Abstract
Refeeding problems have been recognised since the the liberation of starved communities under siege. The main clinical problems may relate to hypophosphataemia, hypomagnesaemia and hypokalaemia with a risk of sudden death; thiamine deficiency with the risk of Wernike’s encephalopathy/Korsakoff psychosis and sodium/water retention. The problems are greatest with oral/enteral feeding and especially with carbohydrate due to it increasing plasma insulin and thus glucose entry into cells. It is difficult to predict patients at risk of refeeding problems so there must be a high clinical suspicion on refeeding any malnourished patient (including any who have had no or very little nutrition for over 5 days). Generous vitamin and electrolyte supplementation may be given while monitoring closely and increasing the calorie intake reasonably rapidly from 10 to 20 kcal/kg/24 hours. Often patients in this category are not hungry, but over the course of a few days, the restoration of their appetite is an indication that the risks of refeeding have been managed and it is now safe to increase the feed aiming for repletion. If problems do occur, the feed should be slowed to the previous day’s amount, reduced further or rarely stopped while fluid and electrolyte issues are corrected.
Keywords: nutrition support, nutrition, nutritional supplementation
Key points.
Refeeding syndrome describes the adverse clinical and biochemical problems that may result from feeding malnourished patients via any route, be it oral, enteral or parenteral.
Clinicians need to be aware of it and assume most malnourished patients are at risk.
Hypophosphataemia is the most commonly used marker for refeeding problems and it commonly occurs when artificial nutritional support is started (especially with carbohydrate) and can rarely cause death.
Hypophosphataemia is more common with oral/enteral feeding than parenteral nutrition.
Hypomagnesaemia, hypokalaemia, hypoglycaemia (occasionally hyper) and thiamine deficiency may occur.
Sodium retention (causing oedema) is common, especially after glucose (and sodium) are given.
Non-protein energy is given as 50/50 carbohydrate (CHO)/lipid and initially at <50% requirements.
Definition
Refeeding syndrome (RFS) is a potentially fatal condition commonly characterised by rapid changes in fluid and electrolyte balance leading to problems of cardiac arrthymias, cardiac and respiratory failure. Other manifestations include acute fatty liver, endocrine and haematological abnormalities, acute thiamine deficiency and neurological syndromes such as delirium and centropontine myelinolysis. Hidden sepsis, a separate dangerous problem, can also occur in malnourished individuals and sometimes is mistaken for a manifestation of RFS.
RFS is thus best described as the adverse clinical and biochemical problems that can result from feeding severely malnourished patients via any route, be it oral, enteral or parenteral.
History
The first records of the problems of refeeding come from when towns/cities were surrounded and the inhabitants starved into surrendering. In AD 70 after the Seige of Jerusalem by the Romans, written about by Flavius Josephus: “they all on the sudden overfilled those bodies that were before empty, and so burst asunder, excepting such only as were skillful enough to restrain their appetites, and by degrees took in their food”, “death was observed in those who overindulged but not in those who restrained their appetite”.1 There are many descriptions of refeeding problems from the World War II2 3 those following the liberation of the Netherlands being the most detailed.4
Evidence for RFS in clinical practice
The lack of a uniform definition of RFS hampers studies. The evidence of refeeding problems from oral, enteral and parenteral feeding is poor. The paper that brought attention to the problems of refeeding in clinical practice was entitled ‘Death resulting from overzealous total parenteral nutrition’,5 in which two deaths were described associated with hypophosphataemia; yet both were being given large amounts of intravenous glucose (500 and 700 g glucose in 24 hours).
The UK National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report of 2010 showed that 60% (455/877) of patients given parenteral nutrition were at risk of refeeding problems, defined using the National Institute for Health and Care Excellence (NICE) criteria, with hypophosphataemia occurring in 18%. Hypokalaemia, hypomagnesaemia and hyperglycaemia were also common.6
Pathophysiology and biochemistry of refeeding
Biochemistry of starvation
With reduced glucose levels resulting from starvation, insulin secretion drops and glucagon levels increase, resulting in higher glycogenolysis to generate energy from carbohydrate sources. However as starvation continues, glycogen stores become depleted, typically within 6 hours to 3 days, then the body shifts to using fat and muscle to produce energy.
Other changes of reductive adaptation include reducing basal metabolic rate and downregulating energy consuming processes such as the activity of the sodium/potassium ATP-pump. This results in changes of electrolyte handling causing leakage of mainly intracellular cations such as potassium, magnesium and phosphate into the circulation where they are lost in urine, while allowing sodium and water to leak into cells.
Despite poor dietary intake of electrolytes, serum levels of these cations during starvation often remain normal (or can even be high if there is a degree of renal failure) as these electrolytes move from the intracellular to the extracellular space,7 so measuring electrolyte levels as a one-off investigation at this point gives little or no useful information to the risk of refeeding problems.
Severe starvation may lead to hepatic steatosis through various mechanisms. Protein deficiency can result in decreased apolipoprotein synthesis, leading to decreased very low-density lipoprotein (VLDL) synthesis and inhibited VLDL transport. Reduced VLDL transport plays a significant role in lipid accumulation in the liver during starvation.8
Biochemical changes of refeeding
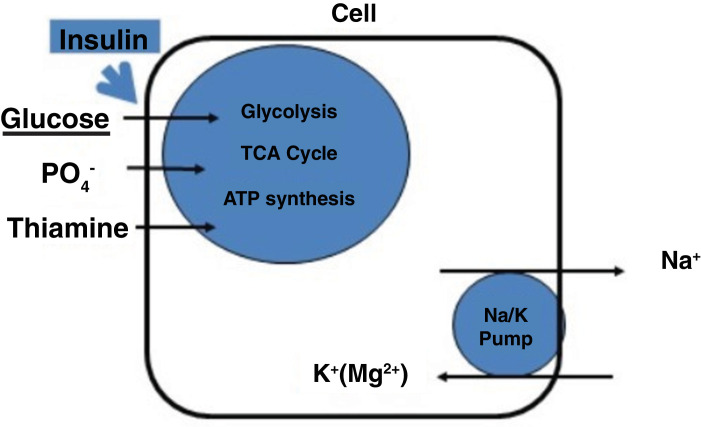
Two key events happen on feeding glucose to patients who have been starving (figure 1).
Figure 1.
Simplified diagram to show key events in refeeding syndrome. TCA, tricarboxylic acid.
First, insulin levels increase which drive glucose and phosphate into cells. This leads to an increased uptake of glucose, phosphate and thiamine (needed for glycolysis and thus ATP manufacture). As a result, serum levels of phosphate along with other cations such as potassium, magnesium, calcium may fall, as will thiamine. Thiamine is a co-factor in many metabolic processes including its essential role in cerebral energy utilisation, it will already be depleted in starvation so refeeding with glucose will result in rapid depletion of the already low stores of thiamine.9
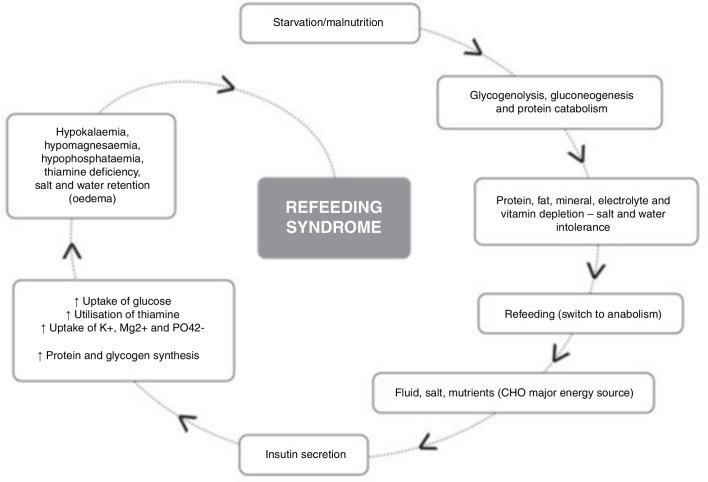
Second, the cell membrane sodium/potassium pump starts actively pumping sodium (using the energy from ATP) out of the cell and taking potassium and magnesium into the cell thus furthering the deficit in circulating electrolytes.10 In addition to this, insulin stimulates sodium reabsorption from the distal nephron.11 Water and sodium efflux into circulation may result in fluid overload and heart failure.12 The combination of a sudden fluid load into the circulation into a system where the heart has been weakened and atrophied due to starvation, along with low circulating electrolytes (giving a propensity to cardiac arrhythmias) can easily lead to pulmonary oedema or signs of heart failure and potentially death (figure 2).
Figure 2.
Diagram summarising events in refeeding syndrome.
Clinical manifestations of refeeding
Hypophosphataemia
This is often considered the hallmark feature of the refeeding syndrome (table 1). Within the cell, phosphate is vital for many cellular pathways including glycolysis and the decarboxcylic acid cycle. Hypophosphataemia results in reduced ATP so many metabolic pathways are slowed/stopped. The low phosphate also reduces the levels of 2,3-diphosphoglycerate so causing the red blood cells to be less able to give up oxygen (shifting the oxygen dissociation curve to the right).
Table 1.
Effects of hypophosphataemia
| Muscular | Weakness (diaphragm), respiratory failure |
| Rhabdomyolysis | |
| Cardiac | Biventricular failure, low blood pressure |
| Arrhythmias, sudden death | |
| Haematological | Low and dysfunctional white blood cells, red blood cells, platelets |
| Haemolysis | |
| Neurological | Weakness, lower motor neurone-type paralysis (loss of reflexes) |
| Cranial nerve palsies | |
| Confusion, ataxia, tremors, fits, coma | |
| Hepatic | Dysfunction (especially alcohol excess) |
Refeeding is not the most common cause of hypophosphataemia. Other reasons include sepsis, stress (postoperative, trauma, burns), recovery from acidosis (eg, diabetic ketoacidosis), alcohol withdrawal, insulin (±glucose) treatment, drugs (chemotherapy, diuretics, biphosphonates, phosphate binding antacids), renal dialysis/transplantation, hyperparathyroidism and respiratory alkalosis.13 14
Thiamine deficiency
The body stores of thiamine (vitamin B1) are sufficient for up to 7 days. It is a co-factor in aerobic glucose consumption. In thiamine deficiency, a combined enzyme defect results in aerobic metabolism impairment and insufficient ATP generation. Furthermore, pyruvate is converted into lactate, resulting in hyperlactaemia and lactic acidosis. As thiamine-dependent metabolic pathways are present in almost all human cells, deficiency can affect many organ systems. It can also exacerbate the hypomagnesaemia, hypokalaemia and hypophosphataemia associated with increased renal losses.15 Thiamine deficiency can lead to the development of Wernicke’s encephalopathy which is a triad of encephalopathy, ataxia and ocular dysfunction (nystagmus). Wernicke’s encephalopathy can progress to the irreversible Korsakoff’s syndrome, which is an amnesic syndrome causing short-term memory loss while preserving long-term memory. Thiamine deficiency can also lead to wet beriberi, affecting the heart and circulatory system causing heart failure, or dry beriberi in which the nerves are damaged leading to weakness or even paralysis. If Wernicke’s is suspected, it is important to give intravenous thiamine as oral doses are rarely sufficient.
Reactivation of the sodium/potassium pump
As the sodium/potassium pump (which has been in a state of reductive adaptation) reactivates, the osmotically active sodium enters the interstitial space and circulation. This effect is compounded by insulin causing sodium retention in the kidney, dysregulation of antidiuretic hormone and aldosterone secretion and the addition of carbohydrate into the diet which leads to a rapid decrease in renal excretion of sodium and water.10 11 This sudden fluid flux into the circulation and inability to excrete a fluid load, particularly in the presence of cardiac dysfunction or failure due to poor nutrition, along with the possibility of low circulating electrolytes giving a predisposition to cardiac arrhythmias, can lead to oedema, left ventricular failure and hypertension. Thus, the first clinical manifestations of RFS are often a raised pulse and respiratory rate. In a patient with low body mass index (BMI), a sinus bradycardia would be expected, so even a pulse above 60 beats/min might indicate a relative tachycardia and should alert the clinician to potential problems.
Hypokalaemia
Potassium is the main intracellular cation. Depletion in starvation and malnutrition is mainly driven by low intake and/or excessive losses. Serum potassium levels may remain normal in starvation because of movement of potassium along chemical gradients to the extracellular space. Insulin is important in stimulation of potassium influx into cells through Na-K ATPase symporter. With initiation of nutrition, the resulting increased secretion of insulin precipitates potassium influx into cells causing a fall in serum potassium levels. Hypokalaemia can cause muscle weakness including the respiratory muscles, atrial and ventricular arrhythmias (QT prolongation), atrioventricular block, a U wave on the ECG and can cause an ileus.
Hypomagnesaemia
Magnesium is a predominantly intracellular cation. Magnesium deficiency in malnutrition and starvation occurs due to poor intake and redistribution. On refeeding, magnesium moves into cells with a resultant drop of serum levels.7 Hypomagnesaemia may cause a tremor, muscle weakness, poor memory and precipitate arrhythmias in susceptible patients.
Moderate abnormalities of liver function
Liver enzyme abnormalities are commonly found both in periods of starvation as well as during the refeeding phase. Excess glucose administered in the early phase of refeeding, particularly after prolonged periods of starvation leads to lipogenesis, again as a result of insulin stimulation. Deposition of fatty acids and triglycerides in the liver can lead to an acute fatty liver often being detected through raised liver transaminases. Moderate abnormalities of liver function (eg, alanine transaminase up to 10 times the upper limit of the normal range) should not delay feeding.16
Other metabolic /clinical abnormalities
Both hyperglycaemia or hypoglycaemia can occur and need to be managed carefully. Glucose intake after a period starvation, can suppress gluconeogenesis through the release of insulin. Excessive administration of glucose can therefore lead to hyperglycaemia and its sequelae including osmotic diuresis, dehydration, metabolic acidosis and ketoacidosis. Conversely, hypoglycaemia can also occur, particularly in the presence of sepsis.17 This may relate to depleted glycogen stores, impaired gluconeogenesis and increased peripheral glucose utilisation. Hypoglycaemia must be detected and corrected quickly as if prolonged can lead to permanent cerebral damage.
Infection
Hypothermia and low blood glucose are often an indication of sepsis and these must be urgently treated. In a case series of 14 patients, 2 patients developed occult sepsis that proved fatal in one case despite intensive therapy unit (ITU) treatment.18 While infection and sepsis are not classical presentations of RFS, it is important to monitor for these during the initial period of refeeding as patients with significant malnutrition are at higher risk of developing severe infections. These patients often do not develop the usual signs of sepsis (eg, fever, neutrophilia or increased C reactive protein). The combination of low BMI, hypoglycaemia and hypothermia is often termed the deadly triad and is a marker for severe infection and should always trigger consideration of antibacterial treatment.19
Who is at risk of refeeding problems
Patients with gastrointestinal disorders who have become severely malnourished often with intestinal failure, and patients with anorexia nervosa, some critical care patients, elderly care patients and oncology patients are at increasing risk of refeeding problems. The classical list includes classic kwashiorkor/marasmus, chronic malnutrition-underfeeding, chronic alcoholism, morbid obesity with massive weight loss, patients unfed in 7–10 days with evidence of stress and depletion, prolonged fasting and prolonged intravenous hydration. It is especially common to find patients who look obese (BMI may be within or above the normal range) but who have lost much of their muscle mass (sarcopoenic obesity) due to a poor intake of food and/or an inflammatory process (eg, sepsis, surgery, etc) and thus become relatively immobile and so are at a high risk of hospital-acquired complications (eg, sepsis, deep vein thrombosis, etc).
How to detect a patient at risk of refeeding problems
The current UK NICE guidelines for nutrition support20 identify criteria as risk factors for developing RFS (box 1). However, the sensitivity of NICE guidelines in predicting RFS is not good.21
Box 1. UK National Institute for Health and Care Excellence risk factors for developing refeeding syndrome20 .
One or more of the following:
Body mass index (BMI) <16 kg/m2;
Unintentional weight loss >15% within last 3–6 months;
Little or no nutritional intake for >10 days;
Low potassium, magnesium or phosphate prior to feeding.
Two or more of the following:
BMI <18.5 kg/m2;
Unintentional weight loss >10% within last 3–6 months;
Little or no nutritional intake for >5 days;
A history of alcohol abuse or drugs including insulin, chemotherapy, antacids or diuretics.
Refeeding index (a score generated from baseline insulin-like growth factor and leptin) has been proposed as a useful biochemical marker for predicting those at risk of RFS.22 A study in 2015 has shown that using highly sensitive baseline Insulin-like growth factor-1 alone is an objective, sensitive and specific biochemical marker in identifying patients who are at high risk of developing refeeding hypophosphataemia in patients starting parenteral nutrition.23
Refeeding hypophosphataemia is more common with oral/enteral feeding than with parenteral feeding
Zeki et al showed that the occurrence of refeeding hypophosphataemia in adult patients fed enterally versus those fed parenterally was more common in those fed enterally (21% vs 8%, p<0.05).21 The main reason is likely to relate to enteral feeding stimulating a greater insulin secretion than parenteral feeding and so the mechanism that drives refeeding hypophosphataemia is amplified. This ‘incretin effect’ was first recognised in the 1960s and describes the greater insulin secretion after a patient is given the same amount of glucose orally and intravenously,24 probably relating to the release of two upper gut peptide hormones gastroinsulinotropic peptide and glucagon-like peptide 1, which act to increase/exaggerate insulin secretion from the pancreatic islet β cells. Refeeding problems must be considered very seriously when starting enteral feeding.
Treatment of patients at risk of refeeding problems
Once detected these patients should be carefully observed/monitored while their circulatory volume (dehydration) is restored (generally using little/no sodium-containing solutions) and their fluid balance/daily weight is monitored. Hypothermia and sepsis if present must be treated.25 Cardiac monitoring is recommended for inpatients with cardiac manifestations and/or ECG changes secondary to hypokalaemia, patients with a QTc >450 ns or where intravenous correction of potassium at a rate of >10 mmol/hour is deemed necessary.26 These severely at-risk patients should ideally be managed by the hospital nutrition support team.
Energy
When the UK NICE guidelines were published there were no good quality trials to enable evidence-based management protocols to be developed so reliance was on expert opinion.20 It suggested that people who had eaten little or nothing for >5 days should have nutrition support introduced at no more than 50% of requirements for the first 2 days; that the prescription for people at high risk of developing refeeding problems could be given nutritional support at a maximum of 10 kcal/kg/day, increasing slowly to meet or exceed full needs by 4–7 days. However, prolonged administration of very low energy feed risks the problem of underfeeding which may exacerbate problems associated with malnutrition. A survey in 2008 showed 39% felt the guidance was appropriate and 36% felt it was too cautious.27
A reasonable strategy to avoid the risks of both refeeding problems as well as underfeeding would be to start feeding cautiously in patients at high risk of refeeding problems at 10–20 kcal/kg/day, while simultaneously correcting electrolyte deficiencies, but then increasing the feeding rate at least daily aiming to achieve feeding to match their metabolic demands over a period of 5–7 days , while keeping a careful watch on clinical and biochemical parameters.20 28 Feeding to full requirements should be achieved within 5–7 days. Specialist dietitians or nutrition support teams are invaluable in providing expert advice on this aspect of care. The non-protein energy should be given as approximately 50% carbohydrate and 50% lipid mix. If signs of refeeding problems or adverse clinical markers ensue, the feed may need to be temporally slowed, reduced or stopped.
Phosphate replacement
A low threshold for starting intravenous correction should be employed and most advocate intravenous replacement for patients at high risk of RFS at PO4 levels of <0.6 mmol.
Intravenous phosphate supplements could be in the form of monobasic potassium phosphate or the more widely used phosphate infusion, Polyfusor. Electrolytes need to be closely monitored as the latter contains significant amounts of sodium and potassium. One litre of Polyfusor contains 100 mmol of PO₄³ˉ, 162 mmol of sodium and 19 mmol of potassium. Excessive doses should also be avoided as they can result in hypocalcaemia and metastatic calcification.29 Small infusions of 10–20 mmol phosphate, that is, 100–200 mL of Polyfusor are advocated and repeated if necessary to reduce the risk of metastatic calcification.
Phosphate must be administered concurrently with low rate feeding, to avoid the risk of desirable electrolytes simply being excreted in the urine, rather than being transported into the cells.
Thiamine, other vitamins and trace elements
It is crucial that thiamine supplementation is started prior to and continued during nutrition support and glucose administration. Oral thiamine can be given at a dose of 200–300 mg/day. One to two tablets of vitamin B co strong can be given three times a day.20 A daily intravenous vitamin B preparation such as Pabrinex can be given intravenously (usually for 3–5 days) in addition to an oral multivitamin supplement.20 A balanced multivitamin/trace element supplement once daily for 10 days is recommended in UK NICE guidelines.20 If signs of Wernicke’s encephalopathy are present the B vitamins need to be given intravenously.
Electrolytes (potassium and magnesium)
A drop of serum potassium by 1 mmol/L is equivalent to a total deficit of approximately 200–400 mmol. Mild asymptomatic hypokalaemia is ideally corrected orally with potassium chloride used to provide up to 50 mmol/day. Intravenous potassium can be used to treat significant hypokalaemia with potassium levels <3.0 mmol/L. Intravenous infusion with potassium chloride concentration of 40 mmol/L is used when using peripheral veins.29 This can be administered at a rate of 10–20 mmol/hour. Higher concentrations of intravenous potassium delivered into a central vein can be used with close cardiac monitoring after specialist advice. The UK NICE guideline recommends providing oral, enteral or intravenous supplements of potassium with the likely requirements being 2–4 mmol/kg/day.
About 80% of plasma magnesium is filtered through glomeruli. Intravenous infusion of magnesium will result in a transient increase in magnesium levels and consequently renal wasting of a substantial proportion of that magnesium. So, in more severe hypomagnesaemia where higher magnesium supplements are required, these should be administered over longer hours to avoid sudden increases in magnesium levels.30 In hypomagnesaemia, 20–40 mmol of magnesium sulfate can be given over 1–2 hours.29 The UK NICE guideline recommends providing oral, enteral or intravenous supplements of magnesium with the likely requirements being 0.2 mmol/kg/day intravenously, 0.4 mmol/kg/day orally.20
Fluid
Should problems with fluid overload occur, an ‘ABC’ approach to resuscitation should be taken and transfer to intensive care unit (ICU) should be considered. If absolutely necessary, diuretics may be required but may have the effect of lowering circulating electrolytes further. If this occurs, central access should be sought and administration of concentrated electrolytes in ICU may be appropriate. The feed should be slowed further while these issues are being managed.
Conclusion
Refeeding problems are common, however are less likely to occur if patients are identified as at risk and if precautions are taken (14% vs 46%).6 Electrolyte (especially hypophosphataemia), sodium and water and thiamine deficiency are common and can be life threatening/life changing so need to be prevented and treated. The ‘start low, go slow’ approach for energy provision recommended by UK NICE guidelines may risk underfeeding which is linked to poor weight gain and the risk of the underlying clinical condition deteriorating leading to organ dysfunction, poor wound healing, increased rates of infection and prolonged hospitalisation.31 In this review, we have advocated an equally cautious start to energy provision but would highlight the importance of a more rapid increase in energy, particularly during the first couple of days, linked to careful blood monitoring and electrolyte/vitamin provision.
Footnotes
Contributors: ADS and JN planned, reasearched and wrote the study jointly.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Josephus F. Chapter XIII, paragraph 4 In: Whiston W, ed The wars of the Jews or history of the destruction of Jerusalem, 2009. [Google Scholar]
- 2. Brozek J, Chapman CB, Keys A. Drastic food restriction; effect on cardiovascular dynamics in normotensive and hypertensive conditions. J Am Med Assoc 1948;137:1569–74. 10.1001/jama.1948.02890520001001 [DOI] [PubMed] [Google Scholar]
- 3. Schnitker MA, Mattman PE, Bliss TL. A clinical study of malnutrition in Japanese prisoners of war. Ann Intern Med 1951;35:69–96. 10.7326/0003-4819-35-1-69 [DOI] [PubMed] [Google Scholar]
- 4. Burger GCE, Drummond JC, Sanstread HR. Part I and II : Malnutrition and starvation in Estern Netherlands. The Hague General State Printing Office, 1948. [Google Scholar]
- 5. Weinsier RL, Krumdieck CL. Death resulting from overzealous total parenteral nutrition: the refeeding syndrome revisited. Am J Clin Nutr 1981;34:393–9. 10.1093/ajcn/34.3.393 [DOI] [PubMed] [Google Scholar]
- 6. Stewart JAD, Mason DG, Smith N, et al. . A mixed bag. An enquiry into the care of hospital patients receiving parenteral nutrition. A report by the National confidential enquiry into patient outcome and death 2010.
- 7. Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001;17:632–7. 10.1016/S0899-9007(01)00542-1 [DOI] [PubMed] [Google Scholar]
- 8. Kneeman JM, Misdraji J, Corey KE. Secondary causes of nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2012;5:199–207. 10.1177/1756283X11430859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Victor M, Adams RD, Collins GH. The Wernicke Korsakoff Syndrome, F.A. Philadelphia: Davis, 1989. [Google Scholar]
- 10. Gozansky DM, Herman RH. Water and sodium retention in the fasted and refed human. Am J Clin Nutr 1971;24:869–71. 10.1093/ajcn/24.7.869 [DOI] [PubMed] [Google Scholar]
- 11. DeFronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia 1981;21:165–71. 10.1007/bf00252649 [DOI] [PubMed] [Google Scholar]
- 12. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ 2008;336:1495–8. 10.1136/bmj.a301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halevy J, Bulvik S. Severe hypophosphatemia in hospitalized patients. Arch Intern Med 1988;148:153–5. 10.1001/archinte.1988.00380010155016 [DOI] [PubMed] [Google Scholar]
- 14. Camp MA, Allon M. Severe hypophosphatemia in hospitalized patients. Miner Electrolyte Metab 1990;16:365–8. [PubMed] [Google Scholar]
- 15. Maiorana A, Vergine G, Coletti V, et al. . Acute thiamine deficiency and refeeding syndrome: similar findings but different pathogenesis. Nutrition 2014;30:948–52. 10.1016/j.nut.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 16. Robinson DP. MARSIPAN: management of really sick patients with anorexia nervosa. 2nd edn, 2014. [Google Scholar]
- 17. Miller SI, Wallace RJ, Musher DM, et al. . Hypoglycemia as a manifestation of sepsis. Am J Med 1980;68:649–54. 10.1016/0002-9343(80)90250-8 [DOI] [PubMed] [Google Scholar]
- 18. Webb GJ, Smith K, Thursby-Pelham F, et al. . Complications of emergency refeeding in anorexia nervosa: case series and review. Acute Med 2011;10:69–76. [PubMed] [Google Scholar]
- 19. Jackson AA. Severe malnutrition chapter 10.4 1054, Oxford textbook of medicine. 4th ed Warrell, Cox & Firth, 2005. [Google Scholar]
- 20. National Institute for Health en Clinical Excellence Nutrition support in adults. Clinical guidelines, 2006. [Google Scholar]
- 21. Zeki S, Culkin A, Gabe SM, et al. . Refeeding hypophosphataemia is more common in enteral than parenteral feeding in adult in patients. Clinical Nutrition 2011;30:365–8. 10.1016/j.clnu.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 22. Elnenaei MO, Alaghband-Zadeh J, Sherwood R, et al. . Leptin and insulin growth factor 1: diagnostic markers of the refeeding syndrome and mortality. Br J Nutr 2011;106:906–12. 10.1017/S0007114511001097 [DOI] [PubMed] [Google Scholar]
- 23. Goyale A, Ashley SL, Taylor DR, et al. . Predicting refeeding Hypophosphataemia: insulin growth factor 1 (IGF-1) as a diagnostic biochemical marker for clinical practice. Ann Clin Biochem 2015;52:82–7. 10.1177/0004563214523739 [DOI] [PubMed] [Google Scholar]
- 24. Mcintyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet 1964;284:20–1. 10.1016/S0140-6736(64)90011-X [DOI] [PubMed] [Google Scholar]
- 25. Ashworth A, Khanum S, Jackson A, et al. . Guidelines for the inpatient treatment of severely malnourished children. World Health Organization, 2003. [Google Scholar]
- 26. Jackson AA. Severe Malnutrition Chapter 10.4 1054 : Oxford textbook of medicine. 4th edn Warrell, Cox & Firth, 2005. [Google Scholar]
- 27. De Silva A, Smith T, Stroud M. Attitudes to NICE guidance on refeeding syndrome. BMJ 2008;337:a680 10.1136/bmj.a680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Culkin A, White R. Refeeding syndrome : A pocket guide to clinical nutrition. 5th edn British Dietetic Association, 2018. [Google Scholar]
- 29. British National formulary (BNF). Available: bnf.nice.org.uk
- 30. al-Ghamdi SM, Cameron EC, Sutton RA. Magnesium deficiency: pathophysiologic and clinical overview. Am J Kidney Dis 1994;24:737–52. 10.1016/s0272-6386(12)80667-6 [DOI] [PubMed] [Google Scholar]
- 31. Garber AK, Sawyer SM, Golden NH, et al. . A systematic review of approaches to refeeding in patients with anorexia nervosa. Int J Eat Disord 2016;49:293–310. 10.1002/eat.22482 [DOI] [PMC free article] [PubMed] [Google Scholar]