Supplemental Digital Content is available in the text.
Key Words: lumbar spine, bone graft substitute, posterior lumbar interbody fusion, glass ceramics, titanium cage, clinical trial
Study Design:
This is a prospective, stratified randomized, multicenter, 4-year follow-up study.
Objective:
The authors aimed to evaluate the long-term clinical efficacy and safety of CaO-SiO2-P2O5-B2O3 glass ceramics (BGS-7) spacers in 1-level posterior lumbar interbody fusion (PLIF) at a 4-year follow-up.
Summary of Background Data:
According to 1-year follow-up results, BGS-7 spacer showed similar fusion rates and clinical outcomes compared with titanium cage. A long-term follow-up study beyond 2 years is necessary to investigate the status of intervertebral bone graft volumes. Moreover, longer follow-up is mandatory to also evaluate the safety and efficacy of BGS-7 spacers, because they remain in the intervertebral space for a long time.
Materials and Methods:
In this prospective, randomized, multicenter, 4-year follow-up study, we evaluated 62 of the 74 patients who underwent 1-level PLIF. During 1-level PLIF, titanium cages filled with autologous local bone were inserted into the control group patients and BGS-7 spacers were inserted to the experimental group patients. Bone fusion was evaluated by plain radiography and thin-section computed tomography. Visual Analog Scale (VAS), the Oswestry Disability Index (ODI), Short Form-36 Health Survey (SF-36), and evaluation of safety were conducted after 48 months.
Results:
Computed tomography scan showed a bone fusion rate of 90.6% in the BGS-7 spacer group and 93.3% in the control group, with no significant differences between groups. The BGS-7 spacer group showed a significantly larger area directly fused to the endplate than the control group (P<0.001). The BGS-7 spacer group showed a significant increase in the fused area compared with the titanium group at 1- and 4-year follow-up. The ODI, SF-36, back pain, and lower limb pain in both groups showed significant improvement after surgery, and no significant differences were observed between the groups. Both groups showed no additional adverse events.
Conclusions:
The 4-year follow-up study showed similar fusion rates and clinical outcomes in both the BGS-7 spacer and autologous bone with a titanium cage in 1-level PLIF. However, the BGS-7 spacer implants showed a larger area of fusion with the endplates than that of autologous bone with a titanium cage. Therefore, the results demonstrated that the BGS-7 spacer can be considered as a novel intervertebral spacer to achieve successful spinal fusion without safety concerns for long-term use.
Lumbar interbody usion is one of the numerous treatment options in patients with degenerative lumbar spinal diseases. Interbody fusion offers the following advantages: (1) it transmits weight through anteriorly positioned interbody cages or spacers and is the most effective method for augmenting intervertebral disk space; (2) it removes the intervertebral disk, which is the source of pain1–4; and (3) cage implantation restores intervertebral height because of the indirect decompression of foraminal stenosis.5
For the lumbar interbody fusion, the intervertebral cages or spacers can be inserted into the disk space after discectomy. Titanium metal is commonly used as a material for the intervertebral cages owing to its biocompatibility, corrosion resistance, and cell adhesion properties, which are associated with high osseointegration.
However, titanium cage can penetrate into the vertebral body, causing subsidence because of the stress-shielding effect and the differences in the modulus of elasticity. Moreover, metallic artifacts due to the titanium render the evaluation of bone fusion status after surgery difficult.6 A previous study reported that the autologous local bone graft in titanium cage for posterior lumbar spinal fusion showed a poor ratio of the fused area of local bone inside the cages (<50%), which indicated the insufficiency for load transmission.7
The intervertebral cages are usually used with a bone graft including autologous bone, allogenic bone, or bone graft extenders. Calcium phosphate ceramics such as hydroxyapatite, beta-tricalcium phosphate, and beta-calcium pyrophosphate have been evaluated as bone graft extenders for spinal fusion.8,9 However, their use in load-bearing areas such as the intervertebral space has been limited because of weak mechanical properties such as brittleness, poor fatigue resistance, and anisotropy. To overcome these limitations, bioactive glass-ceramics with improved mechanical strength have been developed for bone graft and repair. These bioactive glass-ceramics form apatite layers in the physiological condition of the bone and chemically bind to bone directly, which results in improved bone-bonding strength.10–12 Among the many types of bioactive glass ceramics, CaO-SiO2-P2O5-B2O3 glass-ceramics (BGS-7) have been reported to induce osteoblastic differentiation of human mesenchymal stem cells, which results in improved bone-implant contact ratio.13–15 Furthermore, the intravenous administration of BGS-7 in rats did not show any toxicity for 90 days.16 For these reasons, BGS-7 spacer for spinal fusion surgery was developed based on finite element modeling for stress distribution, and it showed great mechanical properties as an intervertebral spacer in an experimental study.17
In the previous study, we reported the efficacy and safety of BGS-7 spacers compared with autograft using titanium cages in the patients who required 1-level posterior lumbar interbody fusion (PLIF).18 The BGS-7 spacers showed similar fusion rates and clinical outcomes as autograft using titanium cages, and the number of adverse events after surgery was similar for both groups. However, the previous study evaluated radiologic status for only 6 months and 1 year after the surgery, thereby lacking long-term results. Generally, an increase in the volume of intervertebral bone grafts after 2 years is not expected based on the diagnosis of pseudoarthrosis at 2 years after the operation. However, Ito et al19 reported an increase in the intervertebral bone graft volumes between the second and the fifth years after surgery. Therefore, long-term follow-up study beyond 2 years is necessary to investigate the status of intervertebral bone graft volumes.
The present work is intended as a follow-up study of previous studies, which is a long-term follow-up evaluation of the safety and effectiveness of BGS-7 spacers compared with autograft using titanium cage in 1-level PLIF.
MATERIALS AND METHODS
Study Design
The previous study was performed for the subjects at 4 institutions to evaluate whether the BGS-7 spacer (NovoMax; CGBio Inc., Seongnam, Korea) implantation is noninferior to the implantation of titanium alloy (Ti-6Al-4V) cages (4CIS; Solco Biomedical, Seoul, Korea) filled with autologous bone in 1-level PLIF for a year.18 The study was conducted between October 28, 2010, and September 13, 2013, and was approved by the Institutional Review Board (IRB) of each institution. This study was carried out to assess changes in bone fusion rate and clinical results at follow-up for at least 48 months from the patients of the previous study. This trial was registered in ClinicalTrials.gov (NCT03532945) and was conducted according to the Declaration of Helsinki and guidelines for Good Clinical Practice.
The inclusion criteria of the previous study were as follows: (1) patient age was between 30 and 80 years; (2) patients who required 1-level PLIF between L1 and S1 among those who required an extensive laminectomy or facetectomy to correct severe disk extrusion or severe spinal stenosis or those who required PLIF owing to grade I or II spondylolisthesis; and (3) those who (only if a signature was obtainable), or whose legal guardian, fully understood the clinical trial details and signed the informed consent form.
The exclusion criteria of the previous study were as follows: (1) patients with osteoporosis with average T scores of L1–L4 at <–3.0 in DEXA bone density tests; (2) women with positive pregnancy tests before the trial or who planned to become pregnant within the following 3 years; (3) patients with a history of malignant tumor or malignant diseases (but the cases of cured disease without relapse for the past 5 years were included in the present study); (4) patients with abnormal blood potassium and phosphorus levels; (5) patients with liver disease, kidney disease, respiratory disease, metabolic disease, or psychological disease; (6) patients deemed to have <1-year life expectancy; (7) patients with mental retardation or whose parents or legal guardians were older or had mental disabilities; and (8) other patients viewed as inappropriate by the staff.
The surgery was performed using pedicle screw fixation and the implantation of the BGS-7 spacers or the autologous bone in titanium cages for the conventional PLIF, as described in our previous study. In brief, a curette and other surgical tools were used to remove the disks completely, and the soft tissue and cartilage of the endplates were removed to trim the bone tissue. Then, the local autogenous bones collected in the decompression process were broken into fine pieces and inserted into interbody spaces at 10 mL per segment. After the insertion of local bone, the 2 medical devices that fit the intervertebral space used for the test (BGS-7 spacer), or the device comparison (titanium cage), were inserted. In the titanium cage, the local bone was crushed and inserted. Additional materials including allograft, demineralized bone matrix, and bone morphogenetic proteins were not used for either group.18
The purpose and methodology of research were explained via email or phone to the 74 study subjects (39 in the test group and 35 in the control group) who were enrolled in the previous study. The study was conducted after obtaining signed consent.
Evaluation
The primary efficacy variable was bone fusion by computed tomography (CT) at 48 months after surgery. The other variables analyzed included bone fusion by plain radiography after 48 months, Oswestry Disability Index (ODI) questionnaire, Short Form-36 Health Survey (SF-36), Visual Analog Scale (VAS) pain assessment, osteolysis, subsidence of BGS-7 spacer or titanium cage, evaluation of the area of fusion with the vertebral endplate, and safety. The evaluation of CT and x-ray used for bone fusion was performed by 2 independent experienced orthopedic surgeons who did not participate in the clinical trial.
3-Dimensional (3D) CT
3D CT (thin cut, <2-mm interval) was performed to evaluate bone fusion and the characteristics of the fusion area. If BGS-7 spacers or autogenous bone in titanium and the vertebral endplate were combined without any gaps or if the bony trabecula was connected, the cases were judged as bone fusion.20
Two spine doctors independently reviewed and scored them as “fusion,” and if >1 fusion was observed, the case was scored as “fusion.” We measured the fusion area of BGS-7 spacers or titanium cage with an upper and lower vertebral endplate. We also evaluated the fusion area where the local bone was inserted outside the titanium cage or BGS-7 spacers.7,21 Osteolysis around the titanium cage or BGS-7 spacers and subsidence to the vertebral endplate were both measured using the sagittal and coronal cuts.
Plain Radiograph
Plain radiography was performed to determine if BGS-7 spacers or the autogenous bone inserted in the titanium cage were in union with the vertebral endplates. Plain radiographs were taken in anteroposterior and lateral, lateral flexion, and degenerative photographs. As in previous studies, the criteria for fusion were as follows: (1) bony trabecula was connected between the autogenous bone inserted in the titanium cage or BGS-7 spacers and the vertebral endplate or the combined part showed no gaps and (2) the difference of the fused segment angle was <3 degrees and the dislocation was <3 mm in flexion and extension photos. On the basis of the aforementioned criteria, if 2 spine doctors review it and both of them read it as “fused,” it is judged as “fusion,” and if >1 fusion was observed, the case was scored as “fusion.”18
ODI Assessment
The ODI questionnaire survey was conducted at ≥48 months after surgery. The overall percentage was calculated by comparing the evaluation scores to the total scores of the items marked except for the items that the subjects did not answer.
SF-36
The subjects were asked to self-evaluate using the SF-36 questionnaire at 48 months after surgery. The raw score for each item was converted into an adjusted or evaluation score. The mean, SD, median, minimum, and maximum values were calculated and analyzed for the total scores in 8 areas and 2 summary areas.
VAS Score
The 100-mm VAS was used to determine the degree of pain experienced by the study subject in 3 areas of the waist and the right and left legs during the activity at 48 months after surgery.
Safety Assessment
Additional adverse events that occurred after the previous study were collected through charts, interviews, and voluntary reporting.18 The adverse events already reported in the previous studies were not investigated. Even if adverse events have already been reported, the contents were collected if symptoms persist or the follow-up was necessary after the end of the study.
Statistical Analysis
The exact binomial distribution was used to obtain 97.5% single-sided confidence interval for the difference in bone fusion rates between the experimental study groups. If the lower limit of the confidence interval was greater than the noninferiority recognition limit of −15%, it was considered as noninferior. A significance level of 0.05 was used for all analyses. R language version 3.3.0 software was used for the statistical analyses. To check the differences between the groups, a 2-sample t test or Wilcoxon rank-sum test was used for continuous variables and χ2 test or Fisher exact test for categorical variables. Fisher exact test was used to identify differences in the incidence of adverse events between the study groups, and the 95% confidence interval value for incidence was calculated.
In confirming the reliability, intraclass correlation coefficient or kappa values of all measured values showed a high degree of agreement, of >0.9 (Online Resource Supplementary Tables 1, 2, Supplemental Digital Content 1, http://links.lww.com/CLINSPINE/A123). Most of the values showed a very high degree of agreement (of about 0.8), but the measured values of the fusion area ratio item showed a degree of agreement of about 0.6 (Online Resource Supplementary Tables 3, 4, Supplemental Digital Content 1, http://links.lww.com/CLINSPINE/A123).
RESULTS
Baseline Characteristics
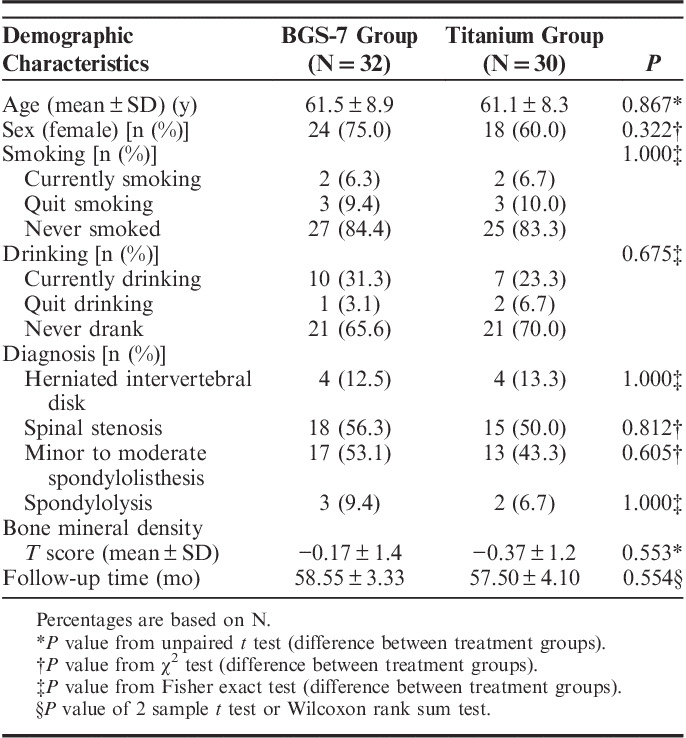
This study was conducted from November 16, 2015, to April 7, 2016, on 62 (83.8%) of the 74 patients who completed the previous study in 4 institutions. There were no significant differences between the 2 groups in mean age, sex, follow-up period, smoking history, preoperative diagnosis, or bone mineral density (Table 1).
TABLE 1.
Summary of Demographic Characteristics (Efficacy Analysis Population)
Bone Fusion and Bone Fusion Area Analysis by 3D CT
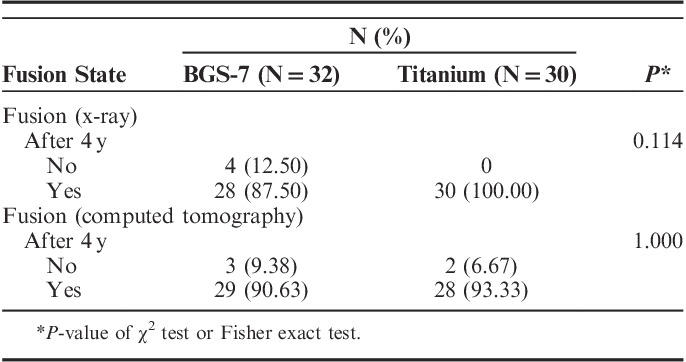
In the previous study, the 3D CT analysis showed that bone fusion at 12 months after the operation was 89.7% (35/39) in the experimental group and 91.2% (31/35) in the control group.17,18 Our analysis by 3D CT showed that after 48 months or more after the operation, the bone fusion rate was 90.6% (29/32 people) in the experimental group and 93.3% (28/30 people) in the control group, with no significant differences between the groups (P=1.0; Table 2).
TABLE 2.
Summary of X-ray and Computed Tomography Measures
According to the results of comparing the fusion area at 1 and 4 years after operation, we observed that both the upper and the lower endplates showed no significant differences in the area directly fused to the endplate outside the cage in the experimental and control groups (Online Resource Supplementary Table 5, Supplemental Digital Content 1, http://links.lww.com/CLINSPINE/A123). After 48 months, the area in the upper endplate that directly fused to the cage or the spacer was significantly larger in the experimental group (231.21±64.98 mm2) than in the control group (76.70±20.58 mm2; P<0.001) (Table 3). Moreover, the area in the lower endplate that directly fused to the cage or the spacer was also significantly larger in the experimental group (206.55±80.69 mm2) than in the control group (90.20±14.97 mm2; P<0.001).
TABLE 3.
Summary of Inside Cage Fusion Area Measures
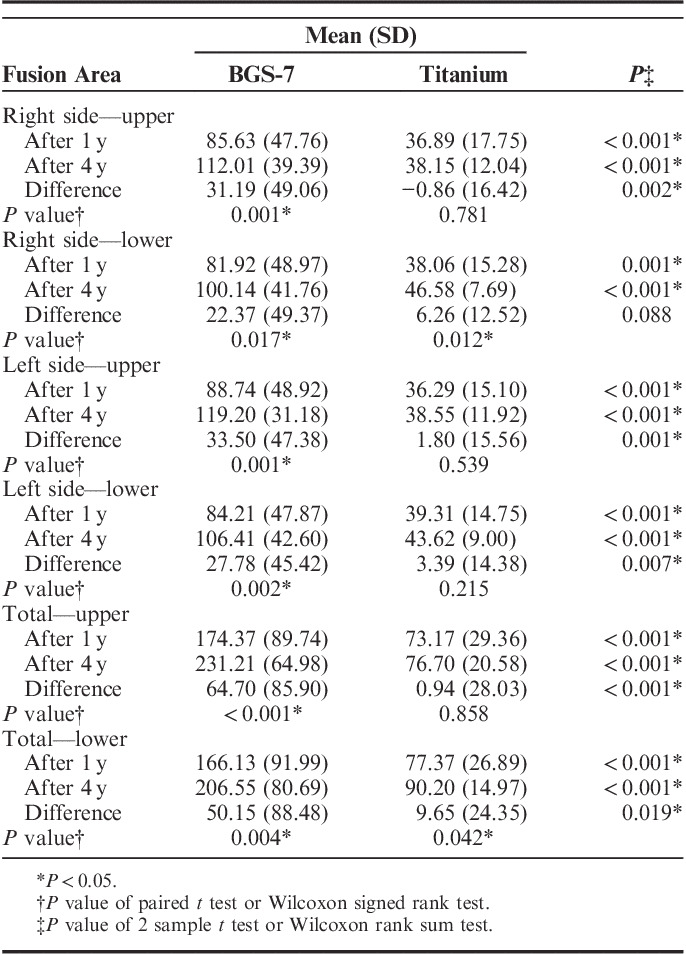
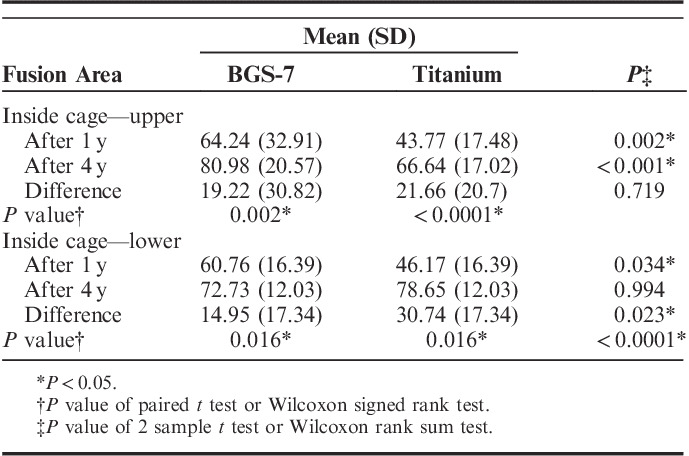
In addition, the differences in the fusion area between 1 and 4 years in each group were compared. In the experimental group, it was 64.70±85.90 (P<0.001) and 50.15±88.48 mm2 (P=0.02) in the upper endplate and lower endplate, respectively, showing a significant increase in fusion area (Table 3). In contrast, the control group did not show a significant increase in the fusion area of the upper endplate (0.94±28.03 mm2; P=0.858), but the fusion area of the lower endplate showed a significant increase (9.65±24.35 mm2; P=0.042). Overall, the experimental group showed a significant increase in the fusion area (Tables 3, 4).
TABLE 4.
Summary of Fusion Area Ratio Measures
Osteolysis and Endplate Subsidence Assessment Results From 3D CT
In the previous study, osteolysis around BGS-7 spacers and the titanium cage was 7.7% in the experimental group and 5.9% in the control group, as measured by 3D CT scan at 1 year after the operation, with no significant differences between the groups.18 In this study, osteolysis around the cage and spacer was not observed in either group at 48 months or more. In the previous study, bone fusions in 1 case of osteolysis in the experimental group and patients with pseudoarthrosis and osteolysis were conducted only with conservative management without any additional interventions and were found to have been resolved in our analysis (Figs. 2, 3).
FIGURE 2.
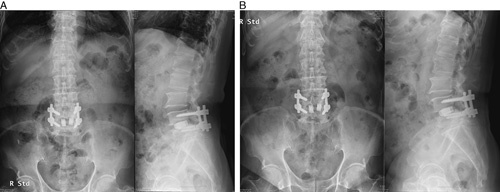
Plain radiographs of the patient who had osteolysis and pseudoarthrosis: postoperative year 1 (A) and postoperative year 4 (B).
FIGURE 3.
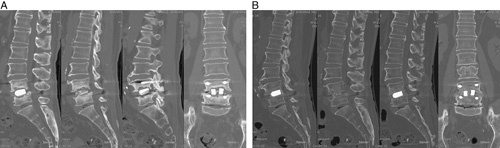
Computed tomography images of the patient who had osteolysis and pseudoarthrosis: postoperative year 1 (A) and postoperative year 4 (B).
Bone Fusion Assessment Results From Plain Radiography
In the previous study, the plain radiography analysis showed that bone fusion at 12 months postoperation was 89.7% (35/39) in the experimental group and 91.4% (32/35) in the control group.18 Our analysis using plain radiography showed that bone fusion after 48 months after the operation was 87.50% (28/32) in the experimental group and 100% (30/30) in the control group. There were no significant differences in the bone fusion between the experimental group and the control group based on radiography (P=0.114; Table 2).
Result of Functional Rating Scale (ODI, SF-36, and VAS Scores)
The ODI at 4 years after operation was 9.67±10.13 for the experimental group and 8.25±8.33 for the control group; no significant differences existed in ODI between the groups (Online Resource Supplementary Table 6, Supplemental Digital Content 1, http://links.lww.com/CLINSPINE/A123). The ODI at 1 year after operation was 27.9±17.6 for the experimental group and 23.2±16.2 for the control group. Thus, there was a statistically significant decrease in ODI at 4 years compared with ODI at 1 year.
The SF-36 evaluation showed that physical health scores were 48.35±9.25 for the experimental group and 48.87±8.91 for the control group and the mental health scores were 49.37±9.34 for the experimental group and 49.14±10.69 for the control group; there were no statistical differences between the groups (Online Resource Supplementary Table 6, Supplemental Digital Content 1, http://links.lww.com/CLINSPINE/A123).
The VAS score for back pain was 15.22±20.07 for the experimental group and 12.30±19.87 for the control group (P=0.40); VAS score for the right leg was 7.19±14.01 for the experimental group and 11.53±19.78 for the control group (P=0.46), and VAS score for the left leg was 13.34±23.27 for the experimental group and 8.58±16.11 for the control group (P=0.55); there were no statistically significant differences between the groups for all VAS scores (Online Resource Supplementary Table 7, Supplemental Digital Content 1, http://links.lww.com/CLINSPINE/A123).
Adverse Events
In this follow-up study, no additional adverse events were reported in either the experimental or the control group.
DISCUSSION
Cage implantation in PLIF surgery for the degenerative spinal disease has several advantages and is gaining widespread popularity. Implanted cages in the intervertebral space recover disk height and distract the foraminal space, thereby decompressing the neural structure indirectly. They also support the anterior column to transmit weight effectively.1–3 Instead of cages filled with autogenous bone graft, spacers made out of variable bone graft substitutes such as bioactive glass-ceramics have been widely researched. BGS-7 spacers show 2-fold higher mechanical strength than that of hydroxyapatite.22 Furthermore, previous in vitro and in vivo studies show that BGS-7 spacers possess high bioactivity and chemical bonding ability.12–15,22
Therefore, a clinical trial was conducted to evaluate if BGS-7 spacers can be used as a substitute for intervertebral cages.18 The fusion rates and clinical results for BGS-7 spacers were similar to titanium cages and showed ignorable adverse events, implying the safety of the BGS-7 spacers.18
The authors evaluated the fusion rates of both the experimental and the control groups using plain radiography and 3D CT. Both groups showed satisfactory fusion rates, and there were no significant differences between the methods. The experimental group showed fusion rates of 89.7% (35/39) in the 1-year 3D CT scan and 90.6% (29/32) in the 4-year 3D CT scan. The 4-year fusion rate of the experimental group was estimated to be 92.3% (36/39); 7 cases were lost in the experimental group at the 4-year follow-up, all of which were judged as fusion at the 1-year follow-up. The increase in bone fusion rates at the 4-year follow-up demonstrated high osteoconductivity of the BGS-7 spacers.
Fusion area in both the upper and the lower endplates is significantly broader in the BGS-7 spacers group than in the titanium cage group because the BGS-7 spacer has broad contact surface and shows direct chemical bonding ability with the bone (Fig. 1). Broad fusion area plays an effective role in the stress distribution of the spacer and the cage in the interbody fusion.2 Furthermore, a spacer with a large footprint has a biomechanical advantage because it efficiently distributes the stress resulting in a low rate of subsidence.23 Therefore, the benefits of a broad fusion area of BGS-7 spacers suggest its potential use in intervertebral spacers.
FIGURE 1.
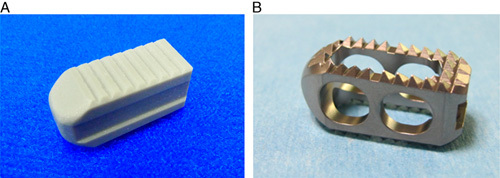
Gross photographs of implants. A and B, Titanium cage.
A significant increase in the fusion area was observed in the BGS-7 spacers group compared with the titanium cage group between 1 and 4 years after the operation. Moreover, a statistically significant increase in the fusion area was observed in both the upper and lower endplates in the experimental group. In the control group, however, the fusion area in the lower endplate showed a significant increase, but the fusion area in the upper endplate did not show any significant improvement. Changes in the fusion area of the BGS-7 spacers are consistent with previously published results in an animal study.12 Although titanium is a biocompatible material that promotes bone growth near the cage surface, it is biologically inert and cannot chemically bind to the bone. On the contrary, BGS-7 is a bioactive material, which facilitates a progressive increase in the fusion area. Therefore, rigid instrumentation maintains the stability between the vertebral bodies and promotes the fusion between the ceramic spacer and the vertebral body, even beyond 1 year after surgery. Moreover, the cases judged as osteolysis and pseudoarthrosis in the first year were judged as fused in the fourth year owing to spontaneous fusion and bone healing (Figs. 2, 3). These results demonstrate that higher osteoconductivity of BGS-7 spacers promotes bone fusion over time.
The authors analyzed the clinical results of experimental and control groups and observed significant improvements in ODI, SF-36, and VAS score relative to the preoperative status in both groups. Although both groups showed significant improvement at the 4-year follow-up compared with the 1-year follow-up, no significant differences existed between the 2 groups in back pain VAS. This suggests functional improvements even 4 years after PLIF was performed with BGS-7 spacers or titanium cage.
Although the 90-day intravenous toxicity results showed no systemic toxicity after administration of CaO-SiO2-P2O5-B2O3,16 long-term residual effects must be investigated for the clinical use of BGS-7 as an intervertebral spacer. Our study shows no adverse events in the experimental group after >4 years. The previous study reported only 1 adverse event (back pain due to osteolysis) attributed to the BGS-7 spacers.18 After 4 years, the patient showed significant improvements in back pain, improvements in intervertebral fusion, and gradual resolution of osteolysis around the spacer without any additional surgical intervention (Fig. 3). Therefore, our study demonstrates that superior chemical bonding ability and osteointegration of the BGS-7 facilitates effective bone fusion around the intervertebral spacer. These properties of the BGS-7 are beneficial for its use as an alternative bone graft extender.
However, this study has limitations. First, the safety of the BGS-7 should be analyzed beyond 4 years, because BGS-7 spacers would remain in the intervertebral spaces for a long time. In terms of cost-effectiveness, the use of BGS-7 spacer or titanium cages can be considered similar, because they are currently sold at similar prices in Korea. However, the cost-effectiveness of the use of BGS-7 spacers compared with other cages should be further investigated with considering the length of hospital stay after surgery or the risk of reoperation owing to complications.
CONCLUSIONS
In conclusion, the long-term follow-up of >4 years shows similar fusion rates and clinical results for both BGS-7 spacers and the titanium cages in 1-level PLIF operation. Furthermore, the BGS-7 spacers show significant progress in bone fusion around the spacers with time, thereby suggesting more effective osteointegration. Therefore, this study demonstrates the safety and potential value of BGS-7 as novel intervertebral spacers and bone graft substitutes.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jspinaldisorders.com.
ACKNOWLEDGMENTS
The authors would like to thank and acknowledge Kun-Woo Park, MD, Ho-Joong Kim, MD, and Jin-Sup Yeom, MD, for helping with study enrollment, surgery, and data collection.
Footnotes
FDA device/drug status: Investigating (Novomax).
Supported by a research grant for clinical studies from CGBio Inc. and BioAlpha Inc.
J.H.L., C.K.L., and B.S.C.: Stock Ownership: Bioalpha (<1%). The remaining authors declare no conflict of interest.
REFERENCES
- 1.Kanayama M, Cunningham BW, Haggerty CJ, et al. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J Neurosurg. 2000;93:259–265. [DOI] [PubMed] [Google Scholar]
- 2.Kumar N, Judith MR, Kumar A, et al. Analysis of stress distribution in lumbar interbody fusion. Spine (Phila Pa 1976). 2005;30:1731–1735. [DOI] [PubMed] [Google Scholar]
- 3.Polly DW, Jr, Klemme WR, Cunningham BW, et al. The biomechanical significance of anterior column support in a simulated single-level spinal fusion. J Spinal Disord. 2000;13:58–62. [DOI] [PubMed] [Google Scholar]
- 4.Lee YC, Zotti MG, Osti OL. Operative management of lumbar degenerative disc disease. Asian Spine J. 2016;10:801–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal SL, Ohnmeiss DD. North American Spine Society (NASS). Intervertebral cages for degenerative spinal diseases. Spine J. 2003;3:301–309. [DOI] [PubMed] [Google Scholar]
- 6.Patel DV, Yoo JS, Karmarkar SS, et al. Interbody options in lumbar fusion. J Spine Surg. 2019;5:S19–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JH, Jeon DW, Lee SJ, et al. Fusion rates and subsidence of morselized local bone grafted in titanium cages in posterior lumbar interbody fusion using quantitative three-dimensional computed tomography scans. Spine (Phila Pa 1976). 2010;35:1460–1465. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Chang BS, Jeung UO, et al. The first clinical trial of beta-calcium pyrophosphate as a novel bone graft extender in instrumented posterolateral lumbar fusion. Clin Orthop Surg. 2011;3:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi J, Lee GW, Nam WD, et al. A Prospective Randomized Clinical Trial Comparing Bone Union Rate Following Anterior Cervical Discectomy and Fusion Using a Polyetheretherketone Cage: Hydroxyapatite/B-Tricalcium Phosphate Mixture versus Hydroxyapatite/Demineralized Bone Matrix Mixture. Asian Spine J. 2015;9:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokubo T, Ito S, Huang ZT, et al. Ca,P-rich layer formed on high-strength bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:331–343. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsuki C, Kamitakahara M, Miyazaki T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J R Soc Interface. 2009;6(suppl 3):S349–S360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Jeung UO, Jeon DH, et al. Quantitative comparison of novel CaO-SiO2-P2O 5-B2O3 glass-ceramics (BGS-7) with hydroxyapatite as bone graft extender in rabbit ilium. Tissue Eng Regen Med. 2010;7:540–547. [Google Scholar]
- 13.Lee JH, Nam H, Ryu HS, et al. Bioactive ceramic coating of cancellous screws improves the osseointegration in the cancellous bone. J Orthop Sci. 2011;16:291–297. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Hong KS, Baek HR, et al. In vivo evaluation of CaO-SiO2-P2O5-B2O3 glass-ceramics coating on Steinman pins. Artif Organs. 2013;37:656–662. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Seo JH, Lee KM, et al. Fabrication and evaluation of osteoblastic differentiation of human mesenchymal stem cells on novel CaO-SiO2-P2O5-B2O3 glass-ceramics. Artif Organs. 2013;37:637–647. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Ryu HS, Seo JH, et al. A 90-day intravenous administration toxicity study of CaO-SiO2-P2O5-B2O3 glass-ceramics (BGS-7) in rat. Drug Chem Toxicol. 2010;33:38–47. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Baek MH, Kim YE, et al. Finite element modeling of stress distribution in intervertebral spacers of different surface geometries. Artif Organs. 2013;37:1014–1020. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Kong CB, Yang JJ, et al. Comparison of fusion rate and clinical results between CaO-SiO2-P2O5-B2O3 bioactive glass ceramics spacer with titanium cages in posterior lumbar interbody fusion. Spine J. 2016;16:1367–1376. [DOI] [PubMed] [Google Scholar]
- 19.Ito Z, Imagama S, Kanemura T, et al. Volumetric change in interbody bone graft after posterior lumbar interbody fusion (PLIF): a prospective study. Eur Spine J. 2014;23:2144–2149. [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Lee JH, Lee JH. A comparison of dynamic views using plain radiographs and thin-section three-dimensional computed tomography in the evaluation of fusion after posterior lumbar interbody fusion surgery. Spine J. 2013;13:1200–1207. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Lee JH, Park JW, et al. Fusion rates of a morselized local bone graft in polyetheretherketone cages in posterior lumbar interbody fusion by quantitative analysis using consecutive three-dimensional computed tomography scans. Spine J. 2011;11:647–653. [DOI] [PubMed] [Google Scholar]
- 22.Ki Hyoung K, Changju H, Jae Hyup L, et al. Treatment of bone defects in rabbit tibiae using CaO-SiO2-P2O5-B2O3 bioactive ceramics: radiological, biomechanical, and histological evaluation. Tissue Eng Regen Med. 2009;6:811–818. [Google Scholar]
- 23.Faizan A, Kiapour A, Kiapour AM, et al. Biomechanical analysis of various footprints of transforaminal lumbar interbody fusion devices. J Spinal Disord Tech. 2014;27:E118–E127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jspinaldisorders.com.


