ABSTRACT
This study analyzed possible associations between the trimester of maternal Zika virus infection (ZIKV) in pregnancy, severity of brain computed tomography (CT) findings and the presence of microcephaly at birth in children with Congenital Zika Syndrome (CZS). It was an analytical study in a cohort of children with CZS. Symptoms of maternal infection were dichotomized into the 1st trimester of pregnancy and other trimesters. Head circumference (HC) at birth was used to calculate the z-score. Mild microcephaly was defined as HC between 2 and ≥3 standard deviations (SD) below the mean for each gestational age and sex, and severe microcephaly when HC <3 SD below average. Brain CT images were evaluated by two radiologists and classified, according to the severity, into mild, moderate and severe. Fisher’s exact, Mann-Whitney and Kruskal-Wallis tests were used to verify the associations between variables. In 108 children, maternal infection in the 1st trimester of pregnancy was associated with more severe brain CT abnormalities (p=0.038), greater severity of microcephaly at birth (p=0.013) and lower HC z-scores at birth (p=0.021). The severity of brain CT lesions was also associated with lower HC z-scores at birth (p<0.001). Maternal ZIKV infection during the first trimester of pregnancy proved to be an important risk factor for a more severe spectrum of CZS, as it is associated with more severe brain CT abnormalities and, consequently, with lower HC z-scores at birth.
Keywords: Zika virus, Zika virus infection, Congenital abnormalities, Brain computed tomography, Microcephaly
INTRODUCTION
At the end of 2015, there was a marked increase in reported cases of microcephaly in newborns in the regions affected by the Zika outbreak in Brazil, especially in the Norteast1. The World Health Organization (WHO) and the Center for Disease Control and Prevention (CDC) recognized the causal relationship between the virus and the presence of birth defects in April 20162,3.
The Congenital Zika Syndrome (CZS) was later described by the presence of a pattern composed of five features that allow the differentiation from other congenital infections: (1) severe microcephaly with partially collapsed skull; (2) thin cerebral cortex with subcortical calcifications; (3) macular healing and retinal pigmentary spotting; (4) congenital contractures; and (5) early hypertonia with extrapyramidal symptoms4,5. However, not all newborns of pregnant women infected with the Zika virus (ZIKV) show clinical signs of the syndrome. The percentage of children with clinical symptomatology or brain images born to pregnant women infected with ZIKV varied from 9% to 42%6,7. Likewise, the absence of signs and symptoms characteristic of ZIKV infection in pregnant women does not exclude the possibility of CZS8,9. Microcephaly and other less frequent signs have been reported in children born to asymptomatic pregnant women8,10.
Some studies have reported a higher frequency of microcephaly when maternal infection occurred in the first gestational trimester6,8,11-13. However, as the studies were descriptive and included a small number of cases, no statistically significant difference was found in these studies6,8,11,12. Additionally, the association between skin rash in the first gestational trimester and severe microcephaly at birth was verified in a retrospective study with 602 confirmed or probable cases of CZS13. However, there seems to be no consensus in the literature in this regard, since Menezes et al. 14, in a retrospective study with 87 confirmed cases of CZS, did not identify this association.
In addition to microcephaly, congenital ZIKV infection is also capable of leading to abnormalities during the developmental stage of the Central Nervous System (CNS), such as brain calcifications, ventriculomegaly, abnormalities of cortical development and cerebellum and brainstem hypoplasias4,14-16. These neuroimaging findings were more common when the infection occurred in the first gestational trimester6,14-16 and it is suggested that the earlier the maternal infection occurs in pregnancy, the greater the risk of involvement and the seriousness of CNS damages15,17.
Only one study in the literature addressed the association between head circumference,measured using the z-score, and neuroimaging abnormalities. In this study, Menezes et al. 14 investigated 87 confirmed cases of congenital ZIKV infection and showed no difference in HC z-scores of infants with or without lysencephaly and hypogyria.
Most previous studies with neuroimaging in CZS are descriptive and performed with few cases, thus with low power to detect statistically significant associations. Furthermore, studies that analyzed the association between abnormalities in brain CT with the gestational trimester of ZIKV infection and the severity of microcephaly are still scarce11,14,18. Based on these considerations, this study aims to answer the following questions: is there an association between maternal ZIKV infection during the first trimester of pregnancy and greater severity of brain CT findings and microcephaly at birth? Is greater severity of head CT abnormalities related to a lower HC z-score at birth?
MATERIAL AND METHODS
This is an analytical study of a cohort of children with CZS born from March 2015 to September 2018 in Maranhao State, Brazil, and followed up at the Reference Center for Neurodevelopment, Assistance and Rehabilitation of Children (NINAR), located in Sao Luis, Maranhao State, Brazil. It is based on a convenience sample. The children were referred to the NINAR Rehabilitation Center for the investigation of the SZC due to the following reasons: a) history of maternal ZIKV infection during pregnancy; b) microcephaly at birth or postnatally; c) neuropsychomotor development delay.
Cases were classified as confirmed, laboratory probable, highly and moderately probable. To identify confirmed cases, the results of the plaque reduction neutralization test (PRNT90), collected after 18 months of age, were used. PRNT was performed based on a previously described protocol, with some adaptations19 to determine the maximum serum dilution (1:8 to 1:4,096) necessary to reduce the formation of ZIKV plaque by 90%. PRNT90 titers ≥1:10 were considered positive. Laboratory probable cases were identified from the serological test of class M specific immunoglobulin (IgM). Enzyme-linked immunosorbent assays (ELISA) based on the envelope proteins were used to screen anti-ZIKV IgM antibodies in the patients’ serum20. For the classification of highly probable cases, brain CT lesions suggestive of CZS were considered (calcifications, reduction in the volume of cerebral parenchyma, ventriculomegaly, malformation of cortical development, malformation/hypoplasia of the cerebellum, malformation/hypoplasia of the brain stem and agenesis/dysgenesis of the corpus callosum)9 and negative serology for the TORCHS group (Toxoplasmosis, Rubella, Cytomegalovirus, Herpes Simplex and Syphilis). The moderately probable cases were defined as the presence of the same brain CT abnormalities mentioned above, but without all serologies for the TORCHS group performed or with inconclusive results, due to the fact that the serum collection for exams was performed in an average age of 18 months, a period in which about 73% of children already have positive cytomegalovirus (CMV) serologies due to postnatal transmission21. Despite this, we do not completely rule out the possibility of vertical transmission, but we considered vertical transmission of CMV in these cases highly unlikely.
The children’s mothers were interviewed and asked about the presence of symptoms related to a possible ZIKV infection during pregnancy. Those who had rash or two or more of the following symptoms were considered symptomatic: fever, headache, joint arthralgia/edema, conjunctivitis and pruritus. The period of probable maternal infection was dichotomized into the first trimester and the 2nd/3rd gestational trimesters. Children whose mothers denied symptoms suggestive of ZIKV during pregnancy were excluded from the analyzes involving the gestational trimester of maternal infection.
Head circumference (HC) was measured by trained health professionals using an inextensible tape positioned over the occipital prominence and the arch of the eyebrows6. The HC measurement at birth was used to calculate the HC z-score, according to the INTERGROWTH-21st22standards for each sex and gestational age, using corrected gestational age for preterm children. Mild microcephaly was defined as HC <2 and ≥3 standard deviations (SD) below the mean for gestational age and sex. Severe microcephaly was considered when HC <3 standard deviations below the mean.
Neuroimaging of children was performed by brain CT scan through volumetric acquisitions in the Siemens Definition AS multi-slice devices (64 channels) and reviewed by two radiologists with experience in neuroimaging. The following classification of severity of damages was proposed: (1) mild, when there were only cerebral calcifications and/or mild/moderate reduction of cerebral parenchyma; (2) moderate, if cortical malformation and/or malformation of the posterior fossa (brainstem and cerebellum) were present, regardless of the presence of injuries considered mild; and (3) severe, which included severe reduction of the brain volume with ex-vacuum or hypertensive ventriculomegaly, regardless of the presence of other abnormalities.
Data were collected from information contained in medical records, interviews with mothers or guardians of the child, medical records and cards for pregnant women and children. The prenatal data collected from interviews were compared with the information contained in the medical records and in the pregnant woman’s card. When data diverged, the team contacted the interviewee to check the inconsistencies. The data were stored in the REDCap software database (Research Eletronic Data Capture, version 9.7.3, Vanderbilt University, Nashville, TN, USA) and exported to the Stata® (version 14.0, StataCorp LP, College Station, TX, USA) for statistical analysis.
Categorical variables were presented as relative and absolute frequencies and continuous variables as median and interquartile range (IQR). Fisher’s exact tests were used to verify the association between categorical variables and the Mann-Whitney and Kruskal-Wallis tests were used for continuous dependent variables without normal distribution. P value for trend was calculated in a linear regression model for continuous variables (head circumference z-score) or in logistic regression models for binary variables (severity of cranial tomography findings and microcephaly at birth) entering gestational trimester of maternal ZIKV infection as an ordinal variable.
Written informed consent was obtained from all mothers. The study was approved by the Institutional Research Ethics Committee of the University Hospital of the Federal University of Maranhao, Certificate of Presentation of Ethical Appreciation (CAAE) Nº 65897317.1.0000.5086.
RESULTS
A total of 108 confirmed or probable cases of CZS were included in this study. Thirty-nine (36.1%) children were classified as confirmed cases (positive PRNT) and three (2.7%) as probable laboratory cases (IgM positive). The remaining children were defined as probable cases of CZS: 33 (30.6%) classified as highly probable and 33 (30.6%) as moderately probable, according to radiological criteria and serology for TORCHS. Fifty-nine percent were males and 14% were born preterm. Only one child with positive PRNT had a positive IgM result for toxoplasmosis. The most common brain CT findings were cerebral calcifications (93.4%), ventriculomegaly (88.6%), reduction of the cerebral parenchyma (85.6%) and malformations of the cortical development (79.1%). Malformations of the cerebellum (19.1%), agenesis/dysgenesis of the corpus callosum (18.2%) and malformations of the brainstem (16.5%) were less frequent.
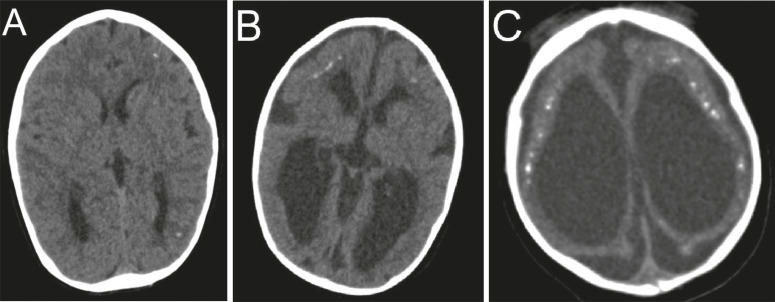
Eighty-one mothers (77.1%) reported signs and symptoms suggestive of ZIKV infection during pregnancy, while 24 (22.9%) were considered asymptomatic. Three women were excluded from the analysis of the gestational trimester of maternal infection, as they were unable to report the presence or absence of signs and symptoms suggestive of ZIKV infection. Of the 81 symptomatic mothers, 65.4% reported symptoms in the first trimester and 34.6% in the second or third trimester of pregnancy. Brain computed tomography images were available in 98.1% of cases. Of the 106 children, 40.6% were classified as severe; 42.4% had moderate abnormalities; and, finally, 17% were classified as mild (Figure 1).
Figure 1. Classification of the severity of brain CTlesions : A) axial section showing sparse calcifications in the cerebral parenchyma, alteration classified as mild, with no signs of volumetric reduction of the brain or malformation of cortical development; B) axial section showing malformation of diffuse cortical development, alteration classified as moderate, in addition to sparse calcifications and moderate reduction in brain volume; C) axial section showing severe brain atrophy (severe alteration), cerebral calcifications and severe ex-vacuum ventriculomegaly.
Greater severity of lesions in brain CT was associated with probable maternal infection in the first trimester of pregnancy (p=0.023). Severe microcephaly was more frequent when maternal infection occurred in the first trimester of gestation (p=0.013). Of the 50 children with a history of probable maternal infection in the first trimester of pregnancy, 56% were born with severe microcephaly (Table 1).
Table 1. Association between the gestational trimester of maternal Zika virus infection with microcephaly at birth and the severity of brain Computed Tomography (CT) lesions in children with Congenital Zika Syndrome.
| Variables | Trimester of maternal Zika virus infection | ||
|---|---|---|---|
|
| |||
| 1º n (%) | 2º/3º n (%) | p value | |
| Severity of brain CT lesions (n=80) a | 0.023h | ||
| Mildb | 4 (7.7) | 9 (32.1) | |
| Moderatec | 23 (44.2) | 9 (32.1) | |
| Severed | 25 (48.1) | 10 (35.8) | |
| Microcephaly at birth (n=74) e | 0.013h | ||
| Not present | 12 (24.0) | 14 (58.3) | |
| Mildf | 10 (20.0) | 4 (16.7) | |
| Severeg | 28 (56.0) | 6 (25.0) | |
a,eTwenty-four cases whose mothers were asymptomatic and three mothers who were unable to report the presence of signs and symptoms suggestive of ZIKV infection were excluded; aA child who did not undergo brain CT scan was excluded; bPresence of cerebral calcifications and/ or slight reduction of the cerebral parenchyma with or without ex-vacuum ventriculomegaly; cPresence of malformation of the cortical development and/or the posterior fossa; dSevere reduction of cerebral parenchyma with ex-vacuum or hypertensive ventriculomegaly; eSeven cases were excluded because they did not have their head circumference recorded at birth; fMild microcephaly was defined as a head circumference <2 and ≥3 standard deviations (SD) below the mean for each gestational age and sex; gSevere microcephaly was defined as a head circumference <3 SD below the mean for each gestational age and sex; hFisher’s exact test.
The HC z-score at birth was significantly lower in children whose mothers reported symptoms suggestive of ZIKV infection in the first trimester of pregnancy (p=0.001). The median of the HC z-score at birth of children with maternal infection in the first gestational trimester was -3.22 and -1.83 when the infection occurred in the 2nd or 3rd trimester (Table 2).
Table 2. Association between the median head circumference z-score at birth with the gestational trimester of maternal Zika virus infection and the severity of brain Computed Tomography (CT) lesions in children with Congenital Zika Syndrome.
| Variables | Head circumference z-score at birth | ||
|---|---|---|---|
|
| |||
| n (%) | Median (IQRf) | p value | |
| Gestational trimester of maternal ZIKV infection (n=74) a | 0.001g | ||
| 1st | 50 (67.6) | -3.22 (2.37) | |
| 2nd/3rd | 24 (32.4) | -1.83 (2.09) | |
| Severity of brain CT lesions (n=92) b | <0.001h | ||
| Mildc | 14 (15.2) | -1.62 (3.68) | |
| Moderated | 39 (42.4) | -2.00 (2.18) | |
| Severee | 39 (42.4) | -4.12 (2.03) | |
aTwenty-four cases whose mothers were asymptomatic, three mothers who were unable to report the presence of signs and symptoms suggestive of ZIKV infection and seven newborns that did not have their head circumference recorded at birth were excluded; bFifteen cases that did not have their head circumference recorded at birth and one that did not undergo brain computed tomography were excluded; cPresence of cerebral calcifications and/or slight reduction of the cerebral parenchyma with or without ex-vacuum ventriculomegaly; dPresence of malformation of the cortical development and/or posterior fossa (cerebellum or brain stem); eSevere reduction of cerebral parenchyma with ex-vacuum or hypertensive ventriculomegaly; fInterquartile range; gMann-Whitney test; hKruskal-Wallis test.
No linear trend was observed between the gestational trimester of maternal ZIKV infection and the severity of cranial tomography findings (p value for trend= 0.06). However, a linear trend was observed between the presence of microcephaly at birth and the head circumference Z score at birth with the gestational trimester of maternal infection (p value for trend=0.005 and 0.001, respectively).
Lower HC z-scores at birth were also associated with more severe brain CT damages (p <0.001). Children classified as having severe abnormalities in the brain CT scan had a median HC z-score of -4.12, those with moderate lesions of -2.00 and those with mild injuries of -1.62 (Table 2).
No significant difference in severity of brain CT damages was detected between the diagnostic classification highly probable and moderately probable groups (P=0.947) (Table 3).
Table 3. Association between the severity of brain computed tomography (CT) lesions and diagnostic classification (highly and moderately probable) in children with Congenital Zika Syndrome.
| Severity of brain CT lesions (n=66) | Diagnostic Classification | ||
|---|---|---|---|
|
| |||
| Highly probabled n (%) | Moderately probablee n (%) | p value | |
| Milda | 7 (21.2) | 7 (21.2) | 0.947f |
| Moderateb | 17 (51.5) | 15 (45.5) | |
| Severec | 9 (27.3) | 11 (33.3) | |
aPresence of cerebral calcifications and/ or slight reduction of the cerebral parenchyma with or without ex-vacuum ventriculomegaly; bPresence of malformation of the cortical development and/or posterior fossa; cSevere reduction of cerebral parenchyma with ex-vacuum or hypertensive ventriculomegaly; dBrain CT lesions suggestive of CZS and negative serology for the TORCHSinfections; three children have positive IgM; eBrain CT lesions suggestive of CZS, but without all serologies for the TORCHS infections performed or with inconclusive results; fFisher’s exact test.
DISCUSSION
Maternal ZIKV infection in the first trimester of pregnancy was associated with greater severity of brain injuries and microcephaly at birth, as well as lower HC z-scores at birth. In addition, greater severity of brain CT findings was also associated with lower HC z-scores at birth.
This study has some limitations. Not all described cases have been confirmed by qRT-PCR or PRNT for ZIKV. However, probable cases were included because they present clinical and neuroimaging findings markedly suggestive of CZS, such as microcephaly at birth, disseminated cortical/subcortical calcifications, cortical malformations, neuronal migration disorders, brainstem and cerebellum hypoplasia and ventriculomegaly4,5,9. In addition, a study carried out with 602 confirmed and probable cases did not show clinically significant differences between the two groups13. We compared the diagnostic classification highly probable and moderately probable groups and found no significant difference in severity of brain CT damages between these two groups. Furthermore, it has recently been shown that a negative PRNT does not exclude CZS. Among mothers with qRT-PCR positive for ZIKV, only 48.5% had a positive PRNT23. Considering that this case series was performed at a referral center, it is possible that children with more severe neurological symptoms were more likely to be referred to that center, which may have led to selection bias.
As a strong point, this study included a greater number of CZS cases than previously performed studies, allowing the detection of statistically significant associations that were still little clarified in the literature.
The proposed classification of brain CT damages was based on the severity of the findings. Severe reduction of cerebral parenchyma and cortical and posterior fossa malformation were considered severe or moderate because they are associated with a more severe spectrum of CZS11. However, it was decided to consider severe reduction of brain volume as the most serious abnormality, due to the global involvement of the brain and to occur, in most cases, with other concomitant findings. In turn, mild ventriculomegaly and calcifications were considered mild, as they were related to milder cases of CZS11.
More severe findings in brain CT scans were associated with reported maternal infection in the first trimester of pregnancy. A similar result was found in a study with 23 children with confirmed or probable CZS16, in which all cases with severe reduction of the cerebral parenchyma had a history of ZIKV maternal infection in the first trimester, but there was no statistically significant association, probably due to the study’s small sample size. Previous cohorts have also identified greater involvement of the central nervous system (CNS) when the infection occurred during the first trimester of pregnancy6,7. In the prospective cohort of Pomar et al.7, brain abnormalities such as reduced brain volume, posterior fossa abnormalities and cortical malformations were significantly more frequent when the child was exposed to ZIKV at the beginning of pregnancy.
The presence of reduced brain volume in children infected in the first trimester can be explained by defects induced by the virus in the neuronal proliferation of radial glial cells and glia, a process that occurs around the 3rd month of pregnancy24. Oliveira-Szejnfield et al. 15 also suggested that more severe brain dysmorphisms would be associated with infection during early pregnancy, as this is a period of rapid brain development. In addition, fetuses infected during the first trimester showed neurological findings suggestive of pathological changes during embryogenesis6, which could justify more severe brain lesions. However, it is important to remember that a late pregnancy infection does not rule out the possibility of brain abnormalities, even if they are less severe6.
In addition to the more severe brain CT lesions, greater severity of microcephaly at birth was also associated with maternal infection in the first trimester of pregnancy. Retrospective studies showed a higher risk of microcephaly at birth when the infection occurred in the first gestational trimester12,25,26, however they did not classify microcephaly as mild or severe. On the other hand, França et al.13 identified in their case series that the earlier the skin rash occurred during pregnancy, the lower the mean head circumference at birth, suggesting a possible association between microcephaly severity and trimester of maternal ZIKV infection in pregnancy.
Lower values of HC z-score were also associated to maternal infection in the first trimester of pregnancy, with a median of -3.22 for children infected during this period. This result is in accordance with a study carried out with 602 definitive or probable cases of CZS, in which the mean of the HC z-score was -3.0 when the skin rash occurred in the first trimester, -2.4 in the second, and -1.5 in the third trimester13. However, in another study carried out with 87 confirmed cases, no significant difference in HC z-scores according to trimester of ZIKV infection during pregnancy was identified14.
Infection with ZIKV in the first gestational trimester is the main risk factor for microcephaly and other CNS abnormalities18. During this period of gestation, ZIKV has an impressive tropism for maternal and fetal cells, having wide capacity to infect the maternal basal decidua and placental cells, break through the fetal barrier and spread to the fetus27. After the fetal infection, the virus targets the developing brain’s neural progenitor cells directly, activating the innate immune response, which can lead to increased cell death, interrupted cell cycle progression, reduced proliferation and premature differentiation, resulting in impaired neurogenesis and provoking changes in the development of the CNS, which are possibly responsible for the development of microcephaly28.
Another finding that contributes to this hypothesis was the association found between greater severity of brain CT lesions and lower HC z-scores at birth. This result differs from that found in a series of 87 confirmed cases14, in which no difference was found in HC z-scores between children with and without abnormalities in neuroimaging. However, in line with our findings, Aragao et al.11 identified a significant difference in head CT findings comparing children with and without microcephaly at birth11. Children with normal HC at birth had brain injuries considered milder, such as calcifications at the cortico-subcortical junction and mild ventriculomegaly. In turn, children with a HC z-score 2 standards deviations below the value expected at birth had more brain abnormalities and injuries considered more serious, such as severe parenchyma reduction, cortical malformation and posterior fossa malformations (brainstem hypoplasia)11.
In conclusion, maternal infection in the 1st trimester of pregnancy is an important risk factor for the development of a more severe spectrum of CZS. When ZIKV infection occurs during this period of significant CNS development, it leads to more severe brain abnormalities and, consequently, to lower HC z-scores at birth and to a greater severity of microcephaly.
In addition, the proposed severity classification of brain CT showed prognostic implications, having the sensitivity to distinguish those children with more severe CZS and more likely to present microcephaly at birth.
Footnotes
FUNDING
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Nº 440573/2016-5), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Nº 88881.130813/2016-01) and Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA) (Nº 008/2016).
REFERENCES
- 1.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde Monitoramento integrado de alterações no crescimento e desenvolvimento relacionadas à infecção pelo vírus Zika e outras etiologias infecciosas, da Semana Epidemiológica 45/2015 até a Semana Epidemiológica 02/2017. [cited 2020 Jul 28];Bol Epidemiol. 2017 48:1–18. http://portalarquivos2.saude.gov.br/images/pdf/2017/fevereiro/27/2017_003.pdf. [Google Scholar]
- 2.Centers for Disease Control and Prevention CDC concludes Zika causes microcephaly and other birth defects. [cited 2020 Jul 28]. https://www.cdc.gov/media/releases/2016/s0413-zika-microcephaly.html.
- 3.World Health Organization WHO statement on the third meeting of the International Health Regulations (2005) (IHR(2005)) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. [cited 2020 Jul 28]. https://www.who.int/en/news-room/detail/14-06-2016-who-statement-on-the-third-meeting-of-the-international-health-regulations-(2005)-(ihr(2005))-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations.
- 4.Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, et al. Characterizing the pattern of anomalies in congenital zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171:288–295. doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Congenital Zika syndrome & other birth defects. [cited 2020 Jul 28]. https://www.cdc.gov/pregnancy/zika/testing-follow-up/zika-syndrome-birth-defects.html.
- 6.Brasil P, Pereira JP, Jr, Moreira ME, Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pomar L, Malinger G, Benoist G, Carles G, Ville Y, Rousset D, et al. Association between Zika virus and fetopathy: a prospective cohort study in French Guiana. Ultrasound Obstet Gynecol. 2017;49:729–736. doi: 10.1002/uog.17404. [DOI] [PubMed] [Google Scholar]
- 8.Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet. 2017;390:2099–2109. doi: 10.1016/S0140-6736(17)31450-2. [DOI] [PubMed] [Google Scholar]
- 9.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Secretaria de Atenção à Saúde . Orientações integradas de vigilância e atenção à saúde no âmbito da Emergência de Saúde Pública de Importância Nacional : procedimentos para o monitoramento das alterações no crescimento e desenvolvimento a partir da gestação até a primeira infância, relacionadas à infecção pelo vírus Zika e outras etiologias infeciosas dentro da capacidade operacional do SUS. Brasília: Ministério da Saúde; 2017. [cited 2020 Jul 28]. http://bvsms.saude.gov.br/bvs/publicacoes/orientacoes_integradas_vigilancia_atencao_emergencia_saude_publica.pdf. [Google Scholar]
- 10.Moura da Silva AA, Ganz JS, Sousa PD, Doriqui MJ, Ribeiro MR, Branco MD, et al. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg Infect Dis. 2016;22:1953–1956. doi: 10.3201/eid2211.160956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aragao MF, Holanda AC, Brainer-Lima AM, Petribu NC, Castillo M, van der Linden V, et al. Nonmicrocephalic infants with congenital Zika syndrome suspected only after neuroimaging evaluation compared with those with microcephaly at birth and postnatally: how large is the Zika virus “iceberg”? Am J Neuroradiol. 2017;38:1427–1434. doi: 10.3174/ajnr.A5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira WK, Cortez-Escalante J, Oliveira WT, Carmo GM, Henriques CM, Coelho GE, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- 13.França GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388:891–897. doi: 10.1016/S0140-6736(16)30902-3. [DOI] [PubMed] [Google Scholar]
- 14.Meneses JD, Ishigami AC, Mello LM, Albuquerque LL, Brito CA, Cordeiro MT, et al. Lessons learned at the epicenter of Brazil’s congenital Zika epidemic: evidence from 87 confirmed cases. Clin Infect Dis. 2017;64:1302–1308. doi: 10.1093/cid/cix166. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira-Szejnfeld PS, Levine D, Melo AS, Amorim MM, Batista AG, Chimelli L, et al. Congenital brain abnormalities and Zika virus: what the radiologist can expect to see prenatally and postnatally. Radiology. 2016;281:203–218. doi: 10.1148/radiol.2016161584. [DOI] [PubMed] [Google Scholar]
- 16.Aragao MF, van der Linden V, Brainer-Lima AM, Coeli RR, Rocha MA, Silva PS, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. i1901BMJ. 2016;353 doi: 10.1136/bmj.i1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pool KL, Adachi K, Karnezis S, Salamon N, Romero T, Nielsen-Saines K, et al. Association between neonatal neuroimaging and clinical outcomes in Zika-exposed infants from Rio de Janeiro, Brazil. JAMA Netw Open. 2019;2:e198124. doi: 10.1001/jamanetworkopen.2019.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soriano-Arandes A, Rivero-Calle I, Nastouli E, Espiau M, Frick MA, Alarcon A, et al. What we know and what we don’t know about perinatal Zika virus infection: a systematic review. Expert Rev Anti Infect Ther. 2018;16:243–254. doi: 10.1080/14787210.2018.1438265. [DOI] [PubMed] [Google Scholar]
- 19.Baer A, Kehn-Hall K. Viral concentration determination through plaque assays: using traditional and novel overlay systems. J Vis Exp. 2014;93:e52065. doi: 10.3791/52065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabe IB, Staples JE, Villanueva J, Hummel KB, Johnson JA, Rose L, et al. Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb Mortal Wkly Rep. 2016;65:543–546. doi: 10.15585/mmwr.mm6521e1. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Hu L, Wu M, Zhong T, Zhou YH, Hu Y. Kinetics of IgG antibody to cytomegalovirus (CMV) after birth and seroprevalence of anti-CMV IgG in Chinese children. 304Virol J. 2012;9 doi: 10.1186/1743-422X-9-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 23.Ximenes RA, Miranda-Filho DB, Brickley EB, Montarroyos UR, Martelli CM, Araújo TV, et al. Zika virus infection in pregnancy: establishing a case definition for clinical research on pregnant women with rash in an active transmission setting. PLoS Negl Trop Dis. 2019;13:e0007763. doi: 10.1371/journal.pntd.0007763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes ML, Carlini CR, Marinowic D, Kalil F, Neto, Fiori HH, Scotta MC, et al. J Pediatr. Vol. 92. Rio J: 2016. Microcephaly and Zika virus: a clinical and epidemiological analysis of the current outbreak in Brazil; pp. 230–240. [DOI] [PubMed] [Google Scholar]
- 25.Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016;375:1–4. doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet. 2016;387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, et al. Zika virus reveals broad tissue and cell tropism during the first trimester of pregnancy. 35296Sci Rep. 2016;6 doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen Z, Song H, Ming GL. How does Zika virus cause microcephaly? Genes Dev. 2017;31:849–861. doi: 10.1101/gad.298216.117. [DOI] [PMC free article] [PubMed] [Google Scholar]