Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by 2019 novel coronavirus (2019-nCoV) has been a crisis of global health, whereas the effective vaccines against 2019-nCoV are still under development. Alternatively, utilization of old drugs or available medicine that can suppress the viral activity or replication may provide an urgent solution to suppress the rapid spread of 2019-nCoV. Andrographolide is a highly abundant natural product of the medicinal plant, Andrographis paniculata, which has been clinically used for inflammatory diseases and anti-viral therapy. We herein demonstrate that both andrographolide and its fluorescent derivative, the nitrobenzoxadiazole-conjugated andrographolide (Andro- NBD), suppressed the main protease (Mpro) activities of 2019-nCoV and severe acute respiratory syndrome coronavirus (SARS-CoV). Moreover, Andro-NBD was shown to covalently link its fluorescence to these proteases. Further mass spectrometry (MS) analysis suggests that andrographolide formed a covalent bond with the active site Cys145 of either 2019-nCoV Mpro or SARS-CoV Mpro. Consistently, molecular modeling analysis supported the docking of andrographolide within the catalytic pockets of both viral Mpros. Considering that andrographolide is used in clinical practice with acceptable safety and its diverse pharmacological activities that could be beneficial for attenuating COVID-19 symptoms, extensive investigation of andrographolide on the suppression of 2019-nCoV as well as its application in COVID-19 therapy is suggested.
Keywords: 2019-nCoV, SARS-CoV, Main protease, Andrographolide
Highlights
-
•
2019-nCoV and SARS-CoV main proteases exhibit similar enzyme kinetics.
-
•
Andrographolide inhibits enzyme activity of 2019-nCoV and SARS-CoV main proteases.
-
•
Andrographolide is covalently linked to active cysteine of 2019-nCoV main protease.
1. Introduction
The outbreak of COVID-19, caused by a 2019-nCoV, is an urgent global health crisis which requires a timely solution. As end of July 2020, there are approximately eighteen million confirmed cases and seven hundred thousand deaths worldwide. Currently, the target-based therapeutics and effective treatment regimens for COVID-19 are still limited. One of the potential strategies is to target the proteins which are essential for life cycle of 2019-nCoV, such as main protease (Mpro) and papain-like protease (PLpro) [1]. Mpro is the viral protease involved in production of functional polyproteins required for viral replication, and no human homolog of this protein exists [2]. Remarkably, protein sequence of 2019-nCoV Mpro is highly similar to that of the SARS-CoV Mpro which is a proven target for reducing viral load of SARS [2,3]. Therefore, 2019-nCoV Mpro is a promising target for therapeutic intervention against COVID-19. Indeed, in vitro and in silico studies on identifying potential Mpro inhibitors are rapidly growing in numbers [[3], [4], [5]]. Moreover, lopinavir/ritonavir, previously identified as HIV protease inhibitors and found to exhibit anti-SARS-CoV activity in vitro and in clinical, have been proposed to bind 2019-nCoV Mpro and are being investigated for COVID-19 treatments [6,7]. However, several clinical trials indicate that they have limited efficacy in treating COVID-19 and caused significant adverse effects, such as gastrointestinal intolerance and hepatotoxicity [8].
Recently, a three-dimensional (3D) structure of 2019-nCoV Mpro including the substrate-binding pocket has been illustrated [4]. In addition, Mpro in SARS-CoV or 2019-nCoV is classified as a cysteine protease which involves an active site cysteine in its catalytic dyad. It has been shown that Mpro could be inactivated by the inhibitors initiating a Michael addition reaction on the active site cysteine [2,4]. Therefore, the Michael acceptor-containing compounds which can fit in the catalytic pocket of 2019-nCoV Mpro may serve as attractive drug candidates for treating COVID-19.
Plant-derived natural products play crucial roles in new drug development [9]. Andrographolide, a lactone diterpenoid compound highly abundant in leaves of medicinal plant Andrographis paniculata, has been demonstrated to exhibit diverse pharmacological activities, including anti-inflammatory, anti-viral, anti-cancer and hepatoprotective effects [10]. Both A. paniculata extract and andrographolide alone are currently used worldwide for treating upper respiratory diseases as well as inflammatory diseases [[10], [11], [12]]. In addition, their clinical trials demonstrate that no significant adverse effects were observed in patients [[10], [11], [12]]. More importantly, andrographolide which contains a Michael acceptor group has been shown to react with the Cys62 of NF-κB-p50 through covalent linkage [13,14], rendering it as a potential inhibitor of 2019-nCoV Mpro. In this study, we investigated the inhibitory effects of andrographolide and its fluorescent derivative on the in vitro activity of 2019-nCoV Mpro.
2. Material and methods
2.1. Chemicals and reagents
Andrographolide and disulfiram were purchased from Sigma-Aldrich (USA). Chemical syntheses of ANDRO-NBD and NCTU-048 were performed based on our previous studies [15,16]. Sequencing grade chymotrypsin was obtained from Promega (USA).
2.2. Protein expression and purification of SARS-CoV Mpro and 2019-nCoV Mpro
Preparation of the recombinant SARS-CoV Mpro was performed following a previous report [17]. Moreover, the DNA sequence of 2019-nCoV Mpro was cloned into a pET-29a vector to encode recombinant protease with a C-terminal His6-tag. Upon plasmid transformation and IPTG induction in BL21 E. coli strain, the collected lysate supernatant was further purified by Ni-NTA affinity column (Qiagen, Germany) and S-100 size-exclusion chromatography column (GE Healthcare, USA) to produce pure 2019-nCoV Mpro.
2.3. Protease activity assay
Activities of 2019-nCoV Mpro and SARS-CoV Mpro were measured following a previous cleavage assay which used a fluorogenic peptide substrate (Abz-TSAVLQSGFRK-Dnp) in phosphate buffered saline (PBS) buffer (20 mM, pH 7.6) at 30 °C for 3 min as [18]. Upon the cleavage by protease, the quencher dinitrophenyl (Dnp) at C-terminal was released from the N-terminal fluorophore aminobenzoyl (Abz). Subsequently, the fluorescence at 423 nm was detected with excitation at 321 nm using a PerkinElmer LS 50B luminescence spectrometer (UK). The reaction concentrations of peptide substrate were ranged from 2 μM to 40 μM in PBS buffer (20 mM, pH 7.6), whereas the concentration of 2019-nCoV Mpro and SARS-CoV Mpro were respectively kept as 0.12 μM and 0.48 μM. Kinetics parameters like K m and kcat were determined by fitting the initial velocities at different substrate concentrations to a Michaelis-Menten equation, as described previously [18].
2.4. Inhibition of Mpro activity and determination of the half-maximal inhibitory concentration (IC50)
To determine inhibition of Mpro activity, inhibitor (0 μM–20 μM) and fluorogenic peptide substrate (5 μM) in PBS buffer (20 mM, pH 7.6) were first equilibrated at 30 °C for 3 min, followed by addition of protease (0.12 μM 2019-nCoV Mpro or 0.48 μM SARS-CoV Mpro) and further incubation at 30 °C for 3 min. As described above, enzymatic activity was determined and the measured velocities at different inhibitor concentrations were fitted to obtain IC50 according to the following equation:
in which ν is the velocity with incubation of inhibitor at different concentration [I] and the v0 is the initial velocity without incubation of inhibitor, while n is the Hill constant.
2.5. Fluorescence detection of main proteases upon inhibition with Andro-NBD
A total of 3 μM Mpro was treated with Andro-NBD in various molar ratios at 25 °C for 1 h and then analyzed by 12% SDS-PAGE. The resulting polyacrylamide gels were scanned for fluorescence at 520 nm under excitation at 488 nm using Amersham Imager 680 (GE healthcare, USA).
2.6. Docking analysis
The 3D structures of viral main protease were downloaded from the protein data bank (PDB, https://www.rcsb.org) which collects the published X-ray structures of SARS-CoV Mpro and 2019-nCoV Mpro [4,19]. Water molecules, ions, co-crystalized ligand were first removed from original structure prior to docking experiment, and chemical structure of andrographolide was added. The resulted complex of protein/andrographolide was subjected to molecular docking using Arguslab 4.0.1 to evaluate the putative ligand-binding site and release of free energy [20]. The optimal docking sites for ligand were accordingly predicted.
2.7. Statistical analysis
All of the data were represented as the mean ± standard derivation (SD) from three independent experiments. ∗ represents p-value <0.05; ∗∗ represents p-value <0.01; ∗∗∗ represents p-value <0.001 by Student’s t-test.
3. Results
3.1. 2019-nCoV Mpro and SARS-CoV Mpro shares similar consensus substrate sequence and enzyme kinetics
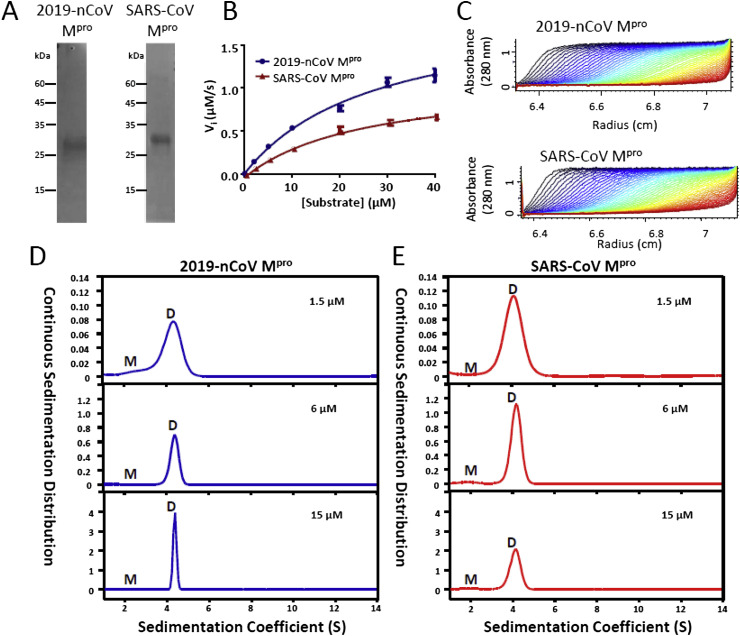
Recent genome analysis of 2019-nCoV revealed the organization of its open reading frame 1 (ORF1) which encodes two polyproteins consisting of all the non-structural proteins needed for virus replication and is very similar to SARS-CoV [21,22] (Fig. S1). In addition, sequence alignment of main proteases from 2019-nCoV, SARS-CoV and MERS-CoV showed that 2019-nCoV Mpro and SARS-CoV Mpro shares 96% identity, whereas the identity between 2019-nCoV Mpro and MERS-CoV Mpro is only 51% (Fig. S2). Because Mpro is the essential protease which is responsible to produce the individual non-structural proteins upon proteolytic cleavage on the primary polyproteins, we further aligned amino acid sequences of all the 12 cleavage sites by 2019-nCoV Mpro (Fig. S3A). Among these sites, 8 of them are completely conserved between 2019-nCoV Mpro and SARS-CoV Mpro, including the peptide sequence between nsp4 and nsp5 (-TSITSAVLQSGFRKMAFP-) (Fig. S3B). Previously, a fluorogenic peptide (Abz-TSAVLQSGFRK-Dnp) which contains both a fluorophore (Abz) and its quencher (Dnp), has been used to determine the protease activity of SARS-CoV Mpro (Fig. S3C) [23]. Upon the cleavage of this peptide probe by protease, the fluorophore (Abz) was separated from its quencher and thus emitted fluorescence under UV excitation. We therefore prepared the recombinant 2019-nCoV Mpro and SARS-CoV Mpro, and measured their protease activities using this fluorogenic probe (Fig. 1 A). Results of the protease activity assay revealed that 2019-nCoV Mpro exhibits higher activity and catalytic efficiency than SARS-CoV Mpro (Fig. 1B and Table 1 ).
Fig. 1.
Enzymatic activities and quaternary structure analyses of the 2019-nCoV Mpro and SARS-CoV Mpro. (A) Expression and purification of 2019-nCoV Mpro and SARS-CoV Mpro. (B) Enzyme activities of 2019-nCoV Mpro and SARS-CoV Mpro were measured using a proteolytic activity assay. (C) Absorbance patterns acquired during the sedimentation velocity experiment. (D) Profiles of 2019-nCoV Mpro quaternary structures at various protein concentration. M as monomer and D as Dimer. (E) Profiles of SARS-CoV Mpro quaternary structures at various protein concentration.
Table 1.
Comparison of kinetics parameters and dimer dissociation constants.
| Enzymes | Kinetics parametersa |
Dimer dissociation constants Kd (μM) | ||
|---|---|---|---|---|
| Km (μM) | kcat (s−1) | kcat/Km (s−1μM−1) | ||
| 2019-nCoV Mpro | 27.56 ± 4.94 | 163.69 ± 15.09 | 5.94 ± 3.05 | 1.23 |
| SARS-nCoV Mpro | 25.83 ± 4.20 | 76.48 ± 6.04 | 2.96 ± 1.44 | 0.35 |
All the experiments were performed in triplicates. Km, Machaelis-Menten constant; kcat, catalytic constant; kcat/Km, specificity constant for hydrolysis of peptide substrate. (Abz-TSAVLQSGFRK-Dnp).
3.2. Sedimentation coefficient distribution suggests a dimeric quaternary structure of 2019-nCoV Mpro
SARS-CoV Mpro was previously shown to function in a dimeric form. We therefore measured the continuous sedimentation coefficient distribution using analytical ultracentrifugation (AUC) to determine whether 2019-nCoV Mpro forms a homodimer (Fig. 1C). The profiles of quaternary structure at different concentrations suggest that 2019-nCoV Mpro exists predominantly in a dimeric structure (Fig. 1D), using SARS-CoV Mpro as a reference (Fig. 1E). In addition, SARS-CoV Mpro was previously shown to increase its monomeric form when existed in acidic environment [23]. However, further AUC experiments of 2019-nCoV Mpro under acidic condition (pH 6) only slightly increased its monomeric ratio. (Fig. S4).
3.3. Andrographolide inhibits protease activities of 2019-nCoV Mpro and SARS-CoV Mpro
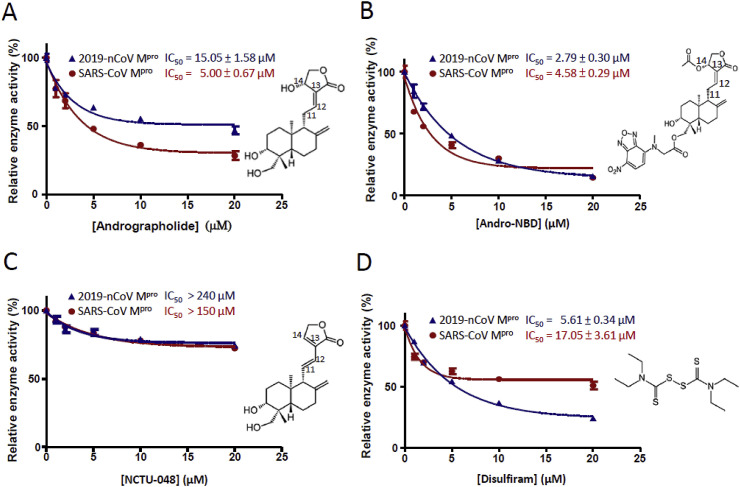
Andrographolide has been reported to inhibit protein function through a Michael addition reaction with the free thiol side chain of cysteine [13,14]. We thus further examined whether the thiol-sensitive andrographolide could inhibit the cysteine proteases like 2019-nCoV Mpro and SARS-CoV Mpro. As shown in Fig. 2 A, andrographolide inhibited protease activity of 2019-nCoV Mpro at IC50 of 15.05 ± 1.58 μM, whereas SARS-CoV Mpro was inhibited by andrographolide with an IC50 of 5.00 ± 0.67 μM. In addition, a previously reported fluorescent probe of andrographolide (Andro-NBD) also inhibited both 2019-nCoV Mpro and SARS-CoV Mpro at IC50 of 2.79 ± 0.30 μM and 4.58 ± 0.29 μM, respectively (Fig. 2B). To investigate whether andrographolide and Andro-NBD inhibited 2019-nCoV Mpro and SARS-CoV Mpro in a Michael addition-dependent manner, a thiol-insensitive andrographolide derivative (NCTU-048) was also examined in the protease activity assay and it exhibited relatively low protease inhibition (Fig. 2C). Moreover, a previously reported SARS-CoV Mpro inhibitor (disulfiram) was also investigated for its inhibition on 2019-nCoV Mpro and the result showed an IC50 of 5.61 ± 0.34 μM (Fig. 2D).
Fig. 2.
Inhibition of 2019-nCoV Mpro activity by andrographolide and its derivatives. Protease activities of 2019-nCoV Mpro or SARS-CoV Mpro in the presence of inhibitors at five different concentrations was measured. IC50 of (A) andrographolide (B) Andro-NBD (C) NCTU-048 and (D) disulfiram were determined and shown respectively. All the experiments were independently carried out in triplicates.
3.4. Andro-NBD and andrographolide form covalent linkages with 2019-nCoV Mpro and SARS-CoV Mpro
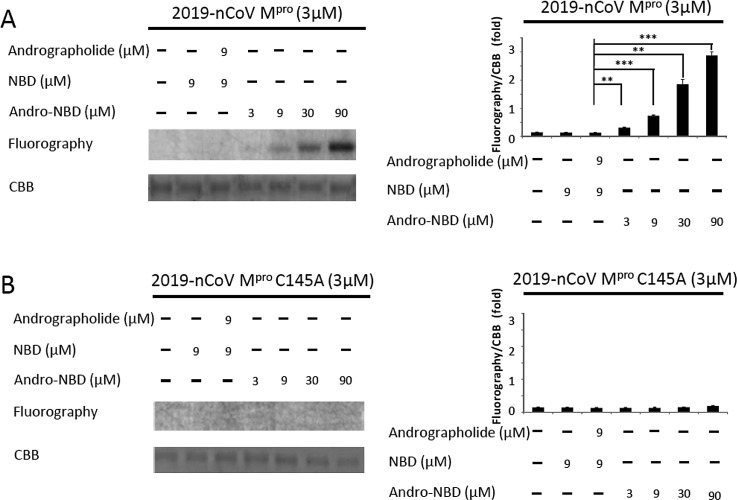
As described above, both andrographolide and Andro-NBD were shown to inhibit the activity of 2019-nCoV Mpro. We have previously demonstrated that Andro-NBD forms a covalent bond with p50 subunit of NF-kB [15]. Whether Andro-NBD also inhibits 2019-nCoV Mpro through a covalent linkage was herein investigated. As shown in Fig. 3 A, protein bands of 2019-nCoV Mpro treated with Andro-NBD at different concentrations exhibited fluorescent signals on SDS-PAGE gels, whereas treatments of nitrobenzoxadiazole (NBD) alone or together with andrographolide did not induce such fluorescence. Similarly, Andro-NBD also formed a covalent linkage with SARS-CoV Mpro (Fig. S5). Furthermore, C145A mutation of 2019-nCoV Mpro abolished the above fluorescence by treatment of Andro-NBD at all concentrations (Fig. 3B). Moreover, the andrographolide-treated 2019-nCoV Mpro was subjected to mass spectrometry analysis for the evidence of covalent linkage. As shown in Fig. S6A, the peptide signal at 2328.0330 m/z matched to the 2019-nCoV Mpro residue (141–159) which is deduced to carry a dehydrated andrographolide (+332 Da) on Cys145. Further MS/MS fragmentation analysis of such mass signal verified the partial amino acid sequences of this Cys145-containing residue. Similarly, the andrographolide-labeled residues of SARS-CoV Mpro was also identified (Fig. S6B). Taken together, we have demonstrated that Andro-NBD and andrographolide form covalent linkages with 2019-nCoV Mpro and SARS-CoV Mpro.
Fig. 3.
Andro-NBD formed covalent linkage with Cys145 of 2019-nCoV Mpro. (A) 2019-nCoV Mpro was incubated with various concentration of Andro-NBD at 25 °C for 1 h and subsequently analyzed by SDS-PAGE. Gel fluorescence was detected and scanned for image. Quantitative data were shown as mean ± SD from three independent experiments. ∗∗ represents p-value <0.01 and ∗∗∗ represents p-value <0.001. (B) The C145A mutant 2019-nCoV Mpro was also incubated with various concentration of Andro-NBD at 25 °C for 1 h and subsequently analyzed by SDS-PAGE. No gel fluorescence was detected.
3.5. Andrographolide can dock into the catalytic pockets of 2019-nCoV Mpro and SARS-CoV Mpro
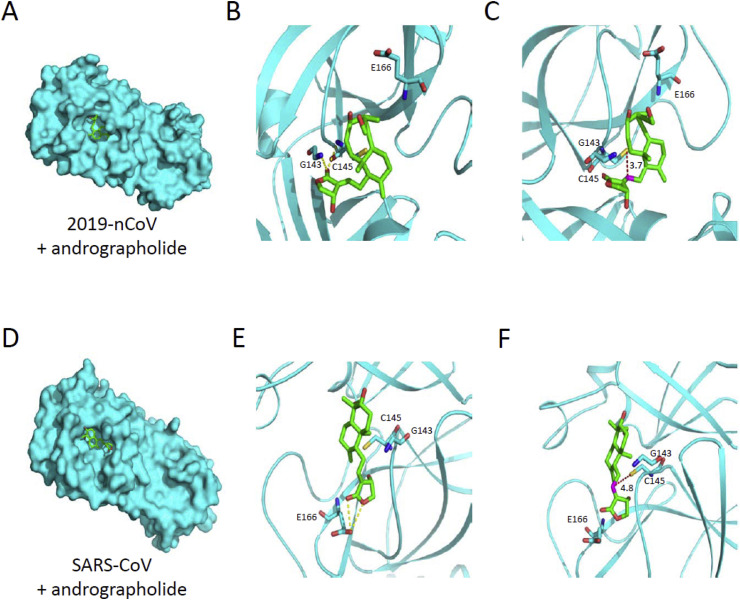
We further carried out the docking experiment using Arguslab modeling program [20] to study whether andrographolide enters the catalytic pockets of 2019-nCoV Mpro (PDB 6LU7) and SARS-CoV Mpro (PDB 1UK4) [4,19]. As shown in Fig. 4 A, modeling results suggested the presence of andrographolide in the catalytic pocket of 2019-nCoV Mpro. The binding affinity of andrographolide in 2019-nCoV Mpro was estimated to be −9.72 kcal/mol (ΔG) and the distance between Cys145 and the acceptor carbon of Michael reaction (C12 of andrographolide) is 3.7 Å (Fig. 4B and C). Similarly, SARS-CoV Mpro exhibits comparable binding affinity of andrographolide at −7.85 kcal/mol and its Cys145 is 4.8 Å away from Michael addition site (Fig. 4E and F). Together, these results support our findings of andrographolide-mediated inhibition and covalent linkage of 2019-nCoV Mpro and SARS-CoV Mpro.
Fig. 4.
Prediction of the putative binding site for andrographolide in 2019-nCoV Mpro and SARS-CoV Mpro. (A) Overall predicted surface model for the complex of 2019-nCoV Mpro (cyan) and andrographolide (green) was established. (B) Amplified region of catalytic pocket highlighting the hydrogen bonds (yellow dot line) between Mpro residues and andrographolide. (C) The distance (red dot line) between Mpro Cys145 (yellow) and Michael acceptor carbon (magenta) of andrographolide is shown. (D) Overall predicted surface model for the complex of SARS-CoV Mpro (cyan) and andrographolide (green) was established. (E, F) Amplified regions of catalytic pocket for complex in (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Our results demonstrated that andrographolide can inhibit the activity of 2019-nCoV Mpro (IC50 = 15.05 ± 1.58 μM; Fig. 2A). Molecular modeling data further support that andrographolide can enter the proposed substrate-binding pocket of 2019-nCoV Mpro (Fig. 4), such observation is consistent with a recent in silico study suggesting that andrographolide can dock in the binding site of 2019-nCoV Mpro [24]. Furthermore, we also found that Andro-NBD, but not compound 048, strongly inhibited the activity of 2019-nCoV Mpro (IC50 = 2.79 ± 0.3 μM for Andro-NBD; > 240 μM for NCTU-048) ( Fig. 2 ). 2019-nCoV Mpro is a cysteine protease known to be inactivated by Michael acceptor inhibitor N3 [4]. Andrographolide belongs to the Michael acceptor category of electrophilic natural compounds and it has been reported to react with Cys62 of NF-κB-p50 at C12-13 exocyclic double bond to form a covalent adduct through a Michael addition [13,14]. Andro-NBD also can covalently bond to the p50, presumably through a similar mechanism [15]. On the other hand, the compound NCTU-048 with much weaker bioactivity than andrographolide is suggested to interact with NF-κB-p50 through different mechanism [25]. Accordingly, Andro-NBD was shown capable of forming covalent linkage with 2019-nCoV Mpro, whereas C145A mutation of protease abolished such covalent bond (Fig. 3). Furthermore, MS-based sequencing analysis suggested that andrographolide forms a covalent bond with active site Cys145 of 2019-nCoV Mpro (Fig. S6). Notably, such covalent linkage of andrographolide with reactive cysteine of protein target has also been reported previously [26]. Together, our data support a model that Andro and Andro-NBD could enter the substrate-binding pocket and initiate Michael reaction with 2019-nCoV Mpro, leading to the inactivation of protease activity.
Lopinavir/ritonavir, the HIV protease inhibitors previously identified with anti-SARS-CoV activity in vitro and in clinical, was proposed to bind 2019-nCoV Mpro and thus has been investigated for COVID-19 treatments [6,7]. Currently, inhibition of 2019-nCoV by Lopinavir/ritonavir has not been experimentally demonstrated. Data of several clinical trials indicate their roles in treating COVID-19 are limited and their significant adverse effects, such as gastrointestinal intolerance and hepatotoxicity, have been observed [8]. Disulfiram, an FDA-approved drug for treating alcohol addiction, has been reported to inhibit 2019-nCoV Mpro [4] but its clinical efficacy against COVID-19 remains to be determined. Therefore, development of 2019-nCoV Mpro inhibitors from clinically available medicine is still in great demand.
Andrographis paniculata along with its major ingredient, andrographolide, have been used as herbal medicine for treating anti-inflammatory diseases in Asia and in Europe [10]. Accumulating evidences from clinical studies indicate that Andrographis paniculata extract and andrographolide reduce symptoms in patients with HIV, upper respiratory infection, ulcerative colitis or rheumatoid arthritis with considerable safety [10,[27], [28], [29], [30]]. Furthermore, experimental data from preclinical studies demonstrated the diverse pharmacological mechanisms of A. paniculata and andrographolide, including those in anti-inflammation, antiviral activity, hepatoprotection, anti-pulmonary fibrosis, and cardiopretection [10,12,[31], [32], [33]] Particularly, their safety profile in clinical uses are well accepted and andrographolide up to 30 μM did not cause cytotoxicity on human peripheral blood mononuclear cells (PBMC) [10,34].
In this study, we revealed that andrographolide and its derivative inhibits the activity of main protease and thus likely to impair the replication of SARS-CoV and 2019-nCoV. Considering that andrographolide is widely demonstrated in clinical application with acceptable safety and exhibits diverse pharmacological activities which could be beneficial for attenuating COVID-19 symptoms, application of andrographolide on COVID-19 therapy certainly merit further investigation.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We specifically thank Dr. Chi-Yuan Chou from the Department of Life Sciences at National Yang-Ming University for providing the SARS-CoV Mpro, the fluorogenic peptide probe and the technical assistance of AUC. We also thank Dr. Chen-Chung Liao of Proteomics Research Center at National Yang-Ming University for the technical assistance of MS analysis and data processing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrc.2020.08.086.
Funding information
This work was supported by grants from the Ministry of Science and Technology in Taiwan (MOST 106-2320-B-010-006-MY3, MOST 106-2311-B-010-004-MY3 and MOST 109-2327-B-010-005, MOST 109-2321-B-010-003).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 2.Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.H. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: Peptidomimetics and small molecule chemotherapy. J. Med. Chem. 2016;59:6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the Past: Possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of M(pro) from COVID-19 virus and discovery of its inhibitors. Nature. 2020 doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 5.Ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 7.Nutho B., Mahalapbutr P., Hengphasatporn K., Pattaranggoon N.C., Simanon N., Shigeta Y., Hannongbua S., Rungrotmongkol T. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry. 2020 doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- 8.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 9.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 10.Dai Y., Chen S.R., Chai L., Zhao J., Wang Y., Wang Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019;59:S17–S29. doi: 10.1080/10408398.2018.1501657. [DOI] [PubMed] [Google Scholar]
- 11.Jantan I., Ahmad W., Bukhari S.N. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015;6:655. doi: 10.3389/fpls.2015.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayakumar T., Hsieh C.Y., Lee J.J., Sheu J.R. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Based Complement Alternat Med. 2013;2013:846740. doi: 10.1155/2013/846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gersch M., Kreuzer J., Sieber S.A. Electrophilic natural products and their biological targets. Nat. Prod. Rep. 2012;29:659–682. doi: 10.1039/c2np20012k. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y.F., Ye B.Q., Li Y.D., Wang J.G., He X.J., Lin X., Yao X., Ma D., Slungaard A., Hebbel R.P., Key N.S., Geng J.G. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J. Immunol. 2004;173:4207–4217. doi: 10.4049/jimmunol.173.6.4207. [DOI] [PubMed] [Google Scholar]
- 15.Hsu Y.H., Hsu Y.L., Liu S.H., Liao H.C., Lee P.X., Lin C.H., Lo L.C., Fu S.L. Development of a bifunctional andrographolide-based chemical probe for pharmacological study. PloS One. 2016;11 doi: 10.1371/journal.pone.0152770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S.H., Lin C.H., Liang F.P., Chen P.F., Kuo C.D., Alam M.M., Maiti B., Hung S.K., Chi C.W., Sun C.M., Fu S.L. Andrographolide downregulates the v-Src and Bcr-Abl oncoproteins and induces Hsp90 cleavage in the ROS-dependent suppression of cancer malignancy. Biochem. Pharmacol. 2014;87:229–242. doi: 10.1016/j.bcp.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S.C., Chang G.G., Chou C.Y. Mutation of Glu-166 blocks the substrate-induced dimerization of SARS coronavirus main protease. Biophys. J. 2010;98:1327–1336. doi: 10.1016/j.bpj.2009.12.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin P.Y., Chou C.Y., Chang H.C., Hsu W.C., Chang G.G. Correlation between dissociation and catalysis of SARS-CoV main protease. Arch. Biochem. Biophys. 2008;472:34–42. doi: 10.1016/j.abb.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand K., Yang H., Yang M., Ding Y., Liu Y., Lou Z., Zhou Z., Sun L., Mo L., Ye S., Pang H., Gao G.F., Bartlam M., Hilgenfeld R., Rao Z. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci U S A. 2003;100:13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joy S., Nair P.S., Hariharan R., Pillai M.R. Detailed comparison of the protein-ligand docking efficiencies of GOLD, a commercial package and ArgusLab, a licensable freeware. Silico Biol. 2006;6:601–605. [PubMed] [Google Scholar]
- 21.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L., Guan Y., Rozanov M., Spaan W.J., Gorbalenya A.E. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou C.Y., Chang H.C., Hsu W.C., Lin T.Z., Lin C.H., Chang G.G. Quaternary structure of the severe acute respiratory syndrome (SARS) coronavirus main protease. Biochemistry. 2004;43:14958–14970. doi: 10.1021/bi0490237. [DOI] [PubMed] [Google Scholar]
- 24.Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen V.S., Loh X.Y., Wijaya H., Wang J., Lin Q., Lam Y., Wong W.S., Mok Y.K. Specificity and inhibitory mechanism of andrographolide and its analogues as antiasthma agents on NF-kappaB p50. J. Nat. Prod. 2015;78:208–217. doi: 10.1021/np5007179. [DOI] [PubMed] [Google Scholar]
- 26.Wang J., Tan X.F., Nguyen V.S., Yang P., Zhou J., Gao M., Li Z., Lim T.K., He Y., Ong C.S., Lay Y., Zhang J., Zhu G., Lai S.L., Ghosh D., Mok Y.K., Shen H.M., Lin Q. A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis. Mol. Cell. Proteomics. 2014;13:876–886. doi: 10.1074/mcp.M113.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang J., Zhang R.M., Zhang Y., Chen Z.B., Zhang Z.M., Xu Q., Yang Y.P., Long Y.Y., Liu L.L., Cai H.Y., Gao J., Lu N., Mao B., Wang L., Li T.Q. [Andrographolide drop-pill in treatment of acute upper respiratory tract infection with external wind-heat syndrome: a multicenter and randomized controlled trial] Zhong Xi Yi Jie He Xue Bao. 2008;6:1238–1245. doi: 10.3736/jcim20081206. [DOI] [PubMed] [Google Scholar]
- 28.Burgos R.A., Hancke J.L., Bertoglio J.C., Aguirre V., Arriagada S., Calvo M., Caceres D.D. Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: a prospective randomized placebo-controlled trial. Clin. Rheumatol. 2009;28:931–946. doi: 10.1007/s10067-009-1180-5. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese C., Berman S.H., Babish J.G., Ma X., Shinto L., Dorr M., Wells K., Wenner C.A., Standish L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res. 2000;14:333–338. doi: 10.1002/1099-1573(200008)14:5<333::aid-ptr584>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn W.J., Targan S.R., Byers V.S., Rutty D.A., Mu H., Zhang X., Tang T. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am. J. Gastroenterol. 2013;108:90–98. doi: 10.1038/ajg.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S., Mishra K.P., Ganju L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017;162:611–623. doi: 10.1007/s00705-016-3166-3. [DOI] [PubMed] [Google Scholar]
- 32.Liao W., Lim A.Y.H., Tan W.S.D., Abisheganaden J., Wong W.S.F. Restoration of HDAC2 and Nrf2 by andrographolide overcomes corticosteroid resistance in chronic obstructive pulmonary disease. Br. J. Pharmacol. 2020 doi: 10.1111/bph.15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu T., Zhang W., Xiao M., Chen H., Jin H. Protective role of andrographolide in bleomycin-induced pulmonary fibrosis in mice. Int. J. Mol. Sci. 2013;14:23581–23596. doi: 10.3390/ijms141223581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao H.C., Chou Y.J., Lin C.C., Liu S.H., Oswita A., Huang Y.L., Wang Y.L., Syu J.L., Sun C.M., Leu C.M., Lin C.H., Fu S.L. Andrographolide and its potent derivative exhibit anticancer effects against imatinib-resistant chronic myeloid leukemia cells by downregulating the Bcr-Abl oncoprotein. Biochem. Pharmacol. 2019;163:308–320. doi: 10.1016/j.bcp.2019.02.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.