Abstract
Patient: Female, 44-year-old
Final Diagnosis: COVID-19
Symptoms: Confusion
Medication:—
Clinical Procedure: —
Specialty: Neurology
Objective:
Rare disease
Background:
Acute hemorrhagic necrotizing encephalitis (AHNE) is a rare manifestation of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. AHNE usually involves the subcortical white matter but not the cortical grey matter. This study describes the disruptive effects of AHNE associated with SARS-CoV-2 on cognitive function in a previously healthy and sound middle-aged woman resulting from alterations in cortical areas involved in the cognitive network.
Case Report:
A 44-year-old previously healthy woman with a history of inter-state travel developed a flu-like illness, followed by acute, steadily progressive cognitive impairment. She was admitted in a comatose state after a first tonic-clonic seizure. Blood tests were non-informative. Cerebral magnetic resonance imaging (MRI) was indicative of AHNE. Cerebrospinal fluid analysis showed mild lymphocytosis with normal protein and normal glucose but an elevated IgG index. After testing positive for SARS-CoV-2, she was administered steroids. Treatment was ineffective, and the patient died.
Conclusions:
SARS-CoV-2 is a potential central nervous system (CNS) pathogen, which may manifest as AHNE. These patients may present with generalized tonic-clonic seizures and frontal dysexecutive syndrome, with cognitive impairment being the presenting feature of neuro-coronavirus disease-2019 (COVID-19). The patient described in this report is unique for acute-onset and isolated cognitive impairments due to SARS-CoV-2 infection in the absence of clinical or radiological respiratory manifestations. These findings may help in the early detection and diagnosis of neuro-COVID-19, especially among clinicians and neurologists working in areas of endemic SARS-CoV-2 infection.
MeSH Keywords: COVID-19; Leukoencephalitis, Acute Hemorrhagic; SARS Virus
Background
In 1896, the eminent Canadian physician Sir William Osler stated that “humanity has but 3 great enemies, fever, famine, and war; of these, by far the greatest and most terrible is fever” [1]. Infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a beta-coronavirus, usually manifests as fever, sore throat, coughing, and interstitial pneumonia, but the virus can also invade the central nervous system (CNS) and the peripheral nervous system (PNS) [2–8]. Neurological manifestations of SARS-CoV-2 infection include hyposmia, hypogeusia, headache, seizures, stroke, bleeding, sinus venous thrombosis, altered consciousness, encephalitis, demyelination, neuropathy, myalgia, and polyradiculitis [9–11], with encephalitis being rare. This report describes the disruptive effects of SARSCoV-2 infection on cognitive function of a previously healthy and sound middle-aged woman, resulting from acute hemorrhagic necrotizing encephalitis (AHNE) affecting various cortical areas involved in the cognitive network [12].
Case Report
A 44-year-old married woman developed a high-grade fever with myalgia, dry cough, hypogeusia, and hypoosmia after returning from a vacation in South India 10 days prior to admission. She took several over-the-counter medications, including cefpodoxime, acetaminophen, and levocetirizine, for 5 days, and her symptoms subsided within 3 days. After becoming afebrile, she became confused, disorientated, and apractic and developed a memory and thought disorder. She was healthy before the fever and had no known contacts with un-well individuals, no addiction or any other comorbidity, and 2 uneventful healthy pregnancies. Her mental history prior to the infection and her family history, including mental disease, were uneventful.
Due to an ongoing complete nation-wide lockdown, her care-givers could not seek medical help until she experienced a first generalized tonic-clonic seizure (GTCS), along with loss of sphincter control, loss of response to audio-visual and pain stimuli, and loss of consciousness (LOC). Because of her recent travel history, she was admitted to the isolation ward of our hospital. Abnormal vital signs on admission included sinus tachycardia and tachypnea, but the patient was afebrile and normotensive.
On neurological exam, the patient was deeply comatose. She had a Glasgow coma scale score (GCS) of E2V1M2 with no signs of meningeal irritation. Both pupils were equally wide and reacted sluggishly to light. All tendon reflexes were depressed. Plantar responses were bilaterally extensor. Fundoscopy showed no evidence of papilledema. Comprehensive cognitive function analysis could not be performed. There were no signs of involvement of cardiorespiratory and other bodily systems.
Blood tests on admission included serum lipids, thyroid function tests, blood cell counts, differential blood cell counts, coagulation tests, electrolytes, renal function tests, liver parameters, C-reactive protein, and blood sedimentation rate. All were within normal limits. She was negative for malaria, dengue, hepatitis B/C, and human immunodeficiency viruses. X-rays of her lungs and non-contrast computed tomography (CT) of her thorax showed no evidence of interstitial pneumonia.
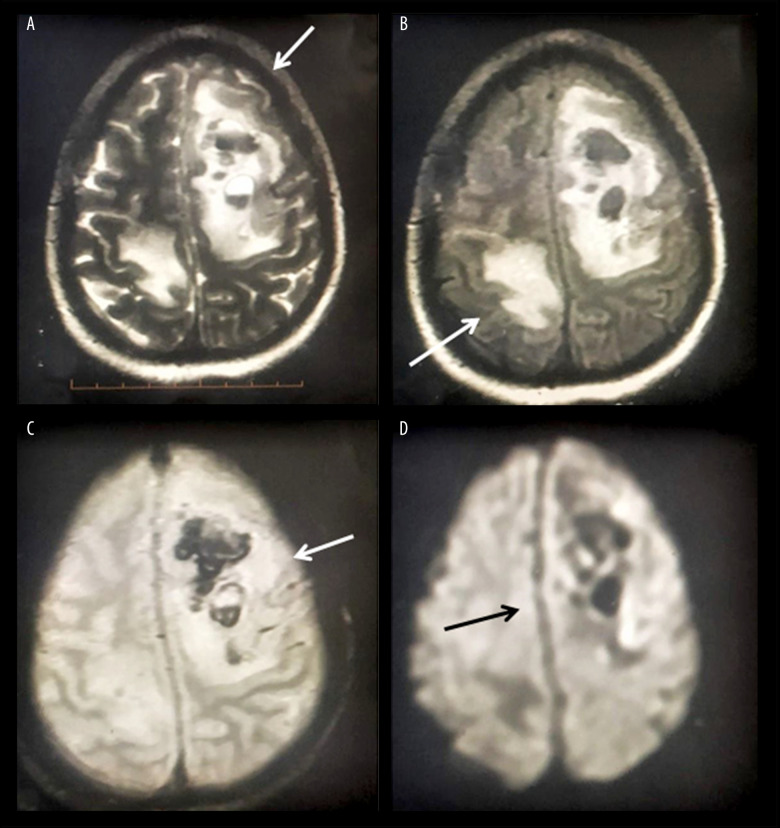
Findings on magnetic resonance imaging (MRI) of the brain were indicative of AHNE (Figure 1). Contrast MR venography ruled out isolated cortical venous thrombosis. Cerebrospinal fluid (CSF) analysis showed mild lymphocytosis (total 20 cells, including 90% lymphocytes) with normal protein (60 mg/dl) and glucose (70 mg/dl) concentrations. Her IgG index was elevated, but there were no oligoclonal bands (OCB). Qualitative polymerase chain reaction (PCR) showed that she was negative for a panel of neuroviruses, including herpes simplex viruses 1 and 2, human herpes viruses 6 and 7, varicella zoster virus, Epstein-Barr virus, Japanese encephalitis virus, cytomegalovirus, mumps virus, enterovirus, adenovirus, and parvovirus-B19). However, real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) assays of nasopharyngeal and oropharyngeal swabs (GeneXpert Xpress STARS CoV2) showed that she was positive for SARS-CoV-2. She was negative for auto-immune diseases and demyelinating conditions, including myelin-oligodendrocyte-glycoprotein disorders (MOG-opathies) and neuro-myelitis spectrum disorders (NMOSD). EEG could not be recorded because she was infected with SARS-CoV-2.
Figure 1.
Cerebral MRI of the index patient, yielding findings suggestive of AHNE. T2-weighted images showing non-enhancing altered intensity lesions in the left high fronto-parietal (arrow) and right posterior parietal (arrows) areas with peri-lesional edema (A, B) and signal blooming in gradient recalled echo (GRE) MRI with mass effect over the adjacent falx towards the right side (C, D).
The patient was initially stabilized with airway and circulatory support along with empirical intravenous ceftriaxone (4 g/d) and vancomycin (2 g/d), followed by acyclovir (1.5 g/d), levetiracetam (1 g/d), saline 0.9% (1.5 l/d), and mannitol (300 ml/d) to lower her intracranial pressure (ICP). Based on a preliminary diagnosis of SARS-CoV-2-associated AHNE, she was administered high-dose intravenous methylprednisolone (1 g/d) for 5 days. Because methylprednisolone was ineffective, intravenous immunoglobulin (IVIG) treatment was planned, but unfortunately she died of her illness prior to this treatment.
Discussion
SARS-CoV-2 is a primary respiratory pathogen, frequently accompanied by neurological complications involving the CNS and PNS [2–8]. CNS manifestations of SARS-CoV-2 infection include meningitis, encephalitis, AHNE, seizures, stroke, cerebral bleeding, sinus venous thrombosis, ataxia, and impaired consciousness [9–11]. PNS manifestations of SARS-CoV2 infection include hyposmia, hypogeusia, neuropathy, myalgia, and polyradiculitis [9–11]. Potential routes of neurotropism and neuroinvasion include retrograde axonal transport via olfactory nerve endings and/or taste buds [6,13] or brain invasion via hematogeneous spread of the virus [14].
In addition to direct damage by the virus, the CNS may be damaged indirectly by virus-induced inflammation, including neuronal damage due to a cytokine storm and accompanied by up-regulation of inflammatory markers [14,15]. The CNS can also be damaged indirectly by hypoxia due to post-acute respiratory distress syndrome (ARDS)/pneumonia [16], a prothrombotic state secondary to endothelial damage [14], embolic events due to SARS-CoV-2-ACE2R-mediated vascular damage and disruption of the blood-brain-barrier (BBB) [2,4,6,14], drugs administered during the infection [14], prolonged ICU stay [14], or septic or cardiogenic shock [14].
GTCS and LOC are likely direct results of viral encephalitis or are indirectly mediated by toxins, resulting from hypoxic encephalopathy, metabolic encephalopathy on a background of multiorgan dysfunction syndrome (MODS), seizures that cause post-ictal confusion, or SARS-CoV-2-associated acute cerebrovascular events [3,7]. Clouded sensorium may delay the diagnosis, as it is still an under-appreciated manifestation of the disease, as the primary focus in SARS-CoV-2 infected patients is the respiratory system. Neurologic manifestations, at least subtle manifestations, may precede respiratory symptoms [17]. For example, the first report of a patient with SARS-CoV-2-associated meningitis/encephalitis found that, despite the absence of virus in nasopharyngeal and oropharyngeal swabs, the CSF was positive for virus by RT-PCR, indicating possible neuroinvasion in the absence of respiratory invasion [18]. The first report of a patient with SARS-CoV-2-associated AHNE suggested that AHNE was induced by a generalized cytokine storm caused by SARS-CoV-2 infection [19], resulting in damage to the BBB, without direct viral invasion or parainfectious demyelination, which are common in AHNE [20]. The cerebral MRI of the first patient with SARS-CoV-2-associated AHNE displayed a hemorrhagic rim enhancing lesions within the thalami, medial temporal lobes, and subinsular regions [19]. AHNE was also reported in a 59-year-old woman with anemia, seizures, and somnolence [21] and, on autopsy, in a polymorbid 77-year-old woman [22].
Neurological substrates showing involvement in the present patient included the dorsolateral prefrontal cortex (DLPFC), the ventromedial prefrontal cortex (VMPFC), the anterior cingulated cortex (ACC), the orbito-frontal cortex (OFC), and the posterior parietal cortex (PPC). Deficits in the DLPFC include executive deficits, inapt approaches to problem solving/analysis, working/procedural memory dysfunction, defective retrieval, impaired attention, lack of initiative and spontaneity, and dis-inhibited responses. Deficits in the VMPFC include dysregulation of social emotions and severe impairment in personal/social decision-making, whereas deficits in the ACC include loss of selective attention. Deficits in the OFC include dysregulated impulses, repetitive behaviors/gestures, and drives, whereas deficits in the PPC include problems with planned movements, visuo-spatial problems, prosopagnosia, and inattention. The lack of fluctuation in the severity of cognitive impairment in our patient suggested acute-onset cognitive impairment rather than delirium. MRI findings were consisted with most of the clinically identified neuro-substrates. The pattern of cognitive impairment in the present patient was comparable to the neurocognitive sequelae of other types of viral encephalitis [23].
Conclusions
This report confirms that SARS-CoV-2 is a potential CNS pathogen. CNS manifestations of SARS-CoV-2 infection can include AHNE presenting as GTCS and frontal dysexecutive syndrome. The present patient is the first to date with acute-onset and isolated cognitive impairments due to SARS-CoV-2 infection in the absence of clinical or radiological respiratory manifestations. These findings may assist in the early detection and diagnosis of neuro-coronavirus disease-2019 (COVID-19), especially among clinicians and neurologists working in areas of endemic SARS-CoV-2 infection. Identifying isolated AHNE may prevent further transmission of SARS-CoV-2 infection, as well as help in early initiation of adequate treatment.
Footnotes
Conflicts of interest
None.
References:
- 1.Osler W. The study of the fevers of the south. JAMA. 1896;26(21):999–1004. [Google Scholar]
- 2.Lahiri D, Mondal R, Deb S, et al. Neuroinvasive potential of a primary respiratory pathogen SARS-CoV2: Exploring the underrecognized. Diabetes Metab Syndr. 2020;14(5):1053–60. doi: 10.1016/j.dsx.2020.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Wang M, Chen S, He Q, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. Lancet Neurol. 2020 [preprint] [Google Scholar]
- 4.Baig AM, Khaleeq A, Ali U, Syneda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–98. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): Encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahiri D, Ardila A. COVID-19 pandemic: A neurological perspective. Cureus. 2020;12(4):e7889. doi: 10.7759/cureus.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: A systematic review and current update. Acta Neurol Scand. 2020;142(1):14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finsterer J, Stollberger C. Update on the neurology of COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.26000. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HJ, Friston K. Structural and functional brain networks: From connections to cognition. Science. 2013;342(6158):1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- 13.Mori I. Transolfactory neuroinvasion by viruses threatens the human brain. Acta Virol. 2015;59(4):338–49. doi: 10.4149/av_2015_04_338. [DOI] [PubMed] [Google Scholar]
- 14.Mishra AK, Lal A, Sahu KK, et al. Letter to the editor regarding “Neurological impact of coronavirus disease (COVID-19): Practical considerations for the neuroscience community”. World Neurosurg. 2020 doi: 10.1016/j.wneu.2020.05.089. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu KK, Mishra AK, Lal A. COVID-2019: Update on epidemiology, disease spread and management. Monaldi Arch Chest Dis. 2020;90(1) doi: 10.4081/monaldi.2020.1292. [DOI] [PubMed] [Google Scholar]
- 16.Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc Neurol. 2020 doi: 10.1136/svn-2020-000431. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poyiadji N, Shahin G, Noujaim D, et al. COVID-19 associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology. 2020;296(2):E119–120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi A. Imaging of acute disseminated encephalomyelitis. Neuroimaging Clin N Am. 2008;18(1):149–61. doi: 10.1016/j.nic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Dixon L, Varley J, Gontsarova A, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoyanov GS, Lyutfi E, Dzhenkov DL, Petkova L. Acute necrotizing encephalitis in viral respiratory tract infection: An autopsy case report. Cureus. 2020;12(5):e8070. doi: 10.7759/cureus.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris L, Griem J, Gummery A, et al. ENCEPH UK study group. Neuropsychological and psychiatric outcomes in encephalitis: A multi-centre case-control study. PLoS One. 2020;15(3):e0230436. doi: 10.1371/journal.pone.0230436. [DOI] [PMC free article] [PubMed] [Google Scholar]