Abstract
Breast cancer is one of the most prevalent cancer types and is accompanied by a high incidence and mortality rate, severely threatening women's health globally. Long non-coding RNA forkhead box D2 adjacent apposite strand RNA 1 (lncRNA FOXD2-AS1) has been identified to function as an oncogene in human cancers; however, it has rarely been investigated in breast cancer. The aim of the present study was to investigate the role of FOXD2-AS1 in breast cancer, and to clarify the underlying mechanisms. The expression of FOXD2-AS1 in breast cancer cell lines was first quantified by reverse transcription-quantitative PCR, and the biological function of FOXD2-AS1 was then determined. Cellular proliferative ability was determined by Cell Counting kit-8 assay, and wound healing and Transwell assays were conducted to assess the cell migratory and invasive ability. Corresponding protein expression levels were determined by western blot analysis. In addition, experimental animal models were established by the subcutaneous injection of MDA-MB-468 cells into the right axillary lymph nodes of BALB/c nude mice, and the effects of FOXD2-AS1 on tumor growth were observed. The results indicated that FOXD2-AS1 expression was upregulated in breast cancer cell lines, and that FOXD2-AS1 downregulation significantly inhibited the proliferation, migration and invasiveness of MCF-7 and MDA-MB-468 cells. S100 calcium binding protein A1 (S100A1) was also upregulated in breast cancer cell lines and was positively regulated by FOXD2-AS1. Furthermore, the inhibition of S100A1 and the overexpression of the serine/threonine-protein kinase, large tumor suppressor homolog 1 (LATS1), inhibited the FOXD2-AS1-induced cellular proliferation, migration and invasiveness in breast cancer. Experimental mouse models revealed that FOXD2-AS1 downregulation significantly inhibited tumor growth, and that the levels of phosphorylated (p-)YAP and p-LATS1 were upregulated by FOXD2-AS1 knockdown, indicating that the inhibition of FOXD2-AS1 activated Hippo/yes-associated protein signaling. On the whole, the findings of the present study suggest that the FOXD2-AS1/S100A1/Hippo axis is involved in the tumorigenesis and progression of breast cancer. In the future, these may contribution to the identification of more effective breast cancer treatments.
Keywords: FOXD2-AS1, S100 calcium binding protein A1, breast cancer, large tumor suppressor homolog 1, Hippo
Introduction
As one of the most prevalent malignancies, breast cancer is a primary cause of mortality among gynecological cancer cases, and with increasing morbidity and mortality rates, it poses a considerable threat to women's health worldwide (1,2). In 2019, statistics from the American Cancer Society estimated 271,270 newly diagnosed cases and 42,260 deaths from breast cancer in the United States (3). The leading causes of the high death rate are distal metastasis and resistance to the existing treatments (4). Despite improvements in early diagnosis and systemic treatment, the incidence of breast cancer and metastasis-related mortality is steadily increasing (5,6). Therefore, there is an urgent need to elucidate the mechanisms responsible for the disordered cellular metastasis and to enhance our understanding of the tumorigenesis and development processes, hence facilitating the identification of more efficient breast cancer treatments.
Long noncoding RNAs (lncRNAs) are a group of RNAs >200 nucleotides in length, which lack protein-coding capacity (7). Numerous studies have revealed that lncRNAs have versatile biological functions in pathological and physiological processes, including tumorigenesis (8-10). lncRNAs are considered to regulate the development of various types of cancer, including breast cancer (10). For instance, LINC01089 is downregulated in breast cancer tissues and cell lines, and LINC01089 overexpression increases tumor cell proliferation, migration and invasiveness. As an oncogene that regulates breast cancer cell proliferation and apoptosis, hepatocellular carcinoma upregulated EZH2-associated lncRNA is closely associated with the clinical progression of breast cancer (11). These results indicate the indispensability of research on lncRNAs and breast cancer.
lncRNA forkhead box D2 adjacent apposite strand RNA 1 (FOXD2-AS1) is a novel non-coding RNA identified to be an oncogene in human cancers. FOXD2-AS1 has been shown to be upregulated in various types of cancer, including glioma, osteosarcoma and papillary thyroid cancer, as well as breast cancer (12-15). A previous study indicated that FOXD2-AS1 participates in regulating the development of breast cancer via the miR-150-5p/PFN2 axis, and that it may be a potential biomarker for the diagnosis and prognosis of breast cancer (15). However, to the best of our knowledge, there are no additional data regarding the investigation of FOXD2-AS1 in breast cancer, and its effects and the underlying mechanisms on the regulation of breast cancer cell invasion and metastasis. Thus, the aim of the present study was to determine the role and potential mechanisms of action of FOXD2-AS1 in breast cancer, and to provide further support for its use in clinical diagnosis and treatment.
Materials and methods
Datasets
The present study evaluated the expression level of FOXD2-AS1 in breast cancer samples using The Cancer Genome Atlas (TCGA) dataset, which was downloaded from the TCGA data portal (https://tcga-data-nci-nih-gov.ez.xjtlu.edu.cn). The TCGA data subset for breast cancer included 246 normal samples and 1,110 tumor samples. The Mann-Whitney test was used to determine statistically significant differences between normal and tumor samples. P<0.05 was considered to indicate a statistically significant difference.
Cell culture
A human normal breast epithelial cell line (MCF-10A) and human breast cancer cell lines (MCF-7, MDA-MB-468, MDA-MB-453 and BT-549) were purchased from the American Type Culture Collection (ATCC). The cells were incubated in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) in a humidified incubator at 37°C (5% CO2) (15,16).
Transfection
To overexpress FOXD2-AS1, an overexpression vector (pcDNA FOXD2-AS1) and its corresponding negative control vector (pcDNA-NC) were synthesized by Shanghai GenePharma Co., Ltd. Short hairpin (sh)RNAs targeting FOXD2-AS1 (100 nM; shRNA-FOXD2-AS1-1 and shRNA-FOXD2-AS1-2) and a negative scramble control shRNA (shRNA) (also purchased from Shanghai GenePharma Co., Ltd.) were used to knock down FOXD2-AS1 expression. In addition, pcDNA-LATS1, shRNA-S100A1-1 and shRNA-S100A1-2 were obtained from Shanghai GenePharma Co., Ltd. to overexpress LATS1 or to knock down S100A1, respectively. The shRNA sequences were as follows: shRNA-FOXD2-AS1-1 targets, GGA CTC CAC TCT TCG CTT A; shRNA-FOXD2-AS1-2 targets, GCT TCC AGG TAT GTG GGA A; shRNA-S100A1-1 targets, GAT CCG GAG ACC CTC ATC AAC GTG TTC TTC CTG TCA GAA ACA CGT TGA TGA GGG TCT CCT TTT TG; shRNA-S100A1-2 targets, GAT CCG TGG ACT TCC AGG AGT ATG TGC TTC CTG TCA GAC ACA TAC TCC TGG AAG TCC ACT TTT TG. Cells were transfected with pcDNA FOXD2-AS1 (15 nM), pcDNA-LATS1 (15 nM), pcDNA-NC (15 nM), shRNA-FOXD2-AS1-1 (500 ng/µl), shRNA-FOXD2-AS1-2 (500 ng/µl), shRNA-S100A1-1 (500 ng/µl), shRNA-S100A1-2 (500 ng/µl), shRNA (500 ng/µl), or co-transfected with pcDNA FOXD2-AS1 and pcDNA-LATS1, or co-transfected with pcDNA FOXD2-AS1 and shRNA-S100A1 using Lipofectamine® 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Lipofectamine 2000 reagent was first mixed with vectors to form a reagent-vector complex, followed by incubation with cells at 37°C for 5 h. The transfection efficacy was assessed by reverse transcription-quantitative PCR (RT-qPCR) after 48 h of transfection.
RNA extraction and RT-qPCR
Total RNA was extracted from all cell lines using TRIzol® reagent (Takara Bio, Inc.) according to the manufacturer's instructions. Total RNA was then reverse transcribed into cDNA using the PrimeScript™ RT Master kit, and the mRNA expression levels were quantified using the SYBR Premix Ex Taq™ kit (Both Takara Bio, Inc.) with a 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The sequences of specific primers used for RT-qPCR were as follows: FOXDA-AS1 forward, 5′-TGG ACC TAG CTG CAG CTC CA-3′ and reverse, 5′-AGT TGA AGG TGC ACA CAC TG-3′; S100A1 forward, 5′-GAG TAT GTG GTG CTT GTG GC-3′ and reverse, 5′-CTT GGA CCG CTA CTC TTG CG-3′; large tumor suppressor homolog 1 (LATS1) forward, 5′-ACC GCT TCA AAT GTG ACT GTG ATG CCA C CT-3′ and reverse, 5′-CTT CCT TGG GCA AGC TTG GCT GAT CCT CT-3′; and GAPDH forward, 5′-GCG AGA TCG CAC TCA TCA TCT -3′ and reverse, 5′-TCA GTG GTG GAC CTG ACC -3′. The data were displayed as 2−ΔΔCq values with GAPDH as the constitutive marker (17). The PCR conditions were as follows: 95°C for 5 min, 40 cycles of 95°C for 20 sec and 62°C for 30 sec, followed by 72°C for 3 min.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was determined using the Cell Counting Kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc). Briefly, cells were seeded into a 96-well plate and incubated for 24 h at 37°C. Following culture for the indicated periods of time (12, 24 and 48 h), 10 µl of the CCK-8 reagent were added to each well and the plate was incubated at 37°C for a further 3 h. The optical density values at 450 nm were then measured using a micro-plate spectrophotometer (Thermo Fisher Scientific, Inc.).
Western blot analysis
The total protein was extracted from the cells using RIPA lysis buffer (Beyotime Institute of Biotechnology) and quantified using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). The same amount of each protein sample (20 µg) was subjected to 10% SDS-PAGE; the proteins were then transferred onto PVDF membranes (EMD Millipore) and was blocked in 5% non-fat milk for 1 h at room temperature. The membranes were then incu-bated with primary antibodies against cyclinE1 (1:1,000; cat. no. ab33911; Abcam), cyclin-dependent kinase-2 (CDK2; 1:1,000; cat. no. ab32147; Abcam), p21 (1:1,000; cat. no. ab109520; Abcam), matrix metalloproteinases (MMP)2 (1:1,000; cat. no. ab92536; Abcam), MMP9 (1:1,000; cat. no. ab38898; Abcam), S100 calcium binding protein A1 (S100A1; 1:1,000; cat. no. 5066; Cell Signaling Technology, Inc.), phosphorylated (p)-yes-associated protein (YAP; 1:1,000; cat. no. 13008; Cell Signaling Technology, Inc.), YAP (1:1,000; cat. no. 15028; Cell Signaling Technology, Inc.), serine/threonine-protein kinase LATS1 (1:1,000; cat. no. 3477; Cell Signaling Technology, Inc.), p-LATS1 (1:1,000; cat. no. 8654; Cell Signaling Technology, Inc.), mammalian STE20-like protein kinase (MST)1 (1:1,000; cat. no. 3682; Cell Signaling Technology, Inc.), MST2 (1:1,000; cat. no. 3952; Cell Signaling Technology, Inc.), and GAPDH (1:2,000; cat. no. ab8245; Abcam) at 4°C overnight. The membranes were washed with Tris-buffered saline with Tween (TBST) and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG secondary antibody (1:2,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) at room temperature for 1.5 h. The protein bands were visual-ized using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech) and quantified using ImageJ software (version 1.46; National Institutes of Health).
Wound healing assay
The cellular migration rate was determined using a wound healing assay. The cells were seeded into a 6-well plate and cultured to 100% confluence. A wound was produced in each monolayer using a 200-µl pipette tip, and the plate was washed 3 times with PBS to remove detached cells. The cells were then cultured in the fresh medium without FBS. Following incubation for 48 h, the wound-healing ability was assessed under a light microscope (magnification ×100; CKX41, Olympus Corporation), and the widths of the wounds were measured at 0 and 48 h.
Transwell assay
The cell invasive rate was determined with a Transwell assay. Cells (4×104/well) in serum-free medium were placed in the upper chamber of each insert [which had been precoated with 40 µl of Matrigel (BD biosciences) at 37°C for 1 h], and complete medium containing 10% FBS was added to the lower 24-well chamber. After 24 h, cells on the upper surface were removed, and cells attached to the lower surface were stained with 0.05% crystal violet (Beijing Solarbio Science & Technology Co., Ltd.) at room temperature for 10 min. The cells were viewed under a light microscope (magnification ×100; CKX41, Olympus Corporation), and the invasive ability of the cells was determined using ImageJ software version 1.46 by counting the number of cells attached to the lower surface.
Cell cycle analysis
The cell cycle distribution was determined by flow cytometry. After being subjected to the indicated treatments, the cells were collected and fixed in 70% ethanol at −20°C overnight. The cells were then washed twice with PBS and incubated in the dark with RNase A and PI staining solution (Roche Diagnostics) at 37°C for 30 min. Finally, the cell samples were analyzed using a FACSCalibur flow cytometer and CellQuest software version 5.1 (both from BD Biosciences).
In vivo experiments
The present study was approved by the First Affiliated Hospital of Zhengzhou University, and the animal experiments were performed according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. A total of 18 5-week-old male BALB/c nude mice were purchased from the Experimental Animal Center of Shanghai Institute for Biological Sciences, and housed in a standard environment (25°C; 50% humidity; 12 h light/dark cycle) with free access to food and water. Each mouse was subcutaneously injected at the right axillary lymph nodes with 1.0×107 MDA-MB-468 cells, which were stably transfected with either the shRNA negative control shRNA or shRNA-FOXD2-AS1. The weights and tumor volumes (tumor volume =1/2 × length × width2) of the mice were monitored every 5 days until the mice were sacrificed. At 20 days after the injection, all the 18 mice were sacrificed by cervical dislocation that caused a sharp section of the spinal cord followed by an instantaneous cardiac arrest. After the cessation of the heartbeat and respiratory arrest of the mice was confirmed, the tumors were excised, photographed and stored for the further investigation.
Statistical analysis
Data are presented as the means ± standard deviation (SD) from ≥3 independent experiments, and each experiment was conducted in triplicate. The data were analyzed using SPSS statistical software version 20.0 (SPSS, Inc.) and the differences among groups were analyzed using one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
FOXD2-AS1 expression is upregulated in breast cancer cells
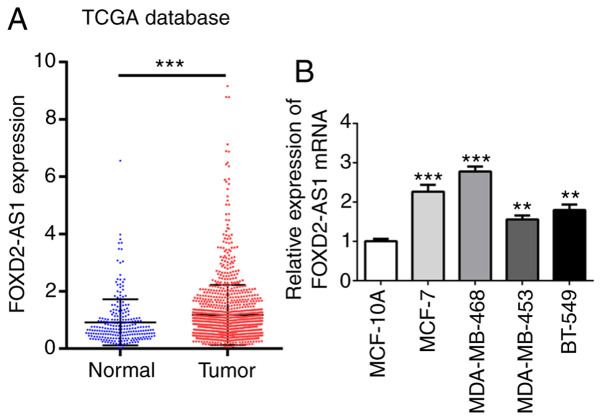
The TCGA database (cancer.gov/tcga) was used to identify the association between FOXD2-AS1 and breast cancer by evaluating the expression profiles of FOXD2-AS1 in breast cancer tissues and normal tissues. The results of TCGA analysis revealed a significantly higher FOXD2-AS1 expression in breast cancer tissues than in normal tissues (Fig. 1A). Human normal breast epithelial cell line (MCF-10A) and human breast cancer cell lines (MCF-7, MDA-MB-468, MDA-MB-453 and BT-549) were also obtained to detect the mRNA levels of FOXD2-AS1. The results revealed that FOXD2-AS1 expression was markedly upregulated in all breast cancer cells, particularly in the MCF-7 (ER-positive breast cancer cell line) and MDA-MB-468 cells (triple-negative breast cancer cell line) (Fig. 1B), which were used for further experiments, even though there may be some variability in the results between the 2 cell lines. These findings indicate that FOXD2-AS1 is upregulated in breast cancer.
Figure 1.
FOXD2-AS1 is upregulated in breast cancer cells. (A) The Cancer Genome Atlas (TCGA) database (cancer.gov/tcga) was used to identify the association of FOXD2-AS1 with breast cancer by collecting the profiles of FOXD2-AS1 in breast cancer tissues and normal tissues. ***P<0.001, vs. normal samples. (B) mRNA level of FOXD2-AS1 in human normal breast epithelial cell line (MCF-10A) and human breast cancer cell lines (MCF-7, MDA-MB-468, MDA-MB-453 and BT-549) was determined by RT-qPCR. **P<0.01 and ***P<0.001 vs. MCF-10A cells.
FOXD2-AS1 knockdown suppresses breast cancer cell proliferation, migration and invasiveness
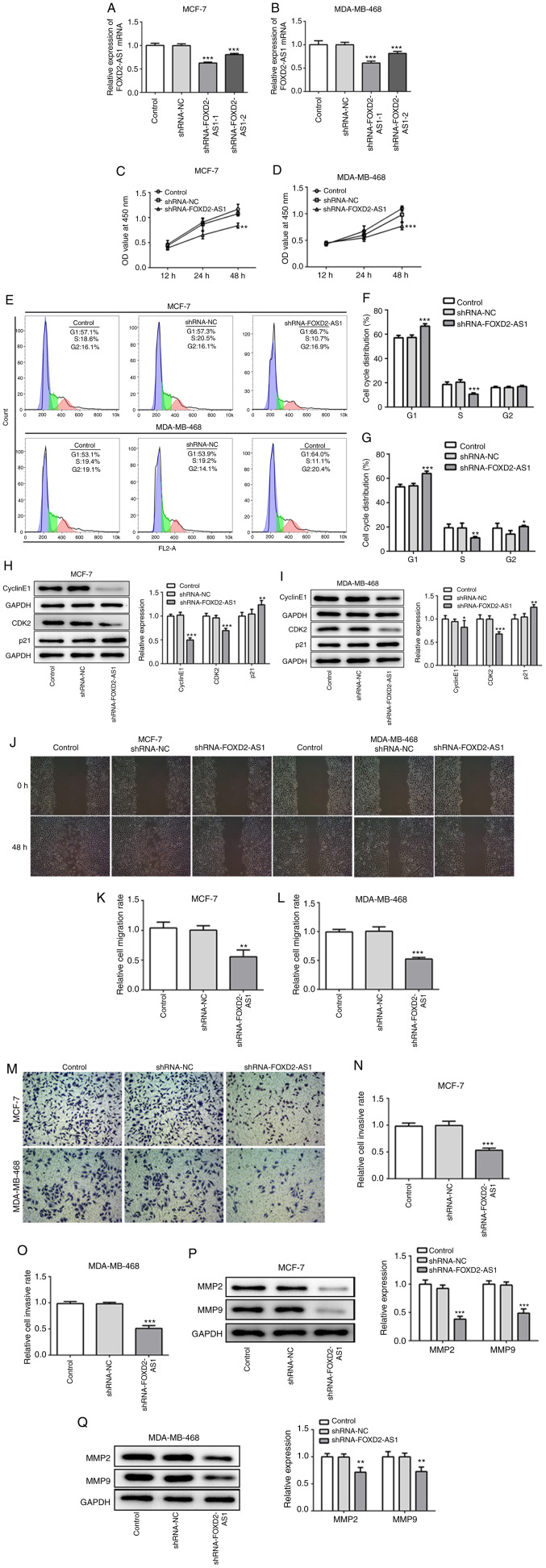
To further elucidate the role of FOXD2-AS1 in breast cancer, FOXD2-AS1 was knocked down in both the MCF-7 and MDA-MB-468 cells. Due to a higher transfection efficacy, shRNA-FOXD2-AS1-1 (referred to as shRNA-FOXD2-AS1) was subsequently used for breast cancer cell experimentation (Fig. 2A and B). The results of CCK-8 assay indicated that FOXD2-AS1 knockdown significantly inhibited the proliferative ability of the MCF-7 and MDA-MB-468 cells (Fig. 2C and D). The cell cycle was then analyzed by flow cytometry, which revealed that FOXD2-AS1 knockdown increased the percentage of cells in the G1 phase, whereas it decreased that in the S phase for both the MCF-7 and MDA-MB-468 cells (Fig. 2E-G). Furthermore, FOXD2-AS1 knockdown decreased the protein expression levels of cyclin E1 and CDK2, and increased the expression of p21 (Fig. 2H and I). These findings indicate that FOXD2-AS1 knockdown suppresses cellular proliferation by regulating the cell cycle, specifically by preventing G1 to S phase progression. Moreover, FOXD2-AS1 knockdown significantly decreased the migration rate (Fig. 2J-L) and the invasiveness (Fig. 2M-O) of the MCF-7 and MDA-MB-468 cells. The expression levels of MMP-2 and -9, which are critical to the migration, invasion and metastasis of breast cancer cells (18), were both downregulated following FOXD2-AS1 knockdown (Fig. 2P and Q), indicating that FOXD2-AS1 may enhance cellular migration and invasiveness by regulating MMP-2 and -9.
Figure 2.
Knockdown of FOXD2-AS1 suppresses cell proliferation, migration and invasion in breast cancer. (A and B) Following transfection with shRNA-FOXD2-AS, the mRNA level of FOXD2-AS1 was measured by RT-qPCR in MCF-7 and MDA-MB-468 cells. (C and D) CCK-8 assay was performed to determine cell proliferation following transfection. (E-G) Cell cycle distribution was determined and analyzed by FACS. (H and I) The protein expression of cyclin E1, CDK2 and p21 was determined by western blot analysis. Knockdown of FOXD2-AS1 suppresses cell proliferation, migration and invasion in breast cancer. (J) Wound healing assay was performed to detect the migration of both MCF-7 and MDA-MB-468 cells. (K and L) Relative migration rate of MCF-7 and MDA-MB-468 cells was quantified, respectively. (M) Transwell assay was performed to detect the invasion of both MCF-7 and MDA-MB-468 cells. (N and O) Relative cell invasive rate of MCF-7 and MDA-MB-468 cells was quantified, respectively. (P) MMP-2 and MMP-9 protein expression in MCF-7 transfected with shRNA-FOXD2-AS1 was detected and quantified. (Q) MMP-2 and MMP-9 protein expression in MDA-MB-468 transfected with shRNA-FOXD2-AS1 was detected and quantified. *P<0.05, **P<0.01 and ***P<0.001, vs. shRNA-NC. CDK2, cyclin-dependent kinase-2; MMP, matrix metalloproteinase.
FOXD2-AS1 knockdown regulates the Hippo/YAP signaling pathway
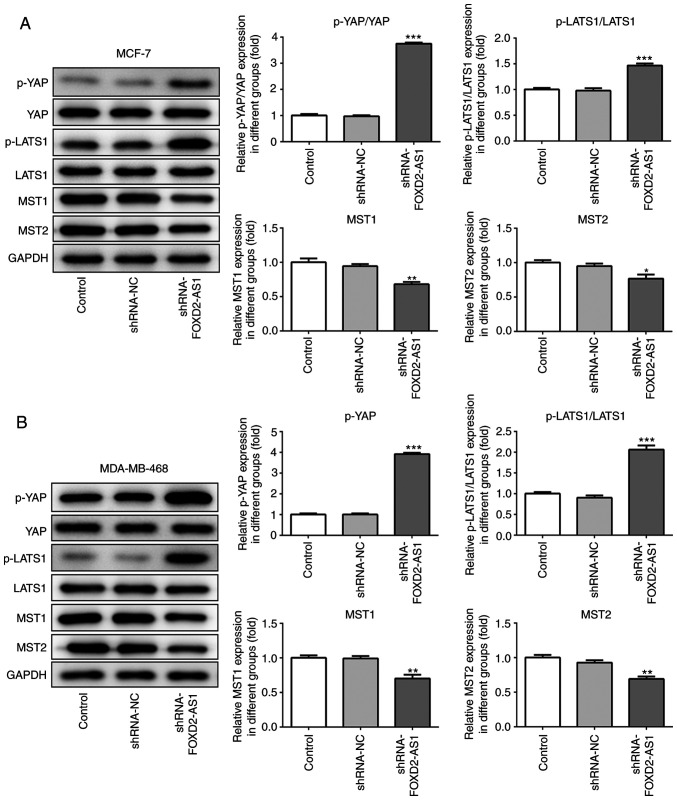
The Hippo/YAP signaling pathway is reportedly involved in the progression of breast cancer (19). In the present study, the levels of specific proteins involved in the YAP/Hippo signaling pathway were assessed in the MCF-7 and MDA-MB-468 cells following FOXD2-AS1 knockdown. Western blot analysis revealed that the levels of p-YAP and p-LATS1 were significantly upregulated, while those of MST1 and 2 were significantly downregulated by FOXD2-AS1 knockdown (Fig. 3A and B). Thus, the results confirmed that FOXD2-AS1 regulates the Hippo/YAP signaling pathway in breast cancer cells.
Figure 3.
Knockdown of FOXD2-AS1 regulates the Hippo/YAP signaling pathway. (A) The protein expression of Hippo/YAP signaling pathway-related genes (p-YAP, YAP, p-LATS1, LATS1, MST1 and MST2) was determined by western blot analysis in MCF-7 cells. (B) The protein expression of Hippo/YAP signaling pathway-related genes (p-YAP, YAP, p-LATS1, LATS1, MST1 and MST2) was determined by western blot analysis in MDA-MB-468 cells. *P<0.05, **P<0.01 and ***P<0.001, vs. shRNA-NC. LATS1, large tumor suppressor homolog 1; MST, mammalian STE20-like protein kinase; YAP, yes-associated protein.
S100A1 mediates FOXD2-AS1-induced breast cancer cell proliferation, migration and invasiveness
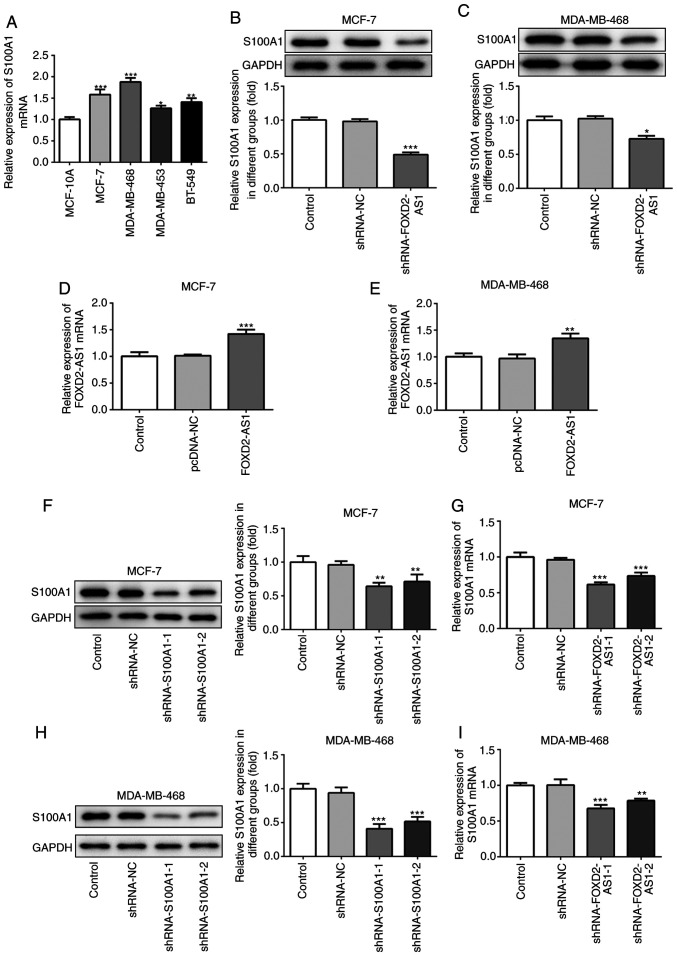
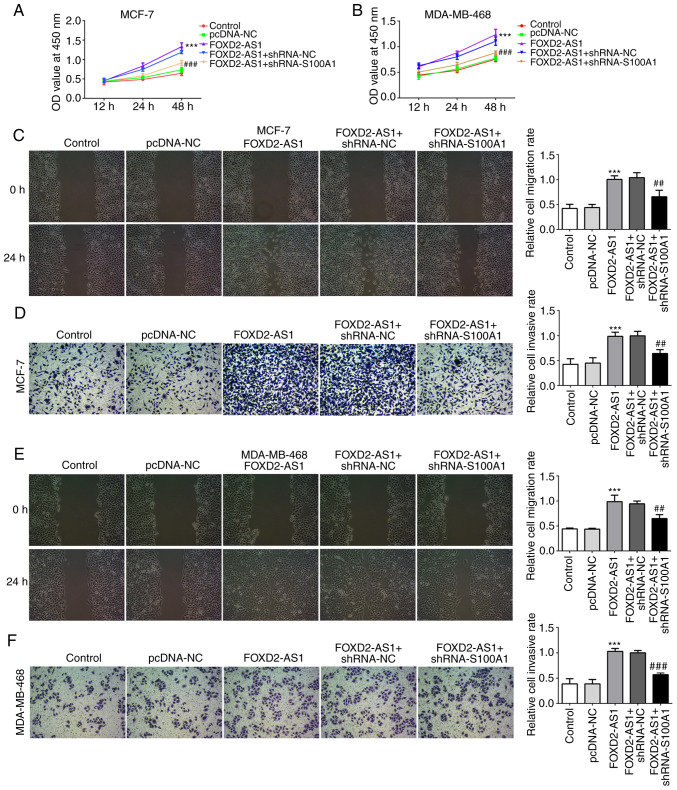
S100A1 is a calcium-binding protein of the S100 protein family, which is not only upregulated in, but is also involved in the progression of ovarian cancer (20). In the present study, the expression of S100A1 was evaluated in the breast cancer cell lines, indicating that S100A1 was significantly upregulated in breast cancer cells (particularly in the MCF-7 and MDA-MB-468 cells) compared with the MCF-10A cells (Fig. 4A). In MCF-7 and MDA-MB-468 cells transfected with shRNA-FOXD2-AS1, it was found that the protein expression level of S100A1 was downregulated (Fig. 4B and C). To further investigate the role of S100A1 in FOXD2-AS1-mediated cellular proliferation, migration and invasiveness, breast cancer cells were transfected with an expression vector, pcDNA-FOXD2-AS1 (Fig. 4D and E), and shRNA-S100A1 to inhibit S100A1 protein and mRNA expression (Fig. 4F-I). As shown in Fig. 5A and B, the overexpression of FOXD2-AS1 significantly promoted the proliferation of MCF-7 and MDA-MB-468 cells, which was subsequently reversed by the downregulation of S100A1. Wound healing and Transwell assays demonstrated that FOXD2-AS1 overexpression significantly increased MCF-7 cell migration and invasiveness, respectively, which were also reversed by the downregulation of S100A1 (Fig. 5C and D). A similar result was observed in the MDA-MB-468 cells (Fig. 5E and F). These results suggest that S100A1 regulates the FOXD2-AS1-mediated proliferation, migration and invasiveness of breast cancer cells.
Figure 4.
S100A1 is upregulated in breast cancer cells. (A) The mRNA level of S100A1 in MCF-10A and human breast cancer cell lines (MCF-7, MDA-MB-468, MDA-MB-453 and BT-549) was determined by RT-qPCR. *P<0.05, **P<0.01 and ***P<0.001, vs. MCF-10A cells. (B and C) In shRNA-FOXD2-AS1-trans-fected MCF-7 and MDA-MB-468 cells, the protein expression of Sl00A1 was detected by western blot analysis. *P<0.05 and ***P<0.001, vs. shRNA-NC. (D and E) Following transfection with pcDNA-FOXD2-AS1, the mRNA level of FOXD2-AS1 in MCF-7 and MDA-MB-468 cells was detected by RT-qPCR. **P<0.01 and ***P<0.001, vs. pcDNA-NC. MCF-7 and MDA-MB-468 cells were transfected with shRNA-S100A1, and the protein expression and mRNA level of S100A1 in (F and G) MCF-7 and (H and I) MDA-MB-468 cells were determined by western blot analysis and RT-qPCR, respectively. **P<0.01 and ***P<0.001, vs. shRNA-NC. S100A1, S100 calcium binding protein A1.
Figure 5.
S100A1 mediates FOXD2-AS1-induced cell proliferation, migration and invasion in breast cancer. Following transfection with pcDNA-FOXD2-AS1, cells were transfected with shRNA-S100A1. (A and B) CCK-8 assay was performed to determine the proliferation of the differently treated cells. (C) Migratory ability of MCF-7 cells in the different treatment groups was determined by wound healing assay. (D) Invasive ability of MCF-7 cells in the different treatment groups was determined by Transwell assay. (E) Migratory ability of MDA-MB-468 cells in the different treatment groups was determined by wound healing assay. (F) Invasive ability of MDA-MB-468 cells in the different treatment groups was determined by Transwell assay. ***P<0.001, vs. pcDNA-NC; ##P<0.01 and ###P<0.001, vs. FOXD2-AS1 + shRNA-NC. S100A1, S100 calcium binding protein A1.
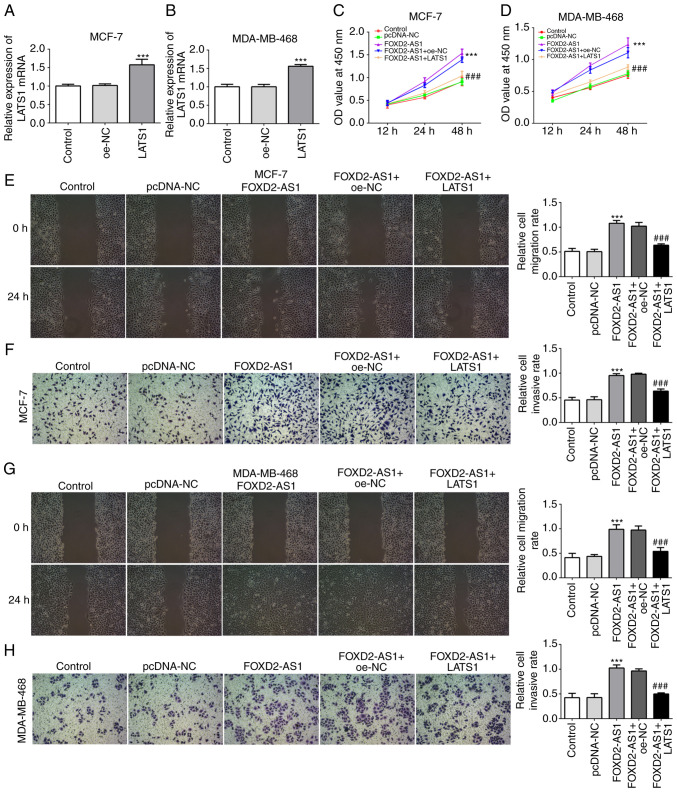
Overexpression of LATS1 inhibits FOXD2-AS-induced cell proliferation, migration and invasiveness
Since the Hippo/YAP signaling pathway is involved in the FOXD2-AS1-mediated characteristics of breast cancer cells, Hippo/YAP signaling was further investigated for its regulatory role in breast cancer cell proliferation, migration and invasiveness. For this purpose, LATS1 was overexpressed in the MCF-7 and MDA-MB-468 cells (Fig. 6A and B), and the results of CCK-8 assay revealed that LATS1 overexpression significantly inhibited FOXD2-AS1-induced cellular proliferation (Fig. 6C and D). Furthermore, the results of wound healing and Transwell assays revealed that LATS1 overexpression significantly inhibited the FOXD2-AS1-induced migration and invasiveness of both the MCF-7 (Fig. 6E and F) and MDA-MB-468 cells (Fig. 6G and H).
Figure 6.
Overexpression of LATS1 inhibits FOXD2-AS-induced cell proliferation, migration and invasion. (A and B) mRNA level of LATS1 in MCF-7 and MDA-MB-468 cells was detected by RT-qPCR in LATS1-overexpressing cells. ***P<0.001, vs. overexpression-NC (oe-NC). Following transfection with pcDNA-FOXD2-AS1, cells were transfected with pcDNA-LATS1 to overexpress LATS1. (C and D) CCK-8 assay was performed to determine the proliferation of the differently treated cells. (E) Migratory ability of MCF-7 cells in the different treatment groups was determined by wound healing assay. (F) Invasive ability of MCF-7 cells in the different treatment groups was determined by Transwell assay. (G) Migratory ability of MDA-MB-468 cells in the different treatment groups was determined by wound healing assay. (H) Invasive ability of MDA-MB-468 cells in the different treatment groups was determined by Transwell assay. ***P<0.001, vs. pcDNA-NC; ###P<0.001, vs. FOXD2-AS1 + shRNA-NC. LATS1, large tumor suppressor homolog 1.
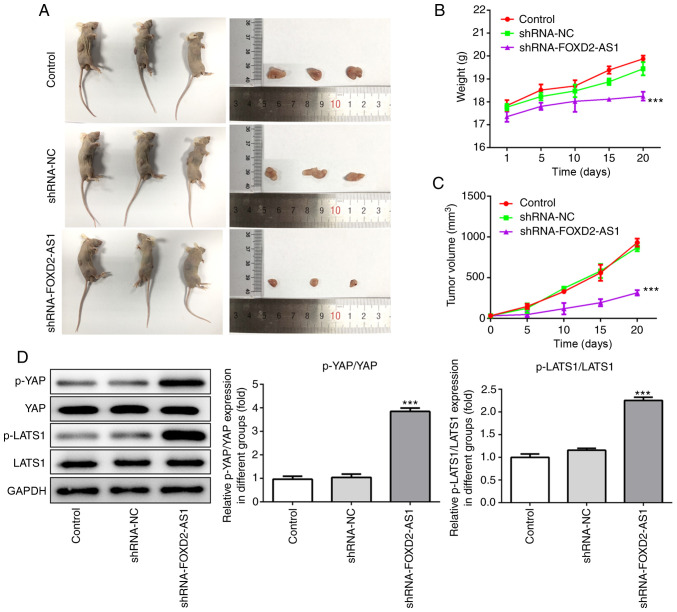
FOXD2-AS1 knockdown suppresses breast cancer tumor progression in vivo
From the aforementioned results, the role of FOXD2-AS1 in both the MCF-7 and MDA-MB-468 cells was confirmed. To explore the role of FOXD2-AS1 in breast cancer in vivo, mice were injected with MDA-MB-468 cells, which were stably transfected with either an shRNA negative control or shRNA-FOXD2-AS1. Following sacrifice, the tumors were excised and photographed (Fig. 7A). Tumors in the shRNA-FOXD2-AS1 group were the smallest in size, directly reflecting the suppressive effect of FOXD2-AS1 knockdown on tumor growth. During the experiment, body weights and tumor volumes were recorded every 5 days. Body weight increased at a slower rate in the shRNA-FOXD2-AS1 group, and the tumor volumes of this group also increased at a slower rate than those in the other groups (Fig. 7B and C). Additionally, western blot analysis of the extracted tumor tissues indicated a significant increase in p-YAP and p-LATS1 expression in the shRNA-FOXD2-AS1 group (Fig. 7D), which was consistent with the in vitro results. These findings thus suggest that FOXD2-AS1 knockdown suppresses the progression of breast cancer and regulates the Hippo/YAP signaling pathway in vivo.
Figure 7.
Knockdown of FOXD2-AS1 suppresses tumor progression of breast cancer in vivo. (A) Each mouse was injected with 1.0×107 MDA-MB-468 cells, which were stably transfected with either shRNA negative control or shRNA-FOXD2-AS1, subcutaneously at the right axillary lymph node. Following sacrifice, the tumors were excised and photographed. (B and C) During the experiment, the mouse weight and tumor volume (tumor volume =1/2 × length × width2) was monitored every 5 days until the mice were sacrificed. (D) Protein expression levels of p-YAP, YAP, p-LATS1 and LATS1 in the extracted tumors were determined by western blot analysis and were then quantified. ***P<0.001, vs. shRNA-NC. LATS1, large tumor suppressor homolog 1; YAP, yes-associated protein.
Discussion
Breast cancer is one of the most common types of human tumors, particularly among females. The high rates of metastasis and recurrence typically result in the deterioration and death of patients with breast cancer (21). Increasing evidence suggests that lncRNAs function as oncogenic or antitumor genes in various tumor types, cells types and the microenvironment, and that they may be used as effective and specific biomarkers for clinical diagnosis and prognosis (22,23). Due to its oncogenic properties, lncRNA FOXD2-AS1 has been investigated in several malignant tumors. In the present study, FOXD2-AS1 expression was found to be upregulated in breast cancer cell lines; thus, it was knocked down in MCF-7 and MDA-MB-468 cell to investigate its role in breast cancer. FOXD2-AS1 knockdown inhibited the proliferation, migration and invasiveness of MCF-7 and MDA-MB-468 cells, and inhibited tumor growth in vivo. Notably, S100A1 expression was also found to be upregulated in breast cancer cells, and further investigation revealed that S100A1 was inhibited following FOXD2-AS1 knockdown, indicating that the expression of FOXD2-AS1 and S100A1 was positively associated in breast cancer cells. Subsequent experiments revealed that the overexpression of FOXD2-AS1 significantly accelerated tumorigenesis by promoting cellular proliferation, migration and invasiveness. However, the effects of FOXD2-AS1 were reversed by the downregulation of S100A1. These results suggest that both FOXD2-AS1 and S100A1 knockdown exert antitumor effects on the progression of breast cancer, and that FOXD2-AS1 may exert its oncogenic functions by regulating S100A1.
S100A1 is a calcium-binding protein belonging to the S100 protein family, which exhibit a range of biological properties surrounding cellular proliferation, metastasis, immune evasion and angiogenesis, and are also involved in tumorigenesis (20,24). For example, S100A4 enhances p53-dependent apoptosis and facilitates more aggressive tumor progression (25). S100A6 has been reported to be upregulated in human osteosarcoma, colorectal carcinoma and hepatocellular carcinoma, which was mostly associated with its suppressive properties towards cancer cell migration and tumor metastasis (26,27). Therefore, S100 proteins play an important role in the development and progression of tumors, highlighting the necessity to further understand their roles and potential underlying mechanisms. In the present study, S100A1 expression was found to be upregulated in breast cancer cell lines. FOXD2-AS1 overexpression was shown to accelerate breast cancer progression by promoting cellular proliferation, migration and invasiveness, and functional experiments demonstrated that the knockdown of S100A1 reversed the effects induced by FOXD2-AS1. Furthermore, S100A1 knock-down suppressed breast cancer progression by inhibiting the proliferation, migration and invasiveness of MCF-7 and MDA-MB-468 cells. In agreement with these findings, S100A1 has been reported to be overexpressed in ovarian cancer, and to be associated with lymph mode metastasis; the overexpression of S100A1 was shown to enhance cellular proliferation and migration, whilst its inhibition exerted an opposite effect on ovarian cancer cells (20). Moreover, high tumor expression levels of S100A1 have been shown to be positively associated with decreased relapse-free survival time in an endometrioid subtype of ovarian and endometrial cancers (28). It was thus hypothesized that S100A1 functions as an important regulator in breast cancer, and may therefore be a promising therapeutic target for this, as well as other types of gynecological cancer.
The Hippo pathway is an important signaling pathway that regulates cellular proliferation and apoptosis, the activation of which is triggered by the phosphorylation of the large tumor suppressor kinases, LATS1 and LATS2. The Hippo pathway is very complex as a number of kinases relay upstream signals to LATS to regulate this pathway. The STE20 protein kinases (MST1/2), as the core components of the Hippo pathway, are considered responsible for the phosphorylation and activation of LATS1/2 (29). YAP, a downstream effector of the Hippo pathway, is highly activated in various types of cancer, and targeting YAP may effectively suppress tumorigenesis (30,31). Both dysregulated Hippo signaling and aberrant YAP activation contribute to cancer progression (32,33). In the present study, FOXD2-AS1 knockdown significantly increased the phosphorylation of YAP and LATS1, indicating that the Hippo signaling pathway was activated by FOXD2-AS1 downregulation. Notably, it was found that MST1/2 expression levels were downregulated by FOXD2-AS1 downregulation, which was contradictory with the activated Hippo pathway. There is evidence to indicate that although MST1/2 are firmly established as the initiating kinases of the Hippo kinase cascade in mammals, it has been observed that MST1/2 are not absolutely required for LATS and YAP regulation by numerous upstream signals. For example, MST1/2 is not involved in YAP regulation in response to other signals such as cAMP (34). Moreover, the knockdown of MST1/2 was previously show to not affect basal YAP phosphorylation in HeLa cells (35). Furthermore, MST1/2 has found to be largely dispensable for YAP regulation, whereas MAPK4s, also considered as Hippo pathway components, exert direct effects on LATS1/2 and YAP phosphorylation and activation (36). Therefore, there is no simple one-to-one linear association in MST1/2 and LATS activation, which may provide a reasonable explanation for the results of the present study. The present results revealed that Hippo/YAP may be involved in the promoting effects of FOXD2-AS1 on cell proliferation, migration and invasiveness. The Hippo/YAP signaling pathway is also associated with the regulation of tumor growth in vivo, and FOXD2-AS1 downregulation was demonstrated to suppress tumor growth in breast cancer-bearing mice by regulating Hippo/YAP signaling pathway.
Furthermore, existing evidence suggests that S100A1 exerts its oncogenic effects by interacting with LATS1 and activating YAP. In view of the positive association between p-LATS1 and S100A1 in clinical samples of hepatocellular carcinoma, LATS1 was considered to be responsible for S100A1-induced cancer progression (37). In the present study, a positive association was observed between FOXD2-AS1 and S100A1 expression, and the knockdown of S100A1 significantly inhibited FOXD2-AS1-induced cellular proliferation, migration and invasion. The overexpression of LATS1 also inhibited FOXD2-AS1-induced cellular activity, suggesting that FOXD2-AS1 is likely to exert its effects by interacting with S100A1 and the LATS1-induced Hippo signaling pathway. Given that MST1/2 was not involved in the activation of LATS1 in the present study, LATS may be activated by other signals; thus, it was hypothesized that S100A1 may be the upstream protein involved in directly activating LATS1. Considering all of the above, the p-LATS1-induced activation of Hippo/YAP signaling is partly dependent on the level of S100A1, which is regulated by FOXD2-AS1.
However, some limitations exist in the present study. First, although it was hypothesized that S100A1 was responsible for the activation of Hippo-YAP signaling, whether Sl00A1 was indispensable for LATS activation or whether its mutation directly affected Hippo-YAP signaling in breast cancer was not elucidated. Secondly, the mechanisms through which FOXD2-AS1 regulates S100A1 were not investigated in the present study. Increasing evidence has revealed that lncRNAs contribute to tumorigenesis by silencing tumor suppressors or activating oncogenes by acting as competing endogenous RNAs (ceRNAs) to sponge miRNAs. In previous studies, FOXD2-AS1 has been reported to regulate cancer progression by sponging various miRNAs, including miR-143, miR-7-5p and miR-185, thus modulating the suppression of mRNAs (38-40). In particular, Chen et al demonstrated that the oncogenic role of FOXD2-AS1 in nasopharyngeal carcinoma was mediated largely in part by sponging miR-363-5p, and subsequently activating S100A1, as S100A1 was confirmed to be a direct target of miR-363-5p in its 3′-UTR mRNA (41). Therefore, it is possible that FOXD2-AS1 may regulate S100A1 by sponging miR-363-5p in breast cancer, thus activating S100A1-induced LATS1 and YAP activation. FOXD2-AS1 may exert its oncogenic effects on breast cancer through the miR-363-5p/S100A1/Hippo pathway; this hypothesis warrants further investigations in the future.
In conclusion, the findings of the present study demonstrate that FOXD2-AS1 is crucial for cellular proliferation, migration and invasiveness, as well as tumor growth in breast cancer. FOXD2-AS1 regulates malignancy by regulating the Hippo/YAP signaling pathway, which is further mediated by the interaction between S100A1 and p-LATS1. The present study suggests that the FOXD2-AS1/S100A1/Hippo axis is involved in the tumorigenesis and progression of breast cancer, which may contribute to the future development of more effective treatments for breast cancer.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
PH made substantial contributions to the conception and design of the study. PH and JX performed data acquisition, data analysis and interpretation. PH drafted the manuscript and critically revised it for important intellectual content. Both authors gave final approval for the published version of the study and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the First Affiliated Hospital of Zhengzhou University, and the animal experiments were performed according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017 racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years Lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69:211–233. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz RS, Erban JK. Timing of metastasis in breast cancer. N Engl J Med. 2017;376:2486–2488. doi: 10.1056/NEJMcibr1701388. [DOI] [PubMed] [Google Scholar]
- 7.Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: Background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 8.Yuan H, Qin Y, Zeng B, Feng Y, Li Y, Xiang T, Ren G. Long noncoding RNA LINC01089 predicts clinical prognosis and inhibits cell proliferation and invasion through the Wnt/β-catenin signaling pathway in breast cancer. Onco Targets Ther. 2019;12:4883–4895. doi: 10.2147/OTT.S208830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua X, Li G, Liu Z, Niu Z. LINK-A lncRNA participates in the pathogenesis of glioma by interacting with survivin. Exp Ther Med. 2019;18:1581–1586. doi: 10.3892/etm.2019.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Sharma S, Watabe K. Roles of lncRNA in breast cancer. Front Biosci (Schol Ed) 2015;7:94–108. doi: 10.2741/s427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Zhou B, Lv Y, Qian Q. LncRNA HEIH regulates cell proliferation and apoptosis through miR-4458/SOCS1 axis in triple-negative breast cancer. Hum Cell. 2019;32:522–528. doi: 10.1007/s13577-019-00273-1. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Li B, Wang C, Luo Y, Zhao M, Chen P. Long non-coding RNA FOXD2-AS1 promotes glioma cell cycle progression and proliferation through the FOXD2-AS1/miR-31/CDK1 pathway. J Cell Biochem. 2019;120:19784–19795. doi: 10.1002/jcb.29284. [DOI] [PubMed] [Google Scholar]
- 13.Ren Z, Hu Y, Li G, Kang Y, Liu Y, Zhao H. HIF-1α induced long noncoding RNA FOXD2-AS1 promotes the osteosarcoma through repressing p21. Biomed Pharmacother. 2019;117:109104. doi: 10.1016/j.biopha.2019.109104. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Hu J, Zhou W, Gao H. LncRNA FOXD2-AS1 accelerates the papillary thyroid cancer progression through regulating the miR-485-5p/KLK7 axis. J Cell Biochem. 2018 Nov 19; doi: 10.1002/jcb.28072. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Qiu N, Xia H, Liang H, Li H, Ao X. Long noncoding RNA FOXD2AS1/miR1505p/PFN2 axis regulates breast cancer malignancy and tumorigenesis. Int J Oncol. 2019;54:1043–1052. doi: 10.3892/ijo.2019.4671. [DOI] [PubMed] [Google Scholar]
- 16.Tseng LM, Chiu JH, Liu CY, Tsai YF, Wang YL, Yang CW, Shyr YM. A comparison of the molecular subtypes of triple-negative breast cancer among non-Asian and Taiwanese women. Breast Cancer Res Treat. 2017;163:241–254. doi: 10.1007/s10549-017-4195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Zhou R, Xu L, Ye M, Liao M, Du H, Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res. 2014;46:753–760. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Liu X, Luo J, Xiao W, Ye X, Chen M, Li Y, Zhang GJ. Notch3 inhibits epithelial-mesenchymal transition by activating Kibra-mediated Hippo/YAP signaling in breast cancer epithelial cells. Oncogenesis. 2016;5:e269. doi: 10.1038/oncsis.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian T, Li X, Hua Z, Ma J, Liu Z, Chen H, Cui Z. S100A1 promotes cell proliferation and migration and is associated with lymph node metastasis in ovarian cancer. Discov Med. 2017;23:235–245. [PubMed] [Google Scholar]
- 21.Wang T. Association between HIF1α 1772 C/T polymorphism and breast cancer susceptibility: A systematic review. Crit Rev Eukaryot Gene Expr. 2017;27:297–304. doi: 10.1615/CritRevEukaryotGeneExpr.2017019947. [DOI] [PubMed] [Google Scholar]
- 22.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S, Wang J, Zhang Z. An emerging understanding of long noncoding RNAs in kidney cancer. J Cancer Res Clin Oncol. 2014;140:1989–1995. doi: 10.1007/s00432-014-1699-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Huo X, Chong Z, Khan H, Liu R, Wang T. A review of S100 protein family in lung cancer. Clin Chim Acta. 2018;476:54–59. doi: 10.1016/j.cca.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Grigorian M, Andresen S, Tulchinsky E, Kriajevska M, Carlberg C, Kruse C, Cohn M, Ambartsumian N, Christensen A, Selivanova G, Lukanidin E. Tumor suppressor p53 protein is a new target for the metastasis-associated Mts1/S100A4 protein: Functional consequences of their interaction. J Biol Chem. 2001;276:22699–22708. doi: 10.1074/jbc.M010231200. [DOI] [PubMed] [Google Scholar]
- 26.Luu HH, Zhou L, Haydon RC, Deyrup AT, Montag AG, Huo D, Heck R, Heizmann CW, Peabody TD, Simon MA, He TC. Increased expression of S100A6 is associated with decreased metastasis and inhibition of cell migration and anchorage independent growth in human osteosarcoma. Cancer Lett. 2005;229:135–148. doi: 10.1016/j.canlet.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Melle C, Ernst G, Schimmel B, Bleul A, von Eggeling F. Colon-derived liver metastasis, colorectal carcinoma, and hepatocellular carcinoma can be discriminated by the Ca(2+)-binding proteins S100A6 and S100A11. PLoS One. 2008;3:e3767. doi: 10.1371/journal.pone.0003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeRycke MS, Andersen JD, Harrington KM, Pambuccian SE, Kalloger SE, Boylan KL, Argenta PA, Skubitz AP. S100A1 expression in ovarian and endometrial endometrioid carcinomas is a prognostic indicator of relapse-free survival. Am J Clin Pathol. 2009;132:846–856. doi: 10.1309/AJCPTK87EMMIKPFS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 30.Kim W, Khan SK, Liu Y, Xu R, Park O, He Y, Cha B, Gao B, Yang Y. Hepatic Hippo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut. 2018;67:1692–1703. doi: 10.1136/gutjnl-2017-314061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Jin Q, Xiao W, Sun C. Tankyrase1 antisense oligodeoxynucleotides suppress the proliferation, migration and invasion through Hippo/YAP pathway in human osteosarcoma cells. Pathol Res Pract. 2019;215:152381. doi: 10.1016/j.prp.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Ehmer U, Sage J. Control of Proliferation and Cancer Growth by the Hippo Signaling Pathway. Mol Cancer Res. 2016;14:127–140. doi: 10.1158/1541-7786.MCR-15-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han H, Yang B, Nakaoka HJ, Yang J, Zhao Y, Le Nguyen K, Bishara AT, Mandalia TK, Wang W. Hippo signaling dysfunction induces cancer cell addiction to YAP. Oncogene. 2018;37:6414–6424. doi: 10.1038/s41388-018-0419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Q, Wang J, Cao Z, Tang Y, Feng C, Huang F. Interaction of S100A1 with LATS1 promotes cell growth through regulation of the Hippo pathway in hepatocellular carcinoma. Int J Oncol. 2018;53:592–602. doi: 10.3892/ijo.2018.4431. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Dong H, Cao W, Xue J. Long noncoding FOXD2-AS1 is activated by CREB1 and promotes cell proliferation and metastasis in glioma by sponging miR-185 through targeting AKT1. Biochem Biophys Res Commun. 2019;508:1074–1081. doi: 10.1016/j.bbrc.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Fu Q, Li S, Liang N, Li F, Li C, Sui C, Dionigi G, Sun H. LncRNA FOXD2-AS1 functions as a competing endogenous RNA to regulate TERT expression by sponging miR-7-5p in thyroid cancer. Front Endocrinol (Lausanne) 2019;10:207. doi: 10.3389/fendo.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An Q, Zhou L, Xu N. Long noncoding RNA FOXD2-AS1 accelerates the gemcitabine-resistance of bladder cancer by sponging miR-143. Biomed Pharmacother. 2018;103:415–420. doi: 10.1016/j.biopha.2018.03.138. [DOI] [PubMed] [Google Scholar]
- 41.Chen G, Sun W, Hua X, Zeng W, Yang L. Long non-coding RNA FOXD2-AS1 aggravates nasopharyngeal carcinoma carcinogenesis by modulating miR-363-5p/S100A1 pathway. Gene. 2018;645:76–84. doi: 10.1016/j.gene.2017.12.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.