarium solani keratitis model evidenced by the substantial fungal retrieval amount and similar histopathogenic figures as found for human fungal keratitis (Figs. 1A–G and 2). The fungal retrieval count was stable with approximately 3 log CFU in all 4 different independent experiments, and all animals in each independent experiment successfully developed an active, progressive infection F. solani keratitis (Figs. 1F and 4). Dendritic ulcers appeared at DPI-1 in our model which is also noted as a common occurrence in patients (Fig. 1A).33 Feathery borders or hyphae edges and the presence of hypopyon in our rabbit model were seen as well and these are also among the most common clinical findings in patients diagnosed with F. solani keratitis (Fig. 1B–D).33 Moreover, our model also showed minimum inflammation and the absence of lid edema, which are similar to reports from infected patients (Fig. 1).33 Most of the studies conducted 8 days of experimental infection model (3 days' waiting period for full-blown infection and 5 days for the treatment).16,17,19,21 However, the delayed diagnosis and the requirement of prolonged treatment duration of F. solani keratitis in real scenario prompted us to study the late treatment effects with prolonged duration in our sustained F. solani rabbit keratitis model.33 The late treatment effects (5 days' waiting period for full-blown infection) of commercially available antifungals for a prolonged duration (7 days) have been studied in our F. solani rabbit keratitis model (Figs. 3B–D and 4).
TABLE 2.
Different Experimental F. solani Rabbit Keratitis Models
| Animal Infection Procedure | Clinical Features | Experimental Evaluation | Fungal Counts | Tested Antifungals and Its Effects | Reference |
| Injection of F. solani spores into the corneal stroma. | F. solani keratitis started 3 days after infection. | Slit-lamp microscopy, histopathology, and viable fungal counts | 2.5 log CFU | Corneal cross-linking was found to be effective. | 13 |
| Inoculation of F. solani spores into the corneal stromal incision | F. solani keratitis started 2 days after inoculation. | Slit-lamp microscopy | No CFU data | Natamycin was found to be effective in controlling F. solani keratitis. | 14 |
| Injection of F. solani spores into the corneal stroma. | Clinical features were evident at 3 days postinfection. | Slit-lamp microscopy, histopathology, and viable fungal counts | Approximately 3.3 log CFU | Combination of corneal collagen cross-linking (PACK-CL) and voriconazole was useful to manage the early stage of F. solani keratitis. | 15 |
| Amphotericin B 0.15%, itraconazole 1% and voriconazole 1% were found to be effective. | 16 | ||||
| Topical caspofungin was effective in controlling F. solani keratitis. | 17 | ||||
| Intrastromal injection of F. solani spores | F. solani corneal ulcer developed 7 days after inoculation. | Slit-lamp microscopy, histopathology, and viable fungal counts | 1.6 log CFU | Intrastromal voriconazole injection was more effective than topical natamycin and topical voriconazole. | 18 |
| Local immunosuppressant for 5 days and inoculation of F. solani spores into the corneal stromal incision | F. solani keratitis started 5 days after inoculation. | Slit-lamp microscopy and viable fungal counts | Fungal count was zero in Sabouraud agar after 1 day incubation in Brain Heart Infusion (BHI) agar | Topical 0.5% povidone-iodine demonstrated no advantages in the management of F. solani keratitis when compared with 5% natamycin. | 19 |
| Intrastromal injection of F. solani spores with local immunosuppression | Small infiltrated lesion was evident as early as 2 days postinfection. | Slit-lamp microscopy, histopathology, and protease analysis | No CFU data | Mechanism of matrix turnover in F. solani keratitis was investigated. | 20 |
| Local immunosuppressant for 5 days and intrastromal injection of F. solani spores | Fungal keratitis developed 3 days after inoculation and severe inflammation was evident at 8 days after inoculation. | Slit-lamp microscopy, histopathology, and viable fungal counts | 2 log CFU | Combination of ultraviolet A and voriconazole was more effective than voriconazole alone. | 21 |
| Topical application of F. solani spores into the scratched cornea after systemic immunosuppression for 3 days | F. solani keratitis started 5 days after inoculation | Slit-lamp microscopy, histopathology, and viable fungal counts | 1.2 log CFU | Combination of voriconazole and epigallocatechin gallate was effective in treating F. solani keratitis. | 22 |
| Intrastromal injection of F. solani spores into the scratched cornea after systemic immunosuppression for 3 days | F. solani keratitis started 3 days after infection. | Slit-lamp microscopy, histopathology, and confocal microscopy | No CFU data | Combination of cryotherapy and antifungal agents was effective in treating F. solani keratitis. | 23 |
CFU, colony-forming unit.
FIG. 4.
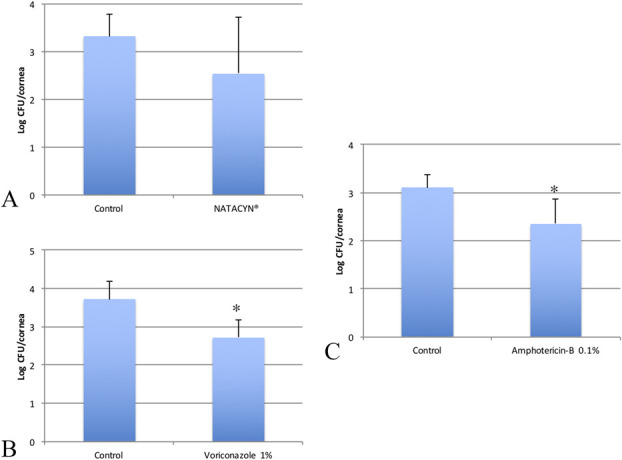
Viable fungal loads in log colony-forming units (CFU) of (A) NATACYN, (B) voriconazole 1%, and (C) amphotericin B 0.1% in the rabbit F. solani keratitis model at DPT-7. Histograms show the comparison of viable fungal counts in log CFU after different treatments. Mean and SDs are shown. (A, NATACYN vs. control, P=0.337) (B, voriconazole 1% vs. control, P=0.004) (C, amphotericin B 0.1% vs. control, P=0.01).
FIG. 3.
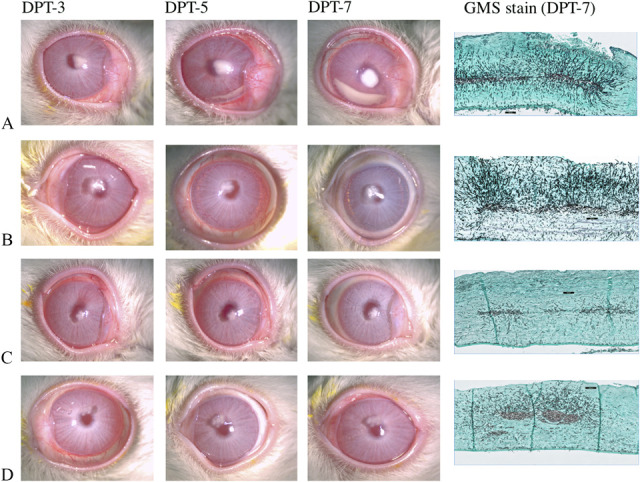
Slit-lamp microscopy and GMS images of (A) control, (B) NATACYN, (C) voriconazole 1%, and (D) amphotericin B 0.1% along the course of rabbit F. solani keratitis treatment efficacy. Rabbit corneal fungal wound was reduced in all the treatments (amphotericin B 0.1% vs. control, P=0.083) along with the significant amount of reduction of fungal wound area in NATACYN (P=0.021) and voriconazole 1% (P=0.02) treatment groups. The best control slit-lamp microscopy and GMS images were chosen as representatives from three independent efficacy experiments. GMS, Grocott-Gömöri Methenamine Silver.
The reports have been published in the literature showing the effectiveness of voriconazole in the animal model,15,16,18,21,22 and most of the animal models used 3 days of a waiting period to initiate the treatment which is indicated for treating superficial infections.15,16,21 A report suggested that filamentous fungi tended to grow toward the anterior chamber as the infection progresses.34 Five days of a waiting period was used in this study to start the treatment for the F. solani to settle in the deep stroma. Histopathology findings also revealed deep stromal or anterior chamber invasion of F. solani (Fig. 2A). To the best of our knowledge, our rabbit fungal keratitis model is the first experimental model showing the anterior chamber invasion of F. solani from corneal stroma. It could be considered as a severe or deep infection model, representing many actual clinical cases which reveal deep-lying infection or anterior chamber involvement, which is complicated to treat due to poor penetration of antifungals and natamycin in particular.35,36
We have compared 3 often used antifungals. Amphotericin B 0.1%–treated rabbit group showed that there was a significant decrease in fungal load evidenced by CFU count and histopathology (Figs. 3D and 4C) as shown by these studies.16,17 However, its clinical use is limited by its toxicity.37 Natamycin, the only US Food and Drug Administration–approved topical antifungal, showed minimal effectiveness in decreasing the fungal burden in histopathology experiments and fungal load in CFU in our study (Figs. 3B and 4A). Similar findings were found in the report that natamycin has poor penetration into the corneal stroma and does not provide good clinical outcomes.35,36 In our study, voriconazole 1% showed the best antifungal activity in terms of CFU, wound area, and histopathological findings (Figs. 3C and 4B).
In conclusion, a new model of clinically relevant F. solani rabbit keratitis model is developed, and we have reported the first experimental antifungal drug efficacy study showing that voriconazole 1% is superior to NATACYN and amphotericin B 0.1%, suggesting the use of voriconazole 1% as a first-line treatment in the clinical management of F. solani. Efficacy testing of these three antifungals against other pathogenic filamentous fungi in this rabbit model is urgently warranted for assistance to ophthalmologists in managing affected patients.
Footnotes
The authors have no conflicts of interest to disclose.
Supported by TCR R1018.
REFERENCES
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: Diagnosis and treatment. Curr fungal Infect Rep 2013;7:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong TY, Ng TP, Fong KS, et al. Risk factors and clinical outcomes between fungal and bacterial keratitis: A comparative study. CLAO J 1997;23:275–281. [PubMed] [Google Scholar]
- 4.Thomas P, Kaliamurthy J. Mycotic keratitis: Epidemiology, diagnosis and management. Clin Microbiol Infect 2013;19:210–220. [DOI] [PubMed] [Google Scholar]
- 5.Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence 2017;8:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egbuta MA, Mwanza M, Babalola OO. A review of the ubiquity of ascomycetes filamentous fungi in relation to their economic and medical importance. Adv Microbiol 2016;6:1140. [Google Scholar]
- 7.Gower EW, Keay LJ, Oechsler RA, et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology 2010;117:2263–2267. [DOI] [PubMed] [Google Scholar]
- 8.Gaujoux T, Borsali E, Goldschmidt P, et al. Fungal keratitis in France. Acta ophthalmologica 2011;89:e215–e216. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh AK, Gupta A, Rudramurthy SM, et al. Fungal keratitis in North India: Spectrum of agents, risk factors and treatment. Mycopathologia 2016;181:843–850. [DOI] [PubMed] [Google Scholar]
- 10.Rogers GM, Goins KM, Sutphin JE, et al. Outcomes of treatment of fungal keratitis at the University of Iowa Hospitals and Clinics: A 10-year retrospective analysis. Cornea 2013;32:1131–1136. [DOI] [PubMed] [Google Scholar]
- 11.Bullock JD, Elder BL, Khamis HJ, et al. Effects of time, temperature, and storage container on the growth of Fusarium species: Implications for the worldwide Fusarium keratitis epidemic of 2004-2006. Arch Ophthalmol 2011;129:133–136. [DOI] [PubMed] [Google Scholar]
- 12.Khor WB, Aung T, Saw SM, et al. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA 2006;295:2867–2873. [DOI] [PubMed] [Google Scholar]
- 13.Galperin G, Berra M, Tau J, et al. Treatment of fungal keratitis from Fusarium infection by corneal cross-linking. Cornea 2012;31:176–180. [DOI] [PubMed] [Google Scholar]
- 14.Dong XH, Gao WJ, He XP. Antifungal efficacy of natamycin in experimental Fusarium solani keratitis. Int J Ophthalmol 2012;5:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Özdemir HB, Kalkanci A, Bilgihan K, et al. Comparison of corneal collagen cross‐linking (PACK‐CXL) and voriconazole treatments in experimental fungal keratitis. Acta Ophthalmol 2019;97:e91–e96. [DOI] [PubMed] [Google Scholar]
- 16.Yavas GF, Öztürk F, Küsbeci T, et al. Antifungal efficacy of voriconazole, itraconazole and amphotericin b in experimental Fusarium solani keratitis. Graefe's Arch Clin Exp Ophthalmol 2008;246:275–279. [DOI] [PubMed] [Google Scholar]
- 17.Ozturk F, Yavas GF, Kusbeci T, et al. Efficacy of topical caspofungin in experimental Fusarium keratitis. Cornea 2007;26:726–728. [DOI] [PubMed] [Google Scholar]
- 18.Nejabat M, Yaqubi N, Khosravi A, et al. Therapeutic effect of intrastromal voriconazole, topical voriconazole, and topical natamycin on Fusarium keratitis in rabbit. J Ophthalmol 2016;2016:8692830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira LAd, Takata TT, Shiguematsu AI, et al. Effect of topical 0.5% povidone-iodine compared to 5% natamycin in fungal keratitis caused by Fusarium solani in a rabbit model: A pilot study. Arq Bras Oftalmol 2008;71:860–864. [DOI] [PubMed] [Google Scholar]
- 20.Gopinathan U, Ramakrishna T, Willcox M, et al. Enzymatic, clinical and histologic evaluation of corneal tissues in experimental fungal keratitis in rabbits. Exp Eye Res 2001;72:433–442. [DOI] [PubMed] [Google Scholar]
- 21.Choi KS, Yoon SC, Rim THT, et al. Effect of voriconazole and ultraviolet: A combination therapy compared to voriconazole single treatment on Fusarium solani fungal keratitis. J Ocul Pharmacol Ther 2014;30:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruban VV, Archana PT, Sundararajan M, et al. Inflammation and oxidative stress in corneal tissue in experimental keratitis due to Fusarium solani: Amelioration following topical therapy with voriconazole and epigallocatechin gallate. Mycoses 2018;61:159–171. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Yang W, Gao M, et al. Experimental study on cryotherapy for fungal corneal ulcer. BMC Ophthalmol 2015;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard DC, Rieg G, Chiang LY, et al. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2004;48:1908–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazlett LD, McClellan S, Kwon B, et al. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest Ophthalmol Vis Sci 2000;41:805–810. [PubMed] [Google Scholar]
- 26.Aung TT, Chor WHJ, Yam JKH, et al. Discovery of novel antimycobacterial drug therapy in biofilm of pathogenic nontuberculous mycobacterial keratitis. Ocul Surf 2017;15:770–783. [DOI] [PubMed] [Google Scholar]
- 27.Lion T. Human Fungal Pathogen Identification. New York, NY: Humana Press; Springer, 2017. [Google Scholar]
- 28.Wang X, Teoh CKG, Chan AS, et al. Biomechanical properties of Bruch's membrane–choroid complex and their influence on optic nerve head biomechanics. Invest Ophthalmol Vis Sci 2018;59:2808–2817. [DOI] [PubMed] [Google Scholar]
- 29.Bharathi MJ, Ramakrishnan R, Vasu S, et al. Epidemiological characteristics and laboratory diagnosis of fungal keratitis: A three-year study. Indian J Ophthalmol 2003;51:315. [PubMed] [Google Scholar]
- 30.Tanure MAG, Cohen EJ, Sudesh S, et al. Spectrum of fungal keratitis at Wills eye hospital, Philadelphia, Pennsylvania. Cornea 2000;19:307–312. [DOI] [PubMed] [Google Scholar]
- 31.Verma S, Tuft S. Fusarium solani keratitis following LASIK for myopia. Br J Ophthalmol 2002;86:1190–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khor W-B, Prajna VN, Garg P, et al. The Asia Cornea Society Infectious Keratitis Study: A prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol 2018;195:161–170. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol 2004;15:321–327. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Chandra J, Mukherjee P, et al. A murine model of contact lens–associated Fusarium keratitis. Invest Ophthalmol Vis Sci 2010;51:1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'day DM, Head WS, Robinson RD, et al. Corneal penetration of topical amphotericin B and natamycin. Curr Eye Res 1986;5:877–882. [DOI] [PubMed] [Google Scholar]
- 36.Pradhan L, Sharma S, Nalamada S, et al. Natamycin in the treatment of keratomycosis: Correlation of treatment outcome and in vitro susceptibility of fungal isolates. Indian J Ophthalmol 2011;59:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster CS, Lass JH, Moran-Wallace K, et al. Ocular toxicity of topical antifungal agents. Arch Ophthalmol 1981;99:1081–1084. [DOI] [PubMed] [Google Scholar]


