Abstract
Patient: Male, 47-year-old
Final Diagnosis: COVID-19 • pneumothorax
Symptoms: Chest discomfort • dry cough • shortness of breath
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Diagnostic/therapeutic accidents
Background:
At the end of 2019, coronavirus (SARS-CoV-2) was recognized as the cause of a cluster of pneumonia cases in Wuhan, a city in China. There are numerous complications associated with COVID-19 infection, such as acute respiratory distress syndrome, renal failure, circulatory shock, and multi-organ failure. Spontaneous pneumothorax following COVID-19 pneumonia is an extremely rare complication.
Case Report:
We report the case of a 49-year-old man with a past medical history of type 2 diabetes mellitus with an initial presentation of cough, shortness of breath, and fever. He was diagnosed with COVID-19 pneumonia and rapidly deteriorated on the day of admission, requiring initiation of mechanical ventilation. The patient recovered clinically and was discharged home. He returned 21 days after discharge with a spontaneous pneumothorax.
Conclusions:
Spontaneous pneumothorax is a rare complication after apparent recovery from COVID-19 pneumonia. It is imperative that treating physicians are aware of this complication in order to recognize it early and treat it promptly.
MeSH Keywords: COVID-19, Pneumothorax, SARS Virus, Pandemics
Background
The outbreak of the COVID-19 virus has caused more than 2 800 000 confirmed cases and more than 193 710 deaths worldwide [1], and the World Health Organization declared the virus a pandemic. Most patients have a mild disease course and recover completely. However, some patients develop serious complications and deteriorate rapidly, requiring ICU admission.
In a study that included 2634 patients who were hospitalized for COVID-19 pneumonia in New York City, 14% were admitted to the intensive care unit and 12% received invasive mechanical ventilation [2]. Several serious complications may develop in this lethal disease which significantly contribute to the mortality rate. These complications include, but are not limited to, acute respiratory distress syndrome (ARDS), renal failure, circulatory shock, encephalopathy, hemorrhagic encephalitis, and, rarely, a spontaneous pneumothorax [3–7]. A spontaneous pneumothorax has been reported in the literature as a possible complication of COVID-19 pneumonia, but the mechanism has not yet been explained. In this report, we discuss a case involving a spontaneous pneumothorax that developed 21 days after clinical recovery from COVID-19 pneumonia.
Objective
To make physicians aware of a spontaneous pneumothorax as a possible complication post COVID-19 pneumonia.
Case Report
Our patient was a 49-year-old man who presented to the hospital with worsening shortness of breath, dry cough, and chest tightness for 3 days. His past medical history was significant for type 2 diabetes mellitus. His initial vitals were as follows: temperature 39.6°C, blood pressure 108/79 mmHg, heart rate 114 beats per minute, respiratory rate 30 breaths per minute, and oxygen saturation of 86% on room air. Laboratory tests were notable for leukocytosis (i.e., white blood cell count of 12.9×103/mcl). The chest x-ray showed bilateral infiltrates, mostly prominent in the lower lobes (Figure 1). The screening was negative for multiple respiratory pathogens, including Influenza A, Influenza B, Respiratory Syncytial Virus, Adenovirus, and Human Parainfluenza Virus. The diagnosis of COVID-19 was confirmed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) testing from nasopharyngeal swab (Cepheid/GenXpert system). The patient was placed on nasal cannula 6 L/min, which only slightly improved his oxygen saturation to 88%. He was subsequently intubated in the intensive care unit due to worsening respiratory failure. The ventilator mode was volume-controlled and the initial settings were as follows: Tidal Volume 380 ml, Respiratory Rate 22, FIO2 80% and Positive End Expiratory Pressure (PEEP) 14. He was treated with piperacillin/tazobactam, vancomycin, azithromycin, and hydroxychloroquine. The patient was intubated for 8 days, and after much improvement, he underwent a successful extubation. During his stay in the intensive care unit, he did not require any other invasive procedures or central lines. After an additional 5 days of supportive care on the medical floor, the patient was discharged home in stable condition.
Figure 1.
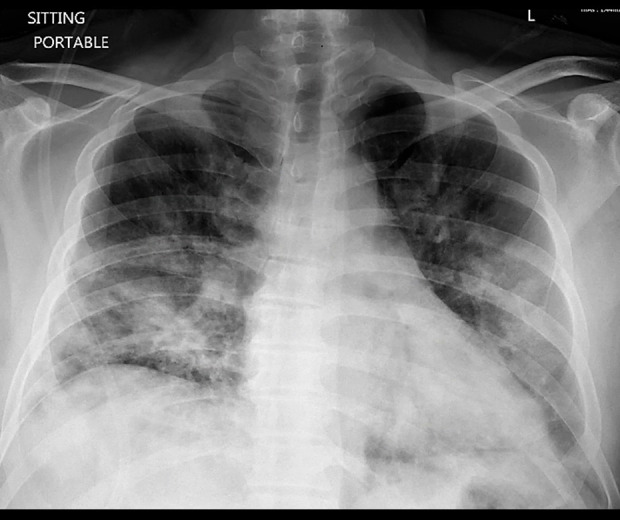
Chest x-ray during the initial admission shows bilateral infiltrate mainly prominent in lower lobes at time of initial presentation.
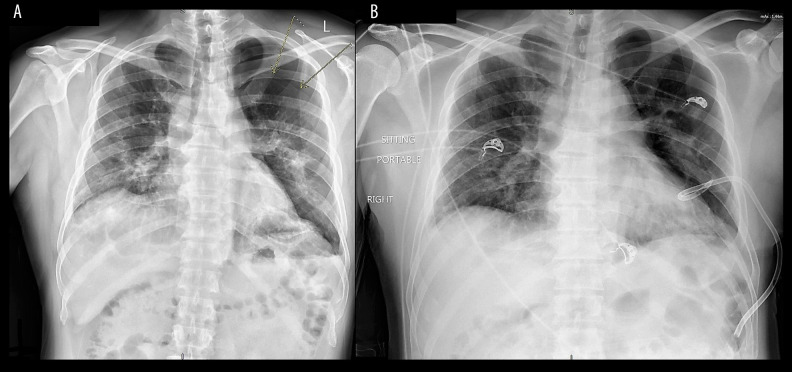
Twenty-one days after discharge, the patient returned to the emergency room because of sudden onset of shortness of breath. He reported no history of trauma. A large left-sided pneumothorax was found on the chest x-ray (Figure 2A). The patient was hemodynamically stable, and his shortness of breath improved after insertion of a pigtail chest tube (Figure 2B). Following the chest tube insertion, a CT scan of the chest without contrast was completed, showing significant residual bilateral pulmonary infiltrates (Figure 3). The patient was eventually discharged home.
Figure 2.
Left-sided pneumothorax (A) and after pigtail insertion (B). There was an interval decrease of the left pneumothorax, from 21 mm to 8 mm in thickness, at the apex after the pigtail insertion.
Figure 3.
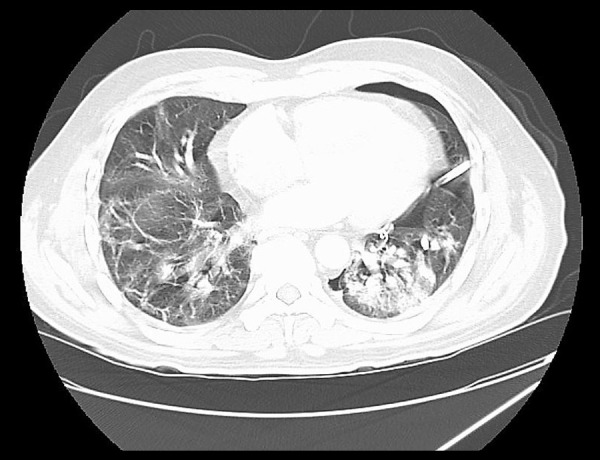
CT scan of the chest without contrast after pigtail insertion. Left pleural catheter is in position with approximately 27% volume left pneumothorax. Bilateral upper and lower lobe airspace infiltrates/consolidation are noted, left more pronounced than right.
Discussion
The spectrum of symptomatic infection with COVID-19 pneumonia ranges from mild to critical. Some patients rapidly develop acute respiratory distress syndrome (ARDS), as well as other serious complications, including renal failure and circula-tory shock [5]. Developing a spontaneous pneumothorax due to COVID-19 pneumonia is rare [5, 6] and the exact pathogenesis is not yet explained. Our patient had a pneumothorax, which occurred after an apparent clinical recovery of COVID-19 pneumonia and without any known precipitating factor or trauma.
Pneumothorax is defined as the presence of air in the pleural cavity. Risk factors for pneumothorax include male sex, smoking, mechanical ventilation, trauma, and the presence of underlying lung disease such as emphysema, cystic fibrosis, necrotizing pneumonia, severe asthma, and lung malignancy [8]. Pneumothorax can be classified as spontaneous (no obvious precipitating factor identified) and nonspontaneous.
Our patient developed spontaneous pneumothorax 21 day after apparent clinical recovery. Most authors believe that subpleural blebs are always the cause of spontaneous pneumothorax [9].
In our patient, there was no bulla or pneumothorax observed in the chest x-ray during initial presentation, after intubation, or at the time of discharge. However, mini-blebs could have formed due to the inflammation, and later ruptured causing the pneumothorax [10]. We also hypothesize that ischemic breakdown of the alveolar wall secondary to micro-thrombi, a feature of this disease [11], could have led to leakage of air into the pleural space. Lastly, the intense cough could have induced rupture of the alveolar wall by increasing the intra-alveolar pressure, called the Maclin effect.
Conclusions
A spontaneous pneumothorax can occur following recovery from COVID-19 pneumonia. It is important that physicians treating COVID-19 pneumonia consider pneumothorax when COVID patients develop sudden deterioration in their respiratory status.
Acknowledgments
We would like to express our very great appreciation to Dr. Nora Bergasa for discussion and developing the hypothesis explaining the pathophysiology of pneumothorax in this case. Also, many thanks to Amelia Vaitekunas and Ruhee Gadhia for proofreading the paper.
Footnotes
Conflicts of interest
None.
References:
- 1.World Health Organization (WHO) https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;232(20):2052–59. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai H, Zhang X, Xia J, et al. High-resolution chest CT features and clinical characteristics of patients infected with COVID-19 in Jiangsu, China. Int J Infect Dis. 2020;95:106–12. doi: 10.1016/j.ijid.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao X, Liu B, Yu Y, et al. The characteristics and clinical value of chest CT images of novel coronavirus pneumonia. Clin Radiol. 2020;75(5):335–40. doi: 10.1016/j.crad.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21(5):541–44. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020;70(9):311–22. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 8.Noppen M. Spontaneous pneumothorax: Epidemiology, pathophysiology and cause. Eur Respir Rev. 2010;19(117):217–19. doi: 10.1183/09059180.00005310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Light RW. Management of spontaneous pneumothorax. Am Rev Respir Dis. 1993;148:245–48. doi: 10.1164/ajrccm/148.1.245. [DOI] [PubMed] [Google Scholar]
- 10.Schramel F, Meyer CJ, Postmus PE. Inflammation as a cause of spontaneous pneumothorax and emphysema-like changes: Results of bronchoalveolar lavage. Eur Respir Rev. 1995;8(Suppl. 19):397s. [Google Scholar]
- 11.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–40. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]