Abstract
The development of processes for electrochemical energy conversion and chemical production could benefit from new strategies to interface chemical redox reactions with electrodes. Here, we employ a diffusible low-potential organic redox mediator, 9,10-anthraquinone-2,7-disulfonic acid (AQDS), to promote efficient electrochemical oxidation of H2 at an off-electrode heterogeneous catalyst. This unique approach to integrate chemical and electrochemical redox processes accesses power densities up to 228 mW/cm2 (528 mW/cm2 with iR-correction). These values are significantly higher than those observed in previous mediated electrochemical H2 oxidation methods, including those using enzymes or inorganic mediators. The approach described herein shows how traditional catalytic chemistry can be coupled to electrochemical devices.
Graphical Abstract

Redox-active organic molecules have been widely employed as electrochemical mediators to enhance electron transfer between an electrode and a catalyst or chemical reagent in solution. Applications include electrochemical organic synthesis1–3 and energy storage and conversion.4–7 Enzymatic biofuel cells, for example, often use organic redox mediators to facilitate redox reactions with enzyme-based catalysts. Representative mediators include (anthra)quinones,8–10 viologens,11–13 and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)14 (Figure 1A). Applications of these methods are typically limited to low-power applications, such as implantable biomedical devices and portable electronics,15–17 because the conditions required to sustain enzyme activity and stability limit the fuel cell performance. Power densities accessed by enzymatic fuel cells are typically 2–3 orders of magnitude lower than those of conventional proton exchange membrane fuel cells (PEMFCs) (Figure 1B). Replacing the relatively fragile enzymes with heterogeneous catalysts capable of tolerating strongly acidic (or basic) fuel cell conditions and elevated temperatures provides a potential strategy to overcome this limitation. Here, we demonstrate this concept by employing 9,10-anthraquinone-2,7-disulfonic acid (AQDS) as a mediator for electrochemical oxidation of H2 at an off-electrode Pt/C catalyst. iR-free power densities of > 500 mW/cm2 are accessible when this anodic process is paired with a mediated cathode, employing a previously reported polyoxometalate-based mediator for O2 reduction.18 This system, which represents the first pairing of an organic mediator with an off-electrode heterogeneous catalyst in a fuel cell anode, leverages the strengths of both chemical and electrochemical redox reactions and establishes an important platform for development of new electrode-driven redox processes.
Figure 1.
Organic redox mediators can play an important role in enzymatic fuel cells (A) and fuel cells with diverse catalysts (B).
Organic mediators have been the focus of substantial recent attention for use in aqueous redox flow batteries (RFBs) and related energy-storage devices.19–25 Quinones, aminoxyls, and other redox-active organic molecules complement the more widely used inorganic mediators, such as V, Fe, and Zn ions, in these devices.6 Aqueous RFBs with organic mediators have been shown to support high power densities (≥ 1 W/cm2),26 and anthraquinones are commonly featured anodic mediators in these applications.27–30 Meanwhile, quinones have been widely used as reagents and catalytic mediators in chemical oxidation reactions,31–32 and they undergo efficient catalytic oxidation33–35 and reduction36–38 over heterogeneous catalysts. A noteworthy example of the latter application is the “anthraquinone process” for industrial production of hydrogen peroxide, which features a two-stage sequence involving catalytic hydrogenation of an anthraquinone derivative followed by autoxidation of the corresponding anthrahydroquinone to generate H2O2 and the quinone derivative.39–40 Collectively, these precedents provided an important foundation for the present study, which takes advantage of anthraquinones and heterogeneous catalysts to mediate the electrochemical oxidation of H2 in an off-electrode compartment. AQDS was selected as a prototypical anode mediator owing to its good electrochemical behavior and the proximity of its redox potential to the thermodynamic H+/H2 potential. Both of these features are evident in the cyclic voltammogram of the disodium salt of AQDS (AQDS-Na2), obtained in the 1 M sulfuric acid medium used in this study. The well-defined oxidation and reduction peaks and quasireversible redox potential of 220 mV vs. NHE (Figure 2) are consistent with previous reports.27
Figure 2.
Cyclic voltammogram of 9,10-anthraquinone-2,7-disulfonic acid, disodium salt (AQDS-Na2) in 1 M H2SO4. Conditions: AQDS-Na2 (10 mM), glassy carbon working electrode, Pt-wire counter electrode, Ag/AgCl reference electrode; 10 mV s−1 scan rate.
The catalytic hydrogenation of anthraquinones has been extensively investigated in organic solvents or neutral aqueous media,36–38 but such conditions are not conducive to fuel cell applications, which favor strongly acidic or basic aqueous conditions. Hydrogenation of quinones in acidic media has only been reported for the parent 1,4-benzoquinone, which has a potential (E1/2 ~ 700 mV vs NHE) more than 450 mV higher than that of AQDS.36 Nonetheless, this precedent employing a supported Pt catalyst prompted us to select a commercial Pt/C catalyst, sourced from Strem Chemicals, for our initial testing of AQDS-Na2 hydrogenation in 1 M sulfuric acid. Monitoring of the hydrogenation of AQDS is complicated by the air sensitivity of AQDSH2 (9,10-anthrahydroquinone-2,7-disulfonic acid), arising from its facile autoxidation to regenerate AQDS. To address this issue, the reaction progress was monitored in a sealed vessel via potentiometric analysis of the batch reaction mixture (Figure 3). The measured potential correlated with the relative concentrations of AQDS and AQDSH2, as expected (see Supporting Information for details), and selective formation of AQDSH2 was confirmed by UV-visible and 1H NMR spectroscopy. The UV-visible spectrum of a sample of hydrogenated AQDS matched the spectrum of electrochemically generated AQDSH2 (see Figures S3 and S4). Two distinct sets of peaks were apparent in the 1H NMR spectrum of a hydrogenated sample that was maintained under N2, and these peaks disappeared upon exposure of the sample to air leaving only signals corresponding to AQDS (see Figure S5).41–43
Figure 3.
Reaction progress for batch hydrogenation of 9,10-anthraquinone-2,7-disulfonic acid, disodium salt catalyzed by Pt/C. Formation of hydroquinone was monitored via potentiometry. Dots represent reaction data, and the line reflects a linear correlation to the data.
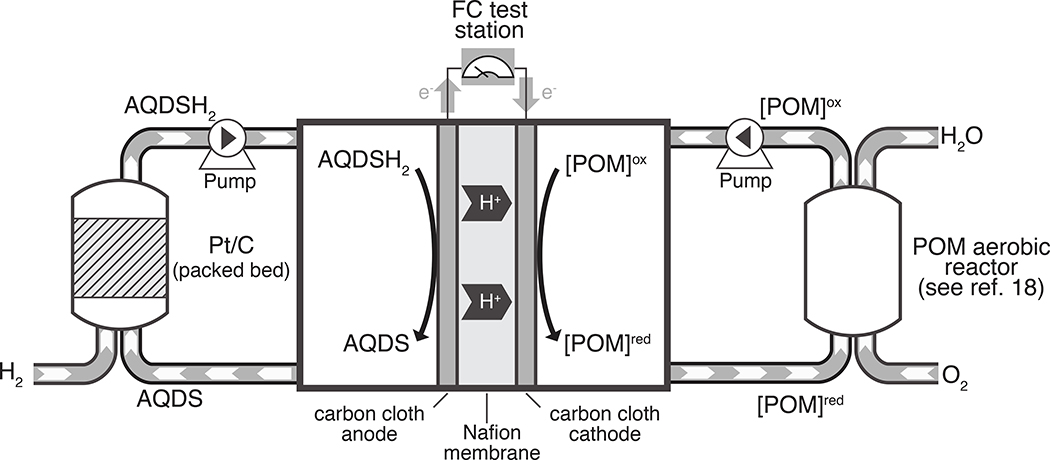
With the successful demonstration of catalytic hydrogenation of AQDS under acidic conditions in batch, we then incorporated the Pt/C catalyst into a packed bed reactor (1/2” o.d. stainless steel tubing) to enable the continuous operation of an AQDS-mediated anode. The packed bed reactor was connected to the anode compartment of a 5 cm2 fuel cell with serpentine flow plates. The membrane electrode assembly (MEA) featured a carbon cloth anode (Avcarb 1071HCB), a Nafion 115 membrane, and a conventional O2 cathode (0.2 mg Pt/cm2 on carbon). A solution of AQDS in 1 M H2SO4 was mixed with H2 at a tee junction and fed into the packed bed reactor to charge the flow anode with reduced anthraquinone. The fuel cell was then operated at 60 °C with the liquid mediator solution cycled through the anode and reactor compartments, while O2 was delivered to the cathode at 0.2 L/min. The preliminary results with this fuel cell arrangement, however, revealed significant complications associated with cathode flooding, arising from water migration through the membrane to the cathode. A power curve obtained during these tests showed significant mass-transport losses (see Figure S7 and Supporting Information for discussion). This phenomenon is not uncommon in liquid fuel cells and has been noted, for example, with other mediated anode44 and direct methanol fuel cell systems.45 To mitigate the impact of water crossover, we elected to replace the gas diffusion cathode with a mediated cathode system consisting of 0.3 M aqueous Na4H3PMo8V4O40, a polyoxometalate (POM) similar to that reported previously.18,46,47 Briefly, the POM solution was heated to 80 °C in a 75 mL vessel sparged with O2, and the oxidized POM solution was flowed to the cathode compartment, where it underwent reduction and then circulated back to the regeneration compartment (see Section 4 of the Supporting Information for details). A schematic illustrating the resulting doubly mediated fuel cell is depicted in Figure 4.
Figure 4.
Schematic of the doubly-mediated fuel cell.
A polarization curve for the AQDS/POM-mediated fuel cell was then collected using 1 M AQDS in 1 M H2SO4 at the anode at a state-of-charge of 85% (i.e., AQDSH2:AQDS ratio of 85:15; Figure 5A). An open-circuit potential of 0.73 V was observed, with a peak power density of 228 mW/cm2 and an iR-corrected value of 528 mW/cm2. Continuous fuel cell operation was established by matching the rate of the chemical reduction of AQDS in the packed bed reactor and the electrochemical oxidation of AQDSH2 at the anode (Figure 5B). Incorporation of a liquid reservoir between the anode and the packed bed reactor permitted in-line potentiometric monitoring of the mediator solution as well as the use of different liquid flow rates for improved performance in the individual units: 40 mL/min through the anode for improved mass transfer and 4 mL/min through the reactor to minimize pressure drop through the powder catalyst bed (for a schematic and photographs of the reactor set-up, see Figure S6). The integrated system sustained a current density of 50 mA/cm2 at a mediator state-of-charge of approximately 70% for 8 hours.48 The contribution of the reactor to sustained system performance was confirmed in a separate experiment by interrupting the hydrogen flow over a period of one hour. The cell potential decreased steadily from 585 mV to 550 mV as the AQDS mediator state-of-charge decreased from 55% to 42%. Once hydrogen flow was reinitiated, reduction of AQDS ensued and the cell potential stabilized (see Figure S8 and Supporting Information for further details). These results demonstrate the steady-state integration of an electrochemical redox process (oxidation of AQDSH2) with an off-electrode chemical redox reaction.
Figure 5.
(A) Polarization and power density curves with 1 M anthraquinone disulfonic acid in 1 M H2SO4 at the anode and 0.3 M Na4H3PMo8V4O40 at the cathode. Pale curves are raw data and bright curves reflect data with iR-compensation. The fuel cell was operated at 60 °C. (B) Steady-state cell potentials observed from constant current operation of the doubly-mediated fuel cell at 50 mA/cm2.
The power densities and currents sustained in these experiments with AQDS are the highest reported to date for mediated electrolysis with hydrogen (Figure 6; experimental details of the mediated fuel cells, as well as power density calculations, are provided in Table S1 of the Supporting Information). Early precedents for mediated fuel cells of this type employed hydrated metal ions as mediators, including Ti3/4+,49 Sn2/4+,50 Fe2/3+,50 Cu0/2+,50 and Mo3/4+,51 in addition to a Si-containing polyoxotungstate51 and EDTA-ligated Fe2/3+.52 Each of these examples used off-electrode Pt or Pd catalysts for reduction of the mediator with H2, although several examples only reported polarization curves, without demonstration of continuous, steady-state fuel cell operation.50 The silicotungstic acid fuel cell accessed the highest power density at 143 mW/cm2. More recently, organic mediators have been used in low power H2 fuel cells to facilitate electron transfer with enzymatic catalysts. In the earliest example, 1.5 mM methyl viologen was paired with the bacterial cell D. vulgaris for a fuel cell with a power density of 0.19 mW/cm2.12 Replacing the methyl viologen with 1.5 mM anthraquinone-2-sulfonic acid (AQS) increased the value to 0.44 mW/cm2. Incorporation of viologen mediators into redox polymer networks with NiFe53 and FeFe54 hydrogenases enabled power densities of approximately 0.2 mW/cm2. The low power densities in these systems partly reflect the mild conditions needed to ensure enzyme stability and activity. For example, the pH typically varies from 4–11 and mediator concentrations are typically under 20 mM and operating temperatures under 40 °C. The ability to use a 1 M mediator concentration, operate the fuel cell at 60 °C, and employ a robust heterogeneous catalyst at pH 0 all contribute to the improved fuel cell performance observed in the present study.
Figure 6.
Comparison of power densities in fuel cells with anodic mediators using H2 as the fuel. Power density was calculated from the highest performance reported in each reference. References, full details of the experimental configurations of the mediated fuel cells, and power density calculations are provided in Table S1 of the Supporting Information.
The results described herein, which demonstrate the ability to produce power in a fuel cell by coupling a conventional catalytic hydrogenation reaction with electrochemical oxidation of an organic mediator, set the stage for a number of future research directions. Improved fuel cell performance should be accessible by using lower potential mediators that operate closer to the thermodynamic potential of the fuel. More broadly, this approach can leverage the widespread use of heterogeneous catalysts in organic chemical transformations. The development of new catalytic transfer (de)hydrogenation reactions between organic molecules and electrochemical mediators represents an important extension of the concepts reported here. For example, preliminary batch experiments show that formic acid and methanol may be used instead of H2 to reduce AQDS to AQDSH2 in the presence of a Pt/C catalyst (Figure S9). These results provide a starting point for development of mediated fuel cells that use more complex fuels than H2 and incorporate organic mediators, in contrast to previously reported systems which utilize inorganic mediators, such as polyoxometalates.55–57 Such systems would have several appealing features, including ability to minimize fuel crossover and avoid gas generation at the electrode surface by moving the catalytic process off the electrode surface.45,58 Efforts to explore such mediated anode systems have been initiated in our lab.
Supplementary Material
ACKNOWLEDGMENT
We thank James Gerken (UW-Madison) for guidance on spectroelectrochemistry. Financial support for this project was provided by the Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences. Supplemental funds from the Wisconsin Alumni Research Foundation (WARF) through the WARF Accelerator Program provided partial support for YP and CWA. NMR spectroscopy facilities were partially supported by the NSF (CHE-1048642) and the NIH (S10 OD012245).
Footnotes
A patent application has been filed, based in part on work reported in this paper.
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website.
Additional experimental details, including method for batch hydrogenation of AQDS and characterization of AQDSH2, operation of the mediated fuel cell, and data for transfer dehydrogenation of HCO2H and MeOH, are included in the Supporting Information. Details for power density calculations for previously reported mediated fuel cells are also provided.
REFERENCES
- (1).Steckhan E Indirect Electroorganic Syntheses – A Modern Chapter of Organic Electrochemistry. Angew. Chem. Int. Ed. 1986, 28, 683–701. [Google Scholar]
- (2).Ogibin YN; Elinson MN; Nikishin GI Mediator Oxidation Systems in Organic Electrosynthesis. Russ. Chem. Rev. 2009, 78, 89–140. [Google Scholar]
- (3).Francke R; Little RD Redox Catalysis in Organic Electrosynthesis: Basic Principles and Recent Developments. Chem. Soc. Rev. 2014, 43, 2492–2521. [DOI] [PubMed] [Google Scholar]
- (4).McCloskey BD; Addison D A Viewpoint on Heterogeneous Electrocatalysis and Redox Mediation in Nonaqueous Li-O2 Batteries. ACS Catal. 2017, 7, 772–778. [Google Scholar]
- (5).Wallace AG; Symes MD Decoupling Strategies in Electrochemical Water Splitting and Beyond. Joule 2018, 2, 1390–1395. [Google Scholar]
- (6).Soloveichik GL Flow Batteries: Current Status and Trends. Chem. Rev. 2015, 115, 11533–11558. [DOI] [PubMed] [Google Scholar]
- (7).Anson CW; Stahl SS Mediated Fuel Cells: Soluble Redox Mediators and their Applications to Electrochemical Reduction of O2 and Oxidation of H2, Alcohols, Biomass, and Complex Fuels. Chem. Rev. 2020, in press (DOI: 10.1021/acs.chemrev.9b00717). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhao X; Jia H; Kim J; Wang P Kinetic Limitations of a Bioelectrochemical Electrode Using Carbon Nanotube-Attached Glucose Oxidase for Biofuel Cells. Biotechnol. Bioeng. 2009, 104, 1068–1074. [DOI] [PubMed] [Google Scholar]
- (9).Zhu Z; Zhang Y-HP Use of Nonimmobilized Enzymes and Mediators Achieved High Power Densities in Closed Biobatteries. Energy Sci. Eng. 2015, 3, 490–497. [Google Scholar]
- (10).Milton RD; Hickey DP; Abdellaoui S; Lim K; Wu F; Tan B; Minteer SD Rational Design of Quinones for High Power Density Biofuel Cells. Chem. Sci. 2015, 6, 4867–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Palmore GTR; Bertschy H; Bergens SH; Whitesides GM A Methanol/Dioxygen Biofuel Cell that Uses NAD+-Dependent Dehydrogenases as Catalysts: Application of an Electro-Enzymatic Method to Regenerate Nicotinamide Adenine Dinucleotide at Low Overpotentials. J. Electroanal. Chem. 1998, 443, 155–161. [Google Scholar]
- (12).Tsujimura S; Fujita M; Tatsumi H; Kano K; Ikeda T Bioelectrocatalysis-based Dihydrogen/Dioxygen Fuel Cell Operating at Physiological pH. Phys. Chem. Chem. Phys. 2001, 3, 1331–1335. [Google Scholar]
- (13).Milton RD; Cai R; Abdellaoui S; Leech D; De Lacey AL; Pita M; Minteer SD Bioelectrochemical Haber-Bosch Process: An Ammonia-Producing H2/N2 Fuel Cell. Angew. Chem. Int. Ed. 2017, 56, 2680–2683. [DOI] [PubMed] [Google Scholar]
- (14).Mano N; de Poulpiquet A O2 Reduction in Enzymatic Biofuel Cells. Chem. Rev. 2018, 118, 2392–2468. [DOI] [PubMed] [Google Scholar]
- (15).Rasmussen M; Abdellaoui S; Minteer SD Enzymatic Biofuel Cells: 30 Years of Critical Advancements. Biosens. Bioelectron. 2016, 76, 91–102. [DOI] [PubMed] [Google Scholar]
- (16).Falk M; Narváez Villarrubia CW; Babanova S; Atanassov P; Shleev S Biofuel Cells for Biomedical Applications: Colonizing the Animal Kingdom. ChemPhysChem 2013, 14, 2045–2058. [DOI] [PubMed] [Google Scholar]
- (17).Xu Q; Zhang F; Xu L; Leung P; Yang C; Li H The Applications and Prospect of Fuel Cells in Medical Field: A Review. Renew. Sust. Energ. Rev. 2017, 67, 574–580. [Google Scholar]
- (18).Ward DB; Gunn NLO; Uwigena N; Davies TJ Performance Comparison of Protonic and Sodium Phosphomolybdovanadate Polyoxoanion Catholytes Within a Chemically Regenerative Redox Cathode Polymer Electrolyte Fuel Cell. J. Power Sources 2018, 375, 68–76. [Google Scholar]
- (19).Wei X; Pan W; Duan W; Hollas A; Yang Z; Li B; Nie Z; Liu J; Reed D; Wang W; Sprenkle V Materials and Systems for Organic Redox Flow Batteries: Status and Challenges. ACS Energy Lett. 2017, 2, 2187–2204. [Google Scholar]
- (20).Winsberg J; Hagemann T; Janoschka T; Hager MD; Schubert US Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem. Int. Ed. 2017, 56, 686–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Luo J; Hu B; Hu M; Zhao Y; Liu TL Status and Prospects of Organic Redox Flow Batteries Toward Sustainable Energy Storage. ACS Energy Lett. 2019, 4, 2220–2240. [Google Scholar]
- (22).Rausch B; Symes MD; Cronin L A Bio-Inspired, Small Molecule Electron-Coupled-Proton Buffer for Decoupling the Half-Reactions of Electrolytic Water Splitting. J. Am. Chem. Soc. 2013, 135, 13656–13659. [DOI] [PubMed] [Google Scholar]
- (23).Liao S; Zong X; Seger B; Pedersen T; Yao T; Ding C; Shi J; Chen J; Li C Integrating a Dual-Silicon Photoelectrochemical Cell into a Redox Flow Battery for Unassisted Photocharging. Nat. Commun. 2016, 7, 11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Li W; Fu H-C; Li L; Cabán-Acevedo M; He J-H; Jin S Integrated Photoelectrochemical Solar Energy Conversion and Organic Redox Flow Battery Devices. Angew. Chem. Int. Ed. 2016, 55, 13104–13108. [DOI] [PubMed] [Google Scholar]
- (25).Zhu YG; Wang X; Jia C; Yang J; Wang Q Redox-Mediated ORR and OER Reactions: Redox Flow Lithium Oxygen Batteries Enabled with a Pair of Soluble Redox Catalysts. ACS Catal. 2016, 6, 6191–6197. [Google Scholar]
- (26).Chen Q; Gerhardt MR; Hartle L; Aziz MJ A Quinone-Bromide Flow Battery with 1W/cm2 Power Density. J. Electrochem. Soc. 2016, 163, A5010–A5013. [Google Scholar]
- (27).Huskinson B; Marshak MP; Suh C; Er S; Gerhardt MR; Galvin CJ; Chen X; Aspuru-Guzik A; Gordon RG; Aziz MJ A Metal-Free Organic-Inorganic Aqueous Flow Battery. Nature 2014, 505, 195–198. [DOI] [PubMed] [Google Scholar]
- (28).Hoober-Burkhardt L; Krishnamoorthy S; Yang B; Murali A; Nirmalchandar A; Prakash GKS; Narayanan SR A New Michael-Reaction-Resistant Benzoquinone for Aqueous Organic Redox Flow Batteries. J. Electrochem. Soc. 2017, 164, A600–A607. [Google Scholar]
- (29).Gerhardt MR; Tong L; Gómez-Bombarelli R; Chen Q; Marshak MP; Galvin CJ; Aspuru-Guzik A; Gordon RG; Aziz MJ Anthraquinone Derivatives in Aqueous Flow Batteries. Adv. Energy Mater. 2017, 1601488. [Google Scholar]
- (30).Kwabi DG; Lin K; Ji Y; Kerr EF; Goulet M-A; De Porcellinis D; Tabor DP; Pollack DA; Aspuru-Guzik A; Gordon RG; Aziz MJ Alkaline Quinone Flow Battery with Long Lifetime at pH 12. Joule 2018, 2, 1894–1906. [Google Scholar]
- (31).Piera J; Bäckvall J-E Catalytic Oxidation of Organic Substrates by Molecular Oxygen and Hydrogen Peroxide by Multistep Electron Transfer – A Biomimetic Approach. Angew. Chem. Int. Ed. 2008, 47, 3506–3523. [DOI] [PubMed] [Google Scholar]
- (32).Wendlandt AE; Stahl SS Quinone-Catalyzed Selective Oxidation of Organic Molecules. Angew. Chem. Int. Ed. 2015, 54, 14638–14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Miyamura H; Shiramizu M; Matsubara R; Kobayashi S Aerobic Oxidation of Hydroquinone Derivatives Catalyzed by Polymer-Incarcerated Platinum Catalyst. Angew. Chem. Int. Ed. 2008, 47, 8093–8095. [DOI] [PubMed] [Google Scholar]
- (34).Jawale DV; Gravel E; Geertsen V; Li H; Shah N; Namboothiri INN; Doris E Aerobic Oxidation of Phenols and Related Compounds Using Carbon Nanotube-Gold Nanohybrid Catalysts. ChemCatChem 2014, 6, 719–723. [Google Scholar]
- (35).Donck S; Gravel E; Li A; Prakash P; Shah N; Leroy J; Li H; Namboothiri INN; Doris E Mild and Selective Catalytic Oxidation of Organic Substrates by a Carbon Nanotube-Rhodium Nanohybrid. Catal. Sci. Technol. 2015, 5, 4542–4546. [Google Scholar]
- (36).Rosenblatt EF Hydrogenation of Quinone with Palladium and Platinum Catalysts. J. Am. Chem. Soc. 1940, 62, 1092–1094. [Google Scholar]
- (37).Khidekel ML; Polkovnikov BD; Taber AM; Balandin AA Catalytic Hydrogenation of Quinones in the Presence of Pt, Pd, and Rh Catalysts. Izv. Akad. Nauk SSSR, Ser. Khim. 1965, 3, 519–521. [Google Scholar]
- (38).Entwistle ID; Johnstone RAW; Telford RP Catalytic Transfer Reduction of Quinones. J. Chem. Research (M) 1977, 1382–1389. [Google Scholar]
- (39).Goor G; Glenneberg J; Jacobi S in Ullmann’s Encyclopedia of Industrial Chemistry (Ed.: Elvers B), Wiley-VCH Verlag GmbH & Co. KGaA, 2007, pp. 443–466. [Google Scholar]
- (40).Wendlandt AE; Stahl SS in Liquid Phase Aerobic Oxidation Catalysis (Eds.: Stahl SS and Alsters P), Wiley-VCH Verlag GmbH & Co. KGaA, 2016, pp. 219–237. [Google Scholar]
- (41).Previous studies have suggested that anthrahydroquinones undergo acid-catalyzed disproportionation to the anthraquinone and the anthrone, but no new peaks were observed by 1H NMR spectroscopy in the present reactions.
- (42).Beck F; Heydecke G On the Mechanism of the Cathodic Reduction of Anthraquinone to Anthrone. Ber. Bunsenges. Phys. Chem. 1987, 91, 37–43. [Google Scholar]
- (43).Wermeckes B; Beck F Acid Catalyzed Disproportionation of Anthrahydroquinone to Anthraquinone and Anthrone. Denki Kagaku oyobi Kogyo Butsuri Kagaku 1994, 62, 1202–1205. [Google Scholar]
- (44).Zhao X; Zhu JY Efficient Conversion of Lignin to Electricity Using a Novel Direct Biomass Fuel Cell Mediated by Polyoxometalates at Low Temperatures. ChemSusChem 2016, 9, 197–207. [DOI] [PubMed] [Google Scholar]
- (45).Ahmed M; Dincer I A Review on Methanol Crossover in Direct Methanol Fuel Cells: Challenges and Achievements. Int. J. Energy Res. 2011, 35, 1213–1228. [Google Scholar]
- (46).While this work was ongoing, we were in the process of developing an alternative mediated cathode concept complementary to the present mediated anode system. The former approach is now published (ref. 47) and would represent an alternative liquid cathode system.
- (47).Preger Y; Gerken JB; Biswas S; Anson CW; Johnson MR; Root TW; Stahl SS Quinone-Mediated Electrochemical O2 Reduction Accessing High Power Density with an Off-Electrode Co-N/C Catalyst. Joule 2018, 2, 2722–2731. [Google Scholar]
- (48).AQDS stability has been demonstrated previously in the context of redox flow battery and decoupled water splitting devices. See ref. 27 and the following: Kirkaldy N; Chisholm G; Chen J-J; Cronin LA Practical, Organic-Mediated, Hybrid Electrolyser that Decouples Hydrogen Production at High Current Densities. Chem. Sci. 2018, 9, 1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Carson WN Jr.; Feldman ML A Redox Type. Proc. Ann. Power Sources Conf. 1959, 13, 111–113. [Google Scholar]
- (50).Oei D-G Chemically Regenerative Redox Fuel Cells. J. Appl. Electrochem. 1982, 12, 41–51. [Google Scholar]
- (51).Kummer JT; Oei D-G A Chemically Regenerative Redox Fuel Cell. II. J. Appl. Electrochem. 1985, 15, 619–629. [Google Scholar]
- (52).Folkesson B Chemically Regenerative Redox Fuel Cells II. Regeneration Reaction Studies. J. Appl. Electrochem. 1990, 20, 907–911. [Google Scholar]
- (53).Plumeré N; Rüdiger O; Oughli AA; Williams R; Vivekananthan J; Pöller S; Schuhmann W; Lubitz W A Redox Hydrogel Protects Hydrogenase from High-Potential Deactivation and Oxygen Damage. Nat. Chem. 2014, 6, 822–827. [DOI] [PubMed] [Google Scholar]
- (54).Oughli AA; Conzuelo F; Winkler M; Happe T; Lubitz W; Schuhmann W; Rüdiger O; Plumeré N A Redox Hydrogel Protects the O2-sensitive [FeFe]-hydrogenase from Chlamydomonas reinhardtii from Oxidative Damage. Angew. Chem. Int. Ed. 2015, 54, 12329–12333. [DOI] [PubMed] [Google Scholar]
- (55).Liu W; Mu W; Deng Y High-Performance Liquid-Catalyst Fuel Cell for Direct Biomass-into-Electricity Conversion. Angew. Chem. Int. Ed. 2014, 53, 13558–13562. [DOI] [PubMed] [Google Scholar]
- (56).Wu W; Liu W; Deng Y Polyoxometalate liquid-catalyzed polyol fuel cell and the related photoelectrochemical reaction mechanism study. J. Power Sources 2016, 318, 86–92. [Google Scholar]
- (57).Gong J; Liu W; Du X; Liu C; Zhang Z; Sun F; Yang L; Xu D; Guo H; Deng Y Direct Conversion of Wheat Straw into Electricity with a Biomass Flow Fuel Cell Mediated by Two Redox Ion Pairs. ChemSusChem 2017, 10, 506–513. [DOI] [PubMed] [Google Scholar]
- (58).Kumar P; Dutta K; Das S; Kundu PP An Overview of Unsolved Deficiencies of Direct Methanol Fuel Cell Technology: Factors and Parameters Affecting its Widespread Use. Int. J. Energy Res. 2014, 38, 1367–1390. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.