Abstract
The decision to commence jejunal feeding in patients with structural abnormalities, which prevent oral or intragastric feeding, is usually straightforward. However, decisions surrounding the need for jejunal feeding can be more complex in individuals with no clear structural abnormality, but rather with foregut symptoms and pain-predominant presentations, suggesting a functional origin. This appears to be an increasing issue in polysymptomatic patients with multi-system involvement. We review the differential diagnosis together with the limitations of available functional clinical tests; symptomatic management options to avoid escalation where possible including for patients on opioids; tube feeding options where necessary; and an approach to weaning from established jejunal feeding in the context of a multidisciplinary approach to minimise iatrogenesis.
Keywords: artificial nutrition support, enteral nutrition, functional bowel disorder, gastroduodenal motility, gastroparesis
Key messages.
Jejunal feeding is primarily evidenced for severe malnutrition, not to improve functional symptoms.
Objective evidence for malnutrition should include whether or not the patient is below the normal body mass index range and/or any weight loss trajectory.
The range of functional differential diagnoses associated with oral and intragastric feed intolerance should be reviewed and symptom and psychosocial management optimised.
The concept of ‘effortful oral feeding as a least worst option’, in the face of ongoing symptoms, is a valid and frequently safer pragmatic alternative to jejunal feeding in patients with functional symptoms.
Chronic pain management and avoiding opioids is important to improve outcomes.
A multidisciplinary approach, addressing psychosocial factors associated with improved outcomes as opposed to factors increasing disability, is essential.
Introduction
Patients with obvious structural abnormalities, such as gastric outlet obstruction, post-gastrectomy or with severe pancreatitis and associated duodenal impingement, may need either short-term bridging or long-term jejunal feeding. In these patients, it is usually clear that post-pyloric feeding is clinically necessary. Such individuals with structural causes precluding oral and intragastric feeding can also often have a more obvious ‘exit strategy’ from short-term bridging jejunal feeding, such as surgery, dilatation or stenting for gastric outlet obstruction, as well as following any drainage of pancreatic pseudo-cysts and settling of pancreatic inflammation.
However, patients and nutrition support teams can face more difficult decisions when considering the appropriateness of jejunal feeding in those with functional feed-related symptoms of nausea and vomiting, early satiety, fullness and pain in the absence of any obvious structural explanation for symptoms or overt structural obstacles to oral or intragastric feeding. In our experience, this can be particularly the case for young multi-system polysymptomatic patients, especially if there are some markers for psychosocial distress and/or disordered eating behaviour, but in whom this is less overtly expressed or acknowledged, and with no clear comorbid mood disorder. Gastroenterology and nutrition teams can feel under considerable pressure to escalate to increasingly invasive approaches, when it is not clear that artificial nutrition support is definitely indicated. In these complex cases, a wide multidisciplinary team approach is required to optimise treatment but also to minimise iatrogenic harm.
Defining objective criteria for jejunal feeding
It is important when considering jejunal feeding in patients with functional symptoms to begin with the fundamental question: “what is the goal of therapy”?
The answer should self-evidently be that we are treating malnutrition. This simple fundamental question and answer can, however, become obscured by an incipient shift away from treating malnutrition, into treating underlying symptoms instead. It is important to keep malnutrition and symptom management as distinct treatment goals. This is because jejunal feeding is a clearly indicated and evidence-based approach for malnutrition, but there is little evidence that it is effective at improving functional symptoms per se if significant malnutrition is not also present. Furthermore, jejunal feeding may simply substitute one set of adverse oral feeding–related problems with mechanical jejunal tube–related problems instead, requiring an increasing number of endoscopic/radiological interventions or other tube-related problems such as infections/leakages, with a significant impact on morbidity. While considering the treatment goals, it is of course important to balance these risks, which, unfortunately, still includes mortality, particularly at the time of permanent jejunal tube insertion.1
Therefore, in patients with functional symptoms, but no objective evidence of malnutrition, the concept of “effortful oral feeding as least worst option”, in the face of ongoing symptoms, can sometimes be the safest and most appropriate approach compared with the risks associated with jejunal feeding. Effortful oral feeding may include changes in food fat content, consistency and particle size and the use of liquid oral nutritional supplements, which can help symptoms in a nutritionally balanced way in some patients.2 3
A further dilemma is what objective criteria to use to intervene with tube feeding for individuals with functional problems?
The National Institute for Clinical Excellence (NICE) defines an objective parameter for consideration of nutrition support as unintentional weight loss greater than 10% within the last 3–6 months.4 The judgement of the degree to which the weight loss is ‘unintentional’ can be problematic in people with functional gastrointestinal problems. Some patients with functional symptoms may have a body image–driven classical eating disorder that would need to be considered and excluded, although usually not. More commonly, others may have “disordered eating” due to fixed beliefs about food, or because of associated trauma, symptoms and distress which compound and adversely affect their tolerance of food. Many of these patients will meet the criteria of ‘Avoidant/Restrictive Food Intake Disorder’, which is now recognised in adults and not just seen as a developmental disorder. However, some patients may not overtly express concerns about food types, but instead restrict intake to avoid the physical symptoms that they perceive when they eat, with no associated overt psychological distress.
Even in people with functional gastrointestinal problems with some degree of weight loss, careful monitoring within a healthy body mass index (BMI) range can often allow a safer approach and prevent unnecessary premature escalation to tube feeding.
When patients with functional symptoms drop below a healthy BMI (NICE guidelines suggest less than 18.5 kg/m²) this should prompt consideration of nutrition support.4 In some patients, however, tube feeding–related functional symptoms and tube-related problems will require a re-evaluation of the risk–benefit balance. For some of these patients, again with careful monitoring, a stable BMI below the healthy range maintained with effortful oral feeding may also be a ‘least worst’ option compared with tube feeding.
Differential diagnosis and management
Functional foregut symptoms (which may present as affecting oral or intragastric feeding) include postprandial regurgitation/vomiting, nausea, early satiety and pain. Organic differentials, primary mood disorders and eating disorders (such as anorexia nervosa and bulimia) as causes for these symptoms should be excluded.5 The following key functional diagnoses should then be considered:
Rumination syndrome
Patients complain of postprandial regurgitation or vomiting shortly or even immediately after eating. The cause is (usually unconscious) habitual abdominal contractions after eating, leading to postprandial regurgitation/vomiting. This can be diagnosed by a concurrent impedance/manometry study with food provocation. Standard in-series testing with manometry then followed by impedance, as is usually performed for reflux testing, will miss the diagnosis and also be unable to distinguish between primary and secondary rumination. If there is associated significant and worsening malnutrition, then short-term nasojejunal feeding may be required for stabilisation as a bridge to therapies, but escalation to long-term invasive jejunal feeding may not be necessary because the underlying cause, once recognised, is readily treated. Therapies which can be successful include open-mouthed diaphragmatic breathing, baclofen, cognitive-behavioural therapy and, in carefully selected patients, a Nissen fundoplication.6
Cyclical vomiting syndrome
This is characterised by bouts of severe hyperemesis, usually with complete resolution of symptoms in between. If frequent, these bouts can occasionally be associated with some weight loss and malnutrition, but more usually weight is maintained. During the hyperemetic bouts, nasojejunal feeding is unlikely to be stable, and since they are usually short-lived, there should not be a need for clinically assisted nutrition. The emphasis in the acute phase therefore is generally on intravenous fluid support and anti-emetics. There may be a personal and/or family history of migraine in which case symptoms can respond to anti-migraine prophylaxis taken between attacks.6
Cannabinoid hyperemesis syndrome
Similarly to cyclical vomiting syndrome, this also presents with bouts of severe hyperemesis and a characteristic symptom of this condition is patients seeking relief from nausea and vomiting through hot showers and baths. Again, this condition does not usually require jejunal feeding either short or long term since it should be entirely reversible by complete cessation of cannabis use, which may require referral to a specialist substance misuse service.6
Chronic unexplained nausea and vomiting
This is distinguished from cyclical vomiting syndrome by the absence of severe hyperemetic bouts, but chronic lower grade persistence of symptoms. The pathophysiology of the chronic symptoms is obscure, but consideration should also be given to a possible somatic symptom syndrome. Over time, this may lead to a degree of malnutrition because of lack of intake. Pro-kinetics and anti-emetics, together with gut–brain neuromodulators such as mirtazapine may have a role alongside addressing any associated psychosocial distress. Jejunal feeding is usually advised against in the absence of objective malnutrition, but there may be a role for oral liquid nutritional supplements to support ‘effortful oral feeding’, which may be better tolerated.6
Functional dyspepsia versus gastroparesis
These two diagnostic categories display considerable overlap and are difficult to fully differentiate.7 8 Together, they represent the largest cohort of people with functional gastrointestinal problems considered for jejunal feeding due to gastric intolerance of feeding and therefore will be addressed in some detail. These conditions likely represent a spectrum of pathophysiology, from more sensory-driven visceral hypersensitivity in the functional dyspepsia–epigastric pain subtype through to a mix of sensory and motor driven mechanisms, including impaired meal-related gastric accommodation, in the functional dyspepsia–postprandial distress subtype and ultimately to global gastric muscle dysfunction in gastroparesis. These are far from water-tight categorisations, however, and both sensory and pain mechanisms may also play a major role in the clinical presentation of gastroparesis. The possible roles of duodenal and pyloric sensorimotor dysfunction have also recently been implicated in gastroparesis.9 10
Classically, the distinction between functional dyspepsia and gastroparesis was made on the finding of a delayed gastric emptying study8; however, this is no longer tenable. First, one-third of patients with functional dyspepsia also have delayed gastric emptying.11 Furthermore, the overall group correlations between gastric emptying and both symptoms and symptom improvement is poor, although can be improved by appropriately timed measurements and stopping acceleratory or slowing drugs.12 13 Nonetheless, at the individual patient level, caution is required in interpreting gastric emptying results and the diagnostic labelling of patients. A diagnostic label of ‘gastroparesis’ based on a gastric emptying test result alone may counterproductively minimise addressing sensory and psychosocial components of symptoms and drive increasing levels of invasive nutrition and other interventions, with the risk of associated iatrogenesis.
First-line approaches to any malnutrition should be to exhaust effortful oral feeding options, and to ensure visceral hypersensitivity, pain and psychosocial components are addressed, within a multidisciplinary setting. Again, this should include dietetic advice on meal frequency and portions, food fat content, consistency and particle size and the use of oral nutrition supplements to support effortful oral feeding. Anti-emetics, pro-kinetics, gut–brain neuromodulators and the possible role of buspirone in functional dyspepsia with impaired meal accommodation should be considered.6 Pain management approaches, including clinical psychology and the avoidance of opioids, are important. Once these components have been assessed and addressed, then for malnourished patients with a methodologically sound and significantly delayed gastric emptying study, in whom gastric muscle failure is likely the over-riding mechanism generating symptoms, jejunal feeding can be highly beneficial.
A short-term trial of at least a few weeks’ nasojejunal feeding for patients with predominantly gastric muscle failure should be considered before escalating to a longer-term invasive tube. This enables determination of how well the patient tolerates small bowel feeding and confirming weight gain.
If the patient is intolerant of jejunal feeding due to pain, nausea or vomiting, this should prompt evaluation for the presence of small bowel involvement before escalating to invasive tube feeding. Distal small bowel obstructing structural pathology should already have been excluded by this stage, but if in doubt re-evaluate with a small bowel contrast study (the contrast can be administered down the jejunal tube if needed). Functional problems with small bowel motility will be considered in the next section.
If jejunal tube feeding is well tolerated with weight gain, then a longer-term invasive tube can be considered. The most widely available option currently is a percutaneous endoscopic gastrostomy with jejunal extension (PEG-J), which enables both jejunal feeding and concurrently gastric venting if needed for persistent vomiting.14 PEG-Js can be limited by frequent jejunal extension displacements, but efforts to achieve a low antral insertion site can be rewarded by maximising jejunal extension stability (due to a short intragastric tube length to the pylorus) and venting when needed (due to gravitational pooling in the antrum).15 Where available, a direct PEJ may be an option to improve stability of jejunal tube position (although can still be subject to retrograde displacement into the stomach), with or without an additional gastric venting tube, if there is persistent vomiting.
Chronic intestinal pseudo-obstruction and enteric dysmotility
Chronic intestinal pseudo-obstruction (CIPO), which can be primary due to neuropathies or myopathies or secondary due to scleroderma, is readily diagnosed by the finding of dilated small bowel on cross-sectional imaging/contrast studies in the absence of any mechanical transition point. With the use of prokinetics and treatment of small intestinal bacterial overgrowth, some patients with concomitant gastric muscle failure and CIPO may be able to tolerate jejunal feeding. However, it is more usually the case that these patients will be intolerant of jejunal feeding and need to be managed with parenteral nutrition long term.16 17 Enteric dysmotility (ED) is characterised by small bowel feeding-related symptoms, normal small bowel diameter on cross-sectional imaging and abnormal small bowel manometry.18 19 Small bowel (antero-duodenal) manometry is even less of an exact science than gastric emptying studies, however, and the correlation between abnormal manometry findings, symptoms and pathology is unclear.17 Notably, in a cohort of patients with CIPO and ED on home parenteral nutrition, the patients with ED were much more likely to be successfully weaned to either oral sip or jejunal feeding.17
Centrally mediated (neuropathic) pain and narcotic bowel syndrome
For patients with a significant pain presentation to their oral/intragastric feed intolerance, the presence of an element of chronic continuous abdominal pain (CCAP) should be elicited. If present, this should lead to examination for the finding of cutaneous allodynia in the area of maximally reported pain (a non-painful stimulus, such as light brush finger strokes, eliciting pain). This is a characteristic finding in neuropathic/centrally mediated pain. Carnett’s sign (very focal increased pain in the rectus sheath on abdominal contraction) should also be examined for to exclude the anterior cutaneous nerve entrapment syndrome, which might respond to a focal subcutaneous injection of local anaesthetic/steroid.
In a large cohort of 103 patients with CCAP, 81% of whom displayed cutaneous allodynia, 50% had exacerbations precipitated by physiological events, including feeding.20 In this context, it is likely that the physiological feed-related exacerbation represents visceral allodynia. Opioid use was associated with worse outcomes and more allodynia, suggesting that opioids were adversely driving some component of the pain neurobiology. The gut–brain neuromodulator duloxetine was the most effective monotherapy, but combination neuromodulators were more effective still. Most patients (90/103, 87%) tolerated an oral diet, two patients received oral supplement drinks and three patients adhered to a liquid diet. Six patients required enteral tube feeding. In a multivariate analysis, there was an initial significant difference between patients with physiological exacerbations experiencing weight loss compared with those who did not, but this did not retain significance after correction for multiple comparisons.
In the presence therefore of CCAP, it is possible that weight loss and physiological exacerbations represent visceral allodynia, and an approach of avoiding/withdrawing opioids and the use of gut–brain neuromodulators may improve feed tolerance without recourse to invasive tube feeding.20
Narcotic bowel syndrome is related to centrally mediated pain and describes a vicious cycle of escalating pain, with escalating opioid doses and increasing opioid-related bowel dysmotility which can include pseudo-obstruction.21 Patients require stabilisation and withdrawal of opioids. Mu-opioid antagonists (such as naloxegol and methylnaltrexone) may also play a role in reducing dysmotility effects of the opioids. These patients would not usually require jejunal feeding, as the symptoms and dysmotility should improve significantly following withdrawal of the opioids.
Multi-system overlaps
It is increasingly common for patients with feeding intolerance to present with multiple functional symptom complexes including chronic fatigue syndrome/myalgic encephalomyelitis, fibromyalgia, postural orthostatic tachycardia syndromes, multiple chemical sensitivities, acoustic and photic sensitivities, hypermobile Ehlers-Danlos syndrome, functional bladder symptoms and functional neurological symptoms. There is considerable overlap between different functional conditions and somatoform disorder/somatic symptom disorder. However, these system functional disorders are often seen as discrete conditions and therefore are often managed within particular medical specialities that focus on those somatic symptoms. It is speculated that the core feature shared by all these symptom complexes is ‘central sensitisation’ of the central nervous system (so-called ‘central sensitivity syndromes’22), together with involvement of the central autonomic network and autonomic nervous system.
These patients are frequently young and may be transitioning from paediatric services. In more severe presentations, patients may develop fear avoidance, reduced function/deconditioning and maladaptive illness behaviour impacting their relationship with caregivers and health staff.
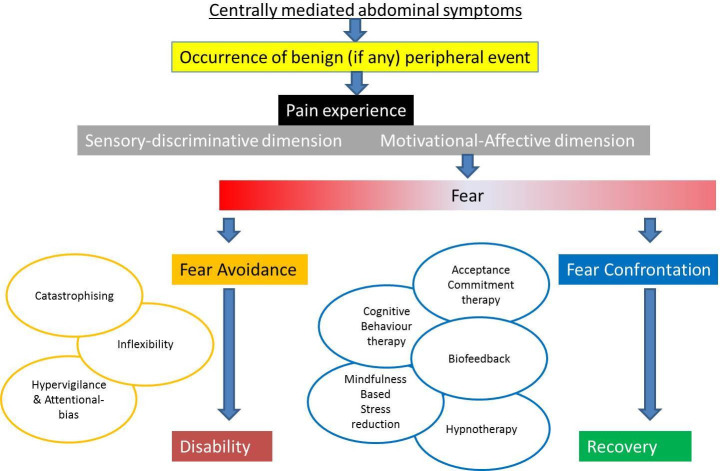
This group of patients appear to be particularly complex and vulnerable to escalating invasiveness of interventions without improvement in function or quality of life, and therefore it is essential that they are managed within a cohesive multidisciplinary team setting. It is also important to ensure that patient and carer expectations are managed from the outset. The fear avoidance model of pain is instructive in understanding factors which can determine a trajectory of either increasing disability or conversely one of rehabilitation and recovery (figure 1 23). A number of psychological approaches shown in figure 1 may be helpful in promoting recovery. Needless to say, de-escalating medicalisation of therapies, including jejunal feeding, and avoiding escalation in the first place is more likely to promote a recovery trajectory.
Figure 1.
Fear avoidance model in chronic pain (adapted from Keefer and Mandal23).
Disordered eating and food intolerances
Food intolerances and allergies can morph into obsessive and phobic behaviours leading to dietary restriction and “disordered eating”, which can lead to malnutrition due to the restrictive nature of the diet. Avoidant/restrictive food intake disorder (ARFID) describes a range of presentations where a person avoids certain food types often leading to a restriction in the overall amount that they eat. The reasons to avoid and/or restrict can include sensitivity to the nature of the food, such as the taste, smell, texture or appearance. Some patients will be fearful of the consequences of eating, especially if they had a previous negative experience such as choking or vomiting with a specific food type, or suffered from abdominal pain following ingestion. This will lead to a restriction of food types eaten and a narrowing of the repertoire of food types eaten in the person’s diet. Other patients have a limited dietary intake because they have a long-term poor appetite, are not particularly interested in food and do not gain pleasure from eating. This often leads to them not eating enough as they have no particular drive to eat and becomes a chore rather than something to look forward to and enjoy.
ARFIDs can present in many different ways but all have a component of either restriction or avoidance of overall food eaten or the range of food. However, unlike anorexia nervosa and bulimia nervosa, the avoidance/restriction of food intake is not associated with concerns about weight and body image. However, people with ARFID are at risk of significant weight loss, leading to impact on their physical health. Their social functioning is also affected and they may struggle at social occasions, where eating often has a prominent place.
Orthorexia is a presentation which is being increasingly recognised in those that are restricting their intake. It is associated with obsessive behaviour about healthy eating, leading to psychological distress, social impairment and deterioration of physical health. People with orthorexia show obsessive-compulsive behaviours in the planning, selection, preparation and eating of healthy food. There is often an unhealthy obsession with what is considered ‘clean’ or ‘pure’ food which can lead to physical health problems when food that provide essential nutrients are excluded from diet because they are seen as ‘impure’. The perception of only eating ‘pure’ food often gives the sense of being in control and being able to manage negative thoughts.
In these cases, avoiding escalation to jejunal tube feeding where possible, and early involvement of liaison psychiatry, clinical psychology and dietitian services in a multidisciplinary team is essential. A time-limited trial of tube feeding may nonetheless be required in severe malnutrition for stabilisation of nutritional status; however, nasogastric rather than jejunal tube feeding will usually suffice. An ‘exit strategy’ with a plan to ultimately wean tube feed support in tandem with mental health interventions should be considered from the outset.
When is a patient considered to have ‘failed’ jejunal feeding and what then?
When assessing the outcome of jejunal feeding, it is clearly important to come back to the fundamental question of ‘what is the goal of therapy’. The primary answer should still be the reversal of malnutrition, and so the primary assessment of success or failure of jejunal feeding should be whether or not malnutrition has improved.
Jejunal feeding should be deemed to have failed if there is ongoing worsening of malnutrition. The decision whether or not to escalate to parenteral nutrition at this point is subject to a similar algorithm as the decision-making process outlined above for jejunal feeding in the first place. The clearest indicator to escalate to parenteral nutrition in people with functional intestinal problems is CIPO with dilated small bowel. The evidence that this is appropriate in other functional disorders is far less compelling and may be subject to significant harms although it may become a pragmatic necessity when otherwise faced with severe life-limiting malnutrition; however, the risks of parenteral nutrition need to be considered at the forefront because parenteral nutrition is typically detrimental to quality of life24 and, perhaps more importantly, because parenteral nutrition can have life-threatening complications.25
What ‘exit strategies’ can be considered for jejunal feeding?
For patients with functional gastrointestinal problems with good evidence of primarily gastric muscular failure, long-term jejunal feeding may be necessary. For all the other functional diagnoses reviewed above (with the exception of CIPO), while short-term bridging jejunal feeding may be pragmatically necessary while assessing a patient and addressing the underlying condition(s), long-term jejunal feeding should not be necessary and should ideally be avoided. Once established on jejunal feeding, however, de-escalation to return to oral effortful feeding can be a challenge, but can be revisited with regular community dietetic support in primary care. Slow weaning can be safely achieved by close dietetic supervision of the patient. The inclusion of anthropometric measurements, for example, mid arm muscle circumference, grip strength and triceps skinfold measurements, should be completed by the same dietitian to minimise the risk of intraobserver error. Bioelectrical impedance, where available, can be a useful adjunct to the dietetic consultation. By engaging the patient in the review process and treatment plan, jejunal feed can be reduced over a period of time.
Once weaned, it is important to ensure the MDT provides comprehensive ongoing support for the patient in partnership with primary care. This ‘safety netting’ can obviate the need to re-escalate to jejunal feeding, which should only be considered if the team are presented with further objective parameters of significant malnutrition, even in the face of ongoing symptoms.
Conclusion
The decision as to when it is right to commence jejunal feeding is far from easy in patients with functional symptoms related to oral and intragastric feeding intolerance. It is important to remain mindful at all times of the primary indication for jejunal feeding in addressing significant malnutrition and that it is not an intervention evidenced for symptom management. Moreover, it is important to consider the differential diagnosis and the potential mechanisms of the presenting functional symptoms, to optimise the management approach and avoid unnecessary jejunal feeding when possible. Essential to improving outcomes is a cohesive and rehabilitative focused multidisciplinary team approach.
Footnotes
Contributors: PP, MM, KF, RO and SL all wrote sections of the article and commented and revised other sections. PP is the guarantor of the overall content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Hvas CL, Farrer K, Blackett B, et al. Reduced 30-day gastrostomy placement mortality following the introduction of a multidisciplinary nutrition support team: a cohort study. J Hum Nutr Diet 2018;31:413–21. 10.1111/jhn.12520 [DOI] [PubMed] [Google Scholar]
- 2. Camilleri M, Diet N. Novel diet, drugs, and gastric interventions for gastroparesis. Clin Gastroenterol Hepatol 2016;14:1072–80. 10.1016/j.cgh.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olausson EA, Störsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol 2014;109:375–85. 10.1038/ajg.2013.453 [DOI] [PubMed] [Google Scholar]
- 4. NICE Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition. Available: https://www.nice.org.uk/guidance/cg32/chapter/1-guidance [Accessed 17 Jul 2019].
- 5. Lacy BE, Parkman HP, Camilleri M. Chronic nausea and vomiting: evaluation and treatment. Am J Gastroenterol 2018;113:647–59. 10.1038/s41395-018-0039-2 [DOI] [PubMed] [Google Scholar]
- 6. Stanghellini V, Chan FKL, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology 2016;150:1380–92. 10.1053/j.gastro.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 7. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol 2018;14:140–5. [PMC free article] [PubMed] [Google Scholar]
- 8. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut 2014;63:1972–8. 10.1136/gutjnl-2013-306084 [DOI] [PubMed] [Google Scholar]
- 9. Koduru P, Irani M, Quigley EMM. Definition, pathogenesis, and management of that cursed dyspepsia. Clin Gastroenterol Hepatol 2018;16:467–79. 10.1016/j.cgh.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 10. Gourcerol G, Tissier F, Melchior C, et al. Impaired fasting pyloric compliance in gastroparesis and the therapeutic response to pyloric dilatation. Aliment Pharmacol Ther 2015;41:360–7. 10.1111/apt.13053 [DOI] [PubMed] [Google Scholar]
- 11. Bredenoord AJ, Chial HJ, Camilleri M, et al. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol 2003;1:264–72. 10.1016/S1542-3565(03)00130-7 [DOI] [PubMed] [Google Scholar]
- 12. Vijayvargiya P, Jameie-Oskooei S, Camilleri M, et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut 2019;68:804–13. 10.1136/gutjnl-2018-316405 [DOI] [PubMed] [Google Scholar]
- 13. Vijayvargiya P, Camilleri M, Chedid V, et al. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology 2019;156:1650–60. 10.1053/j.gastro.2019.01.249 [DOI] [PubMed] [Google Scholar]
- 14. Westaby D, Young A, O'Toole P, et al. The provision of a percutaneously placed enteral tube feeding service. Gut 2010;59:1592–605. 10.1136/gut.2009.204982 [DOI] [PubMed] [Google Scholar]
- 15. Palmer LB, McClave SA, Bechtold ML, et al. Tips and tricks for deep jejunal enteral access: modifying techniques to maximize success. Curr Gastroenterol Rep 2014;16:409. 10.1007/s11894-014-0409-x [DOI] [PubMed] [Google Scholar]
- 16. Paine P, McLaughlin J, Lal S. Review article: the assessment and management of chronic severe gastrointestinal dysmotility in adults. Aliment Pharmacol Ther 2013;38:1209–29. 10.1111/apt.12496 [DOI] [PubMed] [Google Scholar]
- 17. Vasant DH, Kalaiselvan R, Ablett J, et al. The chronic intestinal pseudo-obstruction subtype has prognostic significance in patients with severe gastrointestinal dysmotility related intestinal failure. Clin Nutr 2018;37:1967–75. 10.1016/j.clnu.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 18. Lindberg G, Iwarzon M, Törnblom H. Clinical features and long-term survival in chronic intestinal pseudo-obstruction and enteric dysmotility. Scand J Gastroenterol 2009;44:692–9. 10.1080/00365520902839642 [DOI] [PubMed] [Google Scholar]
- 19. Lindberg G, Törnblom H, Iwarzon M, et al. Full-thickness biopsy findings in chronic intestinal pseudo-obstruction and enteric dysmotility. Gut 2009;58:1084–90. 10.1136/gut.2008.148296 [DOI] [PubMed] [Google Scholar]
- 20. Kilgallon E, Vasant DH, Green D, et al. Chronic continuous abdominal pain: evaluation of diagnostic features, iatrogenesis and drug treatments in a cohort of 103 patients. Aliment Pharmacol Ther 2019;49:1282–92. 10.1111/apt.15241 [DOI] [PubMed] [Google Scholar]
- 21. Grunkemeier DMS, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007;5:1126–39. 10.1016/j.cgh.2007.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 2008;37:339–52. 10.1016/j.semarthrit.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 23. Keefer L, Mandal S. The potential role of behavioral therapies in the management of centrally mediated abdominal pain. Neurogastroenterol Motil 2015;27:313–23. 10.1111/nmo.12474 [DOI] [PubMed] [Google Scholar]
- 24. Wilburn J, McKenna SP, Heaney A, et al. Development and validation of the parenteral nutrition impact questionnaire (PNIQ), a patient-centric outcome measure for home parenteral nutrition. Clin Nutr 2018;37:978–83. 10.1016/j.clnu.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 25. Dibb M, Lal S. Home parenteral nutrition: vascular access and related complications. Nutr Clin Pract 2017;32:769–76. 10.1177/0884533617734788 [DOI] [PubMed] [Google Scholar]