Abstract
Esophageal squamous cell carcinoma (ESCC) is a type of digestive tract malignant tumor that severely threatens human health. The long non-coding RNA BRAF activated non-coding RNA (BANCR) and insulin-like growth factor 1 receptor (IGF1R) are associated with various types of cancer; however, it remains unclear whether BANCR can regulate IGF1R expression in ESCC. In the present study, the expression levels of BANCR, IGF1R mRNA and microRNA-338-3p (miRNA/miR-338-3p) in ESCC tissues or cells were detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The levels of IGF1R, E-cadherin, N-cadherin, Vimentin, p-Raf-1, p-MEK1/2 and p-ERK1/2 were measured by western blot analysis. The proliferation, migration and invasion of ESCC cells were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT) or Transwell assays. The relationship between miR-338-3p and BANCR or IGF1R was predicted using starBase2.0 and confirmed by dual-luciferase reporter assay. The role of BANCR in ESCC in vivo was confirmed through a tumor xenograft assay. It was found that BANCR and IGF1R were upregulated, while miR-338-3p was down-regulated in ESCC tissues and cells. Both BANCR and IGF1R knockdown suppressed the proliferation, migration, invasion and epithelial-mesenchymal transition (EMT) of ESCC cells. IGF1R enhancement reversed BANCR knockdown-mediated effects on the proliferation, migration, invasion and EMT of ESCC cells. BANCR regulated the Raf/MEK/ERK pathway by regulating IGF1R expression. Notably, BANCR regulated IGF1R expression by sponging miR-338-3p. Moreover, BANCR silencing inhibited tumor growth in vivo. On the whole, the findings of the present study demonstrate that BANCR inhibition blocks ESCC progression by inactivating the IGF1R/Raf/MEK/ERK pathway by sponging miR-338-3p.
Keywords: esophageal squamous cell carcinoma, BANCR, miR-338-3p, insulin-like growth factor 1 receptor
Introduction
Esophageal cancer (EC) is a refractory and common invasive disease (1,2). Statistically, 17,290 patients were diagnosed with EC and this led to 15,850 deaths in the United States in 2018 (3). Esophageal squamous cell carcinoma (ESCC) is one of the two main pathological types (ESCC and adenocarcinoma) of EC, accounting for approximately 90% of cases (4). At present, radical surgery remains the only treatment strategy for patients with ESCC to achieve long-term survival. However, patients with ESCC are associated with an extremely poor prognosis due to recurrence and metastasis (5,6). Therefore, it is necessary to examine the mechanisms responsible for the development of ESCC and to provide novel strategies for the prevention and treatment of ESCC.
Long non-coding RNAs (lncRNAs) are emerging regulators of gene expression and cell fate (7,8). Studies have demonstrated that many lncRNAs play differential roles in the development of various types of tumor via the activation or inhibition of oncogenes (8,9). The lncRNA, BRAF activated non-coding RNA (BANCR), was discovered in melanoma cells and plays a vital role during melanoma cell migration (10). In recent years, a substantial amount of evidence has indicated that BANCR plays a crucial role in the occurrence and progression of cancers, such as papillary thyroid cancer (11), colorectal cancer (12) and hepatocellular cancer (13). In addition, BANCR was implicated in the progression of ESCC (14,15). However, the underlying molecular mechanisms of BANCR in the progression of ESCC have not yet been well explained.
MicroRNAs (miRNAs or miRs) are short non-coding RNAs (19-25 nucleotides in length) that primarily regulate gene expression at the post-transcriptional level (16). At present, miRNAs are a promising tool or target in diagnostics and clinical treatment (17). Li et al reported that lncRNA ATB regulated kindling-2 expression via miR-200b, which modulated the malignant behaviors of ESCC cells (18). Liu et al demonstrated that lncRNA XIST regulated tumor growth via regulating the miR-34a/MET axis in thyroid cancer (19). In addition, the enhancement of lncRNA B3GALT5-AS1 has been shown to impede colon cancer metastasis by suppressing miR-203 expression (20). A fairly large number of researches have revealed that miR-338-3p plays a vital role in the progression of epithelial ovarian cancer (21), prostate cancer (22) and cervical cancer (23). Furthermore, miR-338-3p has been shown to exert an antitumor effect on ESCC (24). Nevertheless, the potential molecular regulatory mechanisms of miR-338-3p in ESCC remain largely unknown.
The receptor tyrosine kinase insulin-like growth factor 1 receptor (IGF1R) can bind to ligand that can activate the P13K/AKT/mTOR pathway and the Raf/MEK/ERK pathway (25,26). IGF1R has been shown to be significantly elevated in the majority of commonly human cancers. A previous study indicated that IGF1R was also upregulated in ESCC (27). Considering that BANCR and IGF1R were upregulated in ESCC, the present study thus investigated whether BANCR plays a role in the progression of ESCC by regulating IGF1R expression.
Hence, the present study detected the expression patterns of BANCR and IGF1R in ESCC tissues and cells. Moreover, the biological function of BANCR and IGF1R in ESCC cells were investigated. In addition, the molecular mechanisms by which BANCR regulates IGF1R expression were investigated.
Materials and methods
Tissue samples
The protocols were ratified by The Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical University. A total of 40 paired ESCC tissues and adjacent non-tumor tissues were obtained from The Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical University. The excised tissue was immediately frozen in liquid nitrogen and stored at -80°C until use in subsequent experiments. The histology and pathology of all biopsy samples were examined by 2 independent pathologists. The participating patients did not receive chemotherapy or radiotherapy prior to surgery. Informed consents were obtained from all the subjects participating in the study. The characteristics of the patients with ESCC are presented in Table I.
Table I.
Characteristics of patients with esophageal squamous cell carcinoma.
| Parameter | No. of patients (n=40) |
|---|---|
| Sex | |
| Male | 28 |
| Female | 12 |
| Age, years | |
| <60 | 15 |
| ≥60 | 25 |
| Tumor location (thoracic portion) | |
| Upper | 8 |
| Middle | 27 |
| Lower | 5 |
| Tumor size, cm | |
| <4 | 23 |
| ≥4 | 17 |
| Histological grade | |
| Well | 13 |
| Moderate | 17 |
| Poor | 10 |
| Tumor stage | |
| I/II | 26 |
| III | 14 |
| Lymph node metastasis | |
| Yes | 27 |
| No | 13 |
Cells, cell culture and transfection
KYSE450 and KYSE510 cells were obtained from Bena Culture Collection. The normal human esophageal epithelial cell line, HET-1A, was purchased from the American Tissue Culture Collection (ATCC). The above-mentioned cells were maintianed at 37°C in an incubator with 5% CO2. Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) was utilized to culture all cells. Moreover, the medium was replenished with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences), and 1% penicillin/streptomycin (Baomanbio).
Small interference RNA (siRNA) targeting BANCR (si-BANCR-1 and si-BANCR-2), siRNA targeting IGF1R (si-IGF1R-1 and si-IGF1R-2), and their negative control (si-NC) were obtained from GenePharma. For BANCR and IGF1R overexpression plasmids, the full-length sequences of BANCR (NR_047671) and IGF1R (NM_000875) were inserted into pcDNA3.1 vectors (Invitrogen; Thermo Fisher Scientific, Inc.), respectively. miR-338-3p mimic (miR-338-3p) and matching control (miR-NC) were obtained from Guangzhou RiboBio Co., Ltd. The transfection of the KYSE450 and KYSE510 cells was performed using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The concentrations used for transfection were as follows: si-BANCR-1 (50 nM), si-BANCR-2 (50 nM), si-IGF1R-1 (50 nM), si-IGF1R-2 (50 nM), si-NC (50 nM), miR-338-3p (40 nM), miR-NC (40 nM), pcDNA (15 nM), BANCR (15 nM), IGF1R (15 nM). The sequences of the siRNAs were as follows: si-BANCR-1, 5′-GGA CUC CAU GGC AAA CGU UTT-3′; si-BANCR-2, 5′-GGA AAU AGA CUG CAG CAC CAA TT-3′; si-IGF1R-1, 5′-CAA CAG UGG UCA UCA UGG AAC UGU TT-3′; si-IGF1R-2, 5′-UGA CUG UGA AAU CUU CGG CTT-3′; and si-NC, 5′-TTC TCC GAA CGT GTC ACG T-3′. The cells were collected for subsequent analysis after 48 h of transfection.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
For the expression levels of BANCR, miR-338-3p and IGF1R mRNA in ESCC tissues, total RNA was extracted from 40 paired ESCC tissues (at stages I, II and III disease) and adjacent non-tumor tissues for RT-qPCR. ESCC tissues or cells were homogenized using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) to extract total RNA. The complementary DNA (cDNA) of BANCR, miR-338-3p and IGF1R was generated using the Prime ScriptTM RT reagent kit (Takara Bio, Inc.). The Platinum SYBR-Green qPCR SuperMix UDG from Invitrogen; Thermo Fisher Scientific, Inc. was employed for qPCR. The relative expression of BANCR, IGF1R and miR-338-3p was calculated using the 2−ΔΔCq method (28), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or U6 small nuclear RNA (snRNA) were used as the internal reference genes. The primers for amplification were as follows: GAPDH forward, 5′-GAC TCC ACT CAC GGC AAA TTCA-3′ and reverse, 5′-TCG CTC CTG GAA GAT GGT GAT-3′; BANCR forward, 5′-ACA GGA CTC CAT GGC AAA CG-3′ and reverse, 5′-ATG AAG AAA GCC TGG TGC AGT-3′; IGF1R forward, 5′-GCG GTT CTG TTG ATA GTG G-3′ and reverse, 5′-GCC TCG TTC ACC GTC TTA-3′; U6 snRNA forward, 5′-GCT CGC TTC GGC AGC ACA-3′ and reverse, 5′-GAG GTA TTC GCA CCA GAG GA-3′; and miR-338-3p forward, 5′-TGC GGT CCA GCA TCA GTG AT-3′ and reverse, 5′-CCA GTG CAG GGT CCG AGG T-3′.
Western blot analysis
For the levels of IGF1R in ESCC tissues, 3 pairs of tissue samples were selected for western blot analysis. In brief, the ESCC tissues or cells were lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology, Inc.) to obtain total protein. Subsequently, 8-10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was utilized for total protein (30 µg) isolation. Thereafter, the isolated proteins were electrotransferred onto polyvinylidene fluoride (PVDF) membranes (EMD Millipore). Tris-buffered saline with 0.05% Tween-20 (TBST) was employed to block the PVDF membranes. The PVDF membranes were then incubated with primary antibodies at 4°C for overnight. Subsequently, the PVDF membranes were incubated with a goat anti-mouse (#7076, 1:1,000) or rabbit IgG (#4414, 1:1,000) (from Cell Signaling Technology, Inc.) for 1 h at 37°C. The signal was captured with an enhanced chemiluminescence (ECL) detection system (Thermo Fisher Scientific, Inc.). Every protein band was analyzed with the Image Lab™ Software (Bio-Rad Laboratories, Inc.). The primary anti-bodies used were as follows: Anti-IGF1R (sc-463; 1:500, Santa Cruz Biotechnology, Inc.), anti-p-MEK1/2 (#2338, 1:2,000), anti-MEK1/2 (#9122, 1:1,000) (both from Cell Signaling Technology, Inc.), anti-E-cadherin (sc-8426, 1:200), anti-N-cadherin (sc-393933, 1:100), anti-p-ERK1/2 (sc-81492, 1:200), anti-ERK1/2 (sc-81504, 1:200), anti-Vimentin (sc-32322, 1:200), anti-p-Raf-1 (sc-81513, 1:200), anti-Raf-1 (sc-52827, 1:200) and anti-GAPDH (sc-32233, 1:1,000) (all from Santa Cruz Biotechnology, Inc.).
Cell proliferation assay
Cell proliferation was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide (MTT) kit (Promega Corporation) according to the manufacturer's protocol. In brief, the transfected KYSE450 and KYSE510 cells (5,000 cells/well) were seeded into 96-well plates and cultured for 24, 48 or 72 h at 37°C. Thereafter, 20 µl MTT (5 mg/ml) was supplemented to each well and maintained at 37°C for 4 h. Subsequently, 200 µl dimethyl sulfoxide (DMSO) was employed for the dissolution of the formazan crystals. Finally, the color reaction at 490 nm was measured using a Microplate Absorbance Reader (Thermo Fisher Scientific, Inc.).
Transwell assay
A Transwell chamber (8 µm pore filter) from BD Biosciences was employed to evaluate the migration and invasion of the transfected KYSE450 and KYSE510 cells. Transwell assay was performed as previously described (29). The transfected KYSE450 and KYSE510 cells (1×106) were placed into the upper chamber containing RPMI without FBS. Synchronously, RPMI medium with FBS (10%) was added to the lower chamber. For the invasion assay, the Matrigel matrix (BD Biosciences) was filled in the upper chamber. Subsequently, the cells on the lower surface of the membrane were fixed with glutaraldehyde (5%). Crystal violet (Solarbio, Inc.) (0.1%) was then added to stain the cells for 10 min at room temperature. Finally, the number of the migrated and invasive cells was counted under an inverted microscope (Olympus Corp.).
Dual-luciferase reporter assay
The bioinformatics database starBase2.0 (http://starbase.sysu.edu.cn/starbase2/index.php) was employed to predict the binding sites between miR-338-3p and BANCR or IGF1R, as previously described (30). The sequences of wild-type (WT) BANCR (AUGCUGG), mutant (MUT) BANCR (UACGACC), the 3′-untranslated region (UTR) of WT IGF1R (UGCUGG), and the 3′UTR of mut IGF1R (ACGACC) containing assumed miR-338-3p binding sites were synthesized. The sequences were then inserted into the pGL3-control luciferase reporter vectors (Promega Corp.) with a restriction endonuclease for 16 h, respectively. Subsequently, the KYSE450 and KYSE510 cells were co-transfected with luciferase reporter vectors and miR-338-3p or miR-NC. Following transfection for 48 h, the supernatant of the cell lysate was acquired through centrifugation (5.000 × g) for 5 min at 4°C. The relative luciferase units were evaluated with luciferase reporter assay kit (Promega Corp.). The relative fluorescence value was acquired by dividing the relative luciferase units value determined by Renilla luciferase by the relative luciferase units determined by Firefly luciferase.
In vivo experiment
The protocols of the animal experiment in the present study were authorized by The Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical University. The lentivirus-mediated sh-BANCR was obtained from GenePharma. A total of 14 female BALB/c nude mice (4-6 weeks old) were obtained from Shanghai Experimental Animal Center. All mice were randomly divided into 2 groups (n=7). The growth rate of the transplanted tumors was positively associated with body weight. However, the weight growth rate of mice is related to sex; thus, in the present study, female mice were used in xenotransplantation experiments as they are less aggressive and are less likely to inflict injury on one another. The in vivo experiments were performed as previously described (18,31,32). All mice were kept under specific pathogen-free conditions (25±2°C, 50-60% relative humidity, and a 12-h light/dark cycle) and food and water were provided ad libitum. For the stable knockdown of BANCR, the lentiviral-mediated sh-BANCR was transfected with 293T cells (ATCC) and then infected with KYSE450 cells with the supernatant of the 293T cells. The KYSE450 (1×107) cells stably transfected with lentivirus-mediated sh-BANCR or sh-NC were suspended in 100 µl phosphate-buffered saline. The KYSE450 cells were subcutaneously injected into the right flank of each mouse. Tumor volume was measured once a week using a caliper. After 35 days, all mice were euthanized (cervical decapitation) following an injection of xylazine (10 mg/kg, ChemeGen, Inc.) for subsequent analysis. Tumor volume was assessed with the equation: Volume = (length x width2)/2.
Statistical analysis
Data are presented as the means ± standard error. Prism5 software (GraphPad, Inc.) and SPSS 13.0 software (SPSS, Inc.) were utilized to conduct the statistical analysis. The correlation between BANCR and IGF1R was assessed by Spearman's correlation analysis. The Student's t-test or one-way ANOVA followed by Tukey's post hoc test were applied to compare differences between 2 groups and multiple groups, respectively. The overall survival of the patients with ESCC was determined by Kaplan-Meier analysis with the log-rank test. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
BANCR and IGF1R expression exhibit a positive association in human ESCC tissues
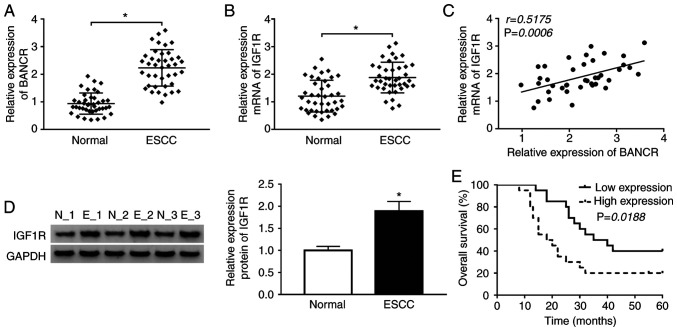
Given that BANCR and IGF1R have been shown to be upregulated in ESCC tissues (14,26), the present study thus examined the association between BANCR and IGF1R in ESCC. The expression levels of BANCR and IGF1R were first examined in 40 paired ESCC tissues and adjacent normal tissues by RT-qPCR. The results indicated that BANCR and IGF1R were upregulated in ESCC tissues when compared with the adjacent normal tissues (Fig. 1A and B). Spearman's correlation analysis revealed that the expression of BANCR positively correlated with that of IGF1R in ESCC tissues (Fig. 1C). Moreover, western blot analysis revealed that the protein level of IGF1R was increased in ESCC tissues compared with adjacent normal tissues (Fig. 1D). In addition, it was found that patients with ESCC with a high BANCR expression exhibited a lower survival rate than patients with ESCC with a low BANCR expression (Fig. 1E). These results suggested that BANCR and IGF1R were involved in the progression of ESCC.
Figure 1.
BANCR and IGF1R are upregulated in human ESCC tissues. (A and B) RT-qPCR was applied to examine the expression levels of (A) BANCR and (B) IGF1R in ESCC tissues and adjacent normal tissues. (C) The correlation between BANCR and IGF1R was determined by Spearman's correlation analysis. (D) The levels of IGF1R protein were detected by western blot analysis. (E) The overall survival of ESCC patients with high or low BANCR expression was determined by Kaplan-Meier analysis followed by the log-rank test. Data are presented as the means ± standard error of 3 experimental results. The Student's t-test was applied to compare differences between groups. *P<0.05. BANCR, BRAF activated non-coding RNA; ESCC, esophageal squamous cell carcinoma.
Knockdown of BANCR inhibits the proliferation, migration, invasion and EMT of human ESCC cells
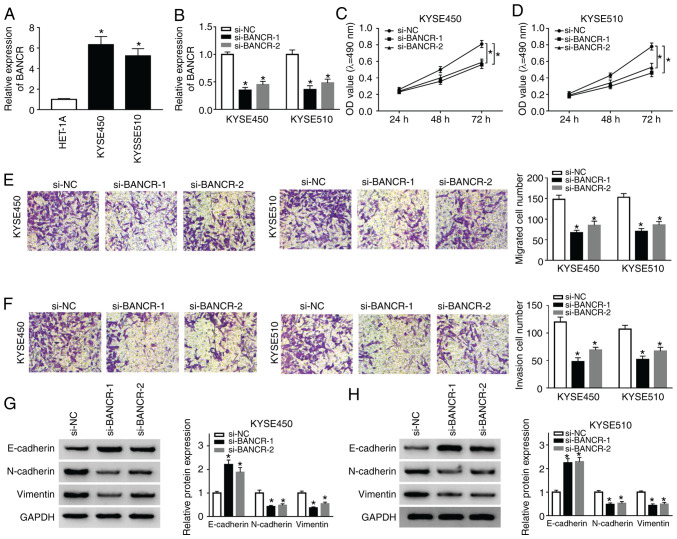
To investigate the function of BANCR in the progression ESCC, the expression of BANCR was examined in ESCC (KYSE450 and KYSE510) and HET-1A cells. The results indicated that the expression of BANCR was significantly higher in ESCC cells than in HET-1A cells (Fig. 2A). Subsequently, the KYSE450 and KYSE510 cells were transfected with si-BANCR-1 and si-BANCR-2 to silence BANCR. As shown in Fig. 2B, the expression of BANCR was decreased in the KYSE450 and KYSE510 cells transfected with si-BANCR-1 and si-BANCR-2. Subsequently, MTT assay was performed and the results revealed the marked suppression of cell proliferation by BANCR silencing in both the KYSE450 and KYSE510 cells (Fig. 2C and D). Moreover, Transwell migration assay revealed that the number of migrated cells was decreased in the BANCR-silenced KYSE450 and KYSE510 cells (Fig. 2E). Consistently, Transwell invasion assay displayed that BANCR silencing inhibited the invasion of both KYSE450 and KYSE510 cells (Fig. 2F). Studies have indicated that KYSE450 and KYSE510 cells can simultaneously express EMT-related markers (E-cadherin, N-cadherin and Vimentin) (33-35); thus, the present study assessed the effects of BANCR depletion on the levels of E-cadherin, N-cadherin and Vimentin by western blot analysis. The results revealed that the level of E-cadherin was distinctly increased in the KYSE450 and KYSE510 cells by BANCR downregulation, while the levels of N-cadherin and Vimentin exhibited an opposite trend (Fig. 2G and H). These results revealed that BANCR repression inhibited the proliferation, migration, invasion and EMT of ESCC cells.
Figure 2.
Effects of BANCR inhibition on the proliferation, migration, invasion and EMT of ESCC cells. (A) The expression of BANCR in ESCC and HET-1A cells was measured by RT-qPCR. (B-H) KYSE450 and KYSE510 cells were transfected with si-NC, si-BANCR-1, or si-BANCR-2. (B) The expression of BANCR in KYSE450 and KYSE510 cells was examined by RT-qPCR. (C and D) The proliferation of KYSE450 and KYSE510 cells was evaluated by MTT assay. (E and F) Transwell assay was conducted to determine the (E) migration and (F) invasion of KYSE450 and KYSE510 cells. (G and H) Western blotting analysis of the expression levels of E-cadherin, N-cadherin, and Vimentin in KYSE450 and KYSE510 cells. Data are presented as the means ± standard error of 3 experimental results. One-way ANOVA was applied to compare differences between groups. *P<0.05. BANCR, BRAF activated non-coding RNA; ESCC, esophageal squamous cell carcinoma.
IGF1R silencing suppresses the proliferation, migration, invasion of and EMT of ESCC cells
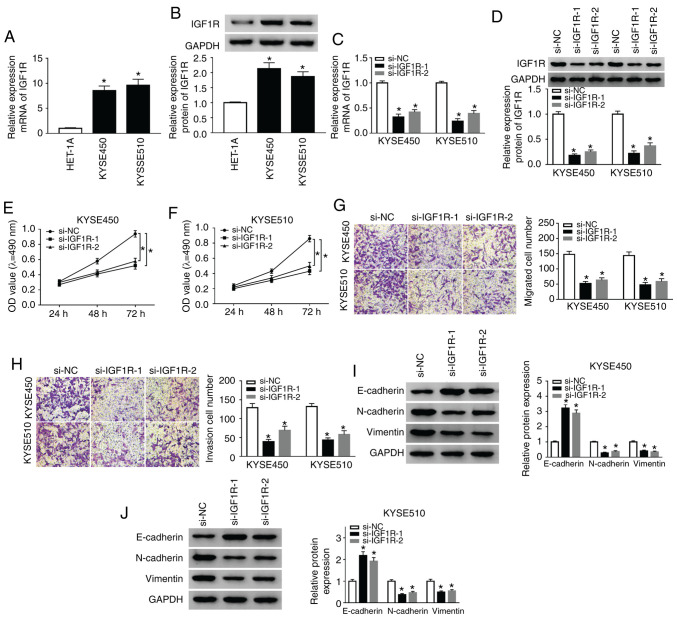
Subsequently, the role of IGF1R in ESCC was examined by loss-of-function experiments. As shown in Fig. 3A, the mRNA expression of IGF1R was markedly elevated in the KYSE450 and KYSE510 cells compared with the HET-1A cells. Consistently, the protein levels of IGF1R were markedly enhanced in the KYSE450 and KYSE510 cells when compared to the HET-1A cells (Fig. 3B). However, IGF1R mRNA and protein levels were decreased in the KYSE450 and KYSE510 cells following si-IGF1R-1 or si-IGF1R-2 transfection (Fig. 3C and D). The results of MTT assay revealed that IGF1R silencing significantly suppressed the proliferation of hte KYSE450 and KYSE510 cells compared with the si-NC control (Fig. 3E and F). Furthermore, Transwell assay revealed that the number of migratory and invasive cells was markedly decreased in the IGF1R-silenced KYSE450 and KYSE510 cells (Fig. 3G and H). Additionally, IGF1R knockdown enhanced the E-cadherin level, and reduced N-cadherin and Vimentin levels in the KYSE450 and KYSE510 cells (Fig. 3I and J). Overall, these results proved that IGF1R depletion suppressed the proliferation, migration, invasion and EMT of ESCC cells.
Figure 3.
Silencing of IGF1R suppresses the proliferation, migration, invasion and EMT of ESCC cells. (A) RT-qPCR was conducted to detect the expression of IGF1R in ESCC and HET-1A cells. (B) Western blot analysis of IGF1R protein expression in ESCC and HET-1A cells was implemented. (C-J) KYSE510 cells were transfected with si-NC, si-IGF1R-1, or si-IGF1R-2. (C and D) RT-qPCR or western blot analysis were used to assess the expression of IGF1R mRNA and protein in KYSE450 and KYSE510 cells. (E-H) MTT or Transwell assays was utilized for the determination of the proliferation, migration, and invasion of KYSE450 and KYSE510 cells. (I and J) The levels of E-cadherin, N-cadherin, and Vimentin in KYSE450 and KYSE510 cells were evaluated by western blot analysis. Data are presented as the means ± standard error of 3 experimental results. One-way ANOVA was applied to compare differences between groups. *P<0.05. BANCR, BRAF activated non-coding RNA; IGF1R, insulin-like growth factor 1 receptor; ESCC, esophageal squamous cell carcinoma.
IGF1R overexpression reverses the effects of BANCR knock-down on the proliferation, migration, invasion and EMT of ESCC cells
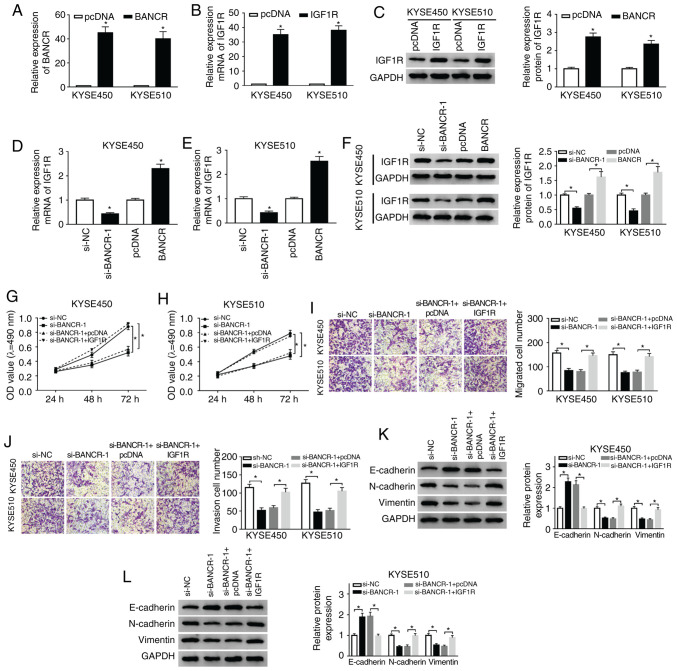
Based on the above-mentioned results, the present study further examined whether BANCR carries out its role in ESCC through IGF1R. The results of RT-qPCR demonstrated that the expression of BANCR was markedly upregulated in the KYSE450 and KYSE510 cells transfected with BANCR over-expression plasmid compared with the control group (Fig. 4A). In addition, the mRNA and protein levels of IGF1R were markedly elevated in the KYSE450 and KYSE510 cells transfected with IGF1R overexpression plasmid (Fig. 4B and C). Moreover, IGF1R mRNA and protein levels were suppressed by BANCR knockdown and enhanced by BANCR overexpression in the KYSE450 and KYSE510 cells (Fig. 4D-F). The results of MTT assay also indicated that IGF1R overexpression markedly attenuated the inhibitory effects of BANCR knockdown on the proliferation of the KYSE450 and KYSE510 cells (Fig. 4G and H). As was expected, the overexpression of IGF1R abrogated the inhibitory effects of BANCR knockdown on the migration and invasion of the KYSE450 and KYSE510 cells (Fig. 4I and J). In addition, IGF1R overexpression reversed the effects of BANCR knockdown on the levels of E-cadherin, N-cadherin and Vimentin in the KYSE450 and KYSE510 cells (Fig. 4K and L). Taken together, these data indicated that BANCR regulated the proliferation, migration, invasion and EMT of ESCC cells via IGF1R.
Figure 4.
Effects of IGF1R overexpression on the proliferation, migration, invasion and EMT of ESCC cells induced by BANCR knockdown. (A) RT-qPCR was performed for the detection of the expression of BANCR in KYSE450 and KYSE510 cells transfected with cDNA or BANCR. (B and C) The levels of IGF1R mRNA and protein in KYSE450 and KYSE510 cells transfected with cDNA or IGF1R were measured by RT-qPCR or western blot analysis. (D-F) Effects of BANCR knockdown or overexpression on the levels of IGF1R mRNA and protein were determined by RT-qPCR or western blot analysis. (G-L) KYSE450 and KYSE510 cells were transfected with si-NC, si-BANCR, si-BANCR+pcDNA, or si-BANCR+IGF1R. (G-J) The proliferation, migration and invasion of KYSE450 and KYSE510 cells were evaluated by MTT or Transwell assays. (K and L) The levels of E-cadherin, N-cadherin and Vimentin in KYSE450 and KYSE510 cells were examined by western blot analysis. Data were presented as the means ± standard error of 3 experimental results. The Student's t-test or one-way ANOVA were applied to compare differences between groups where appropriate. *P<0.05. BANCR, BRAF activated non-coding RNA; IGF1R, insulin-like growth factor 1 receptor; ESCC, esophageal squamous cell carcinoma.
BANCR regulates IGF1R expression by targeting miR-338-3p
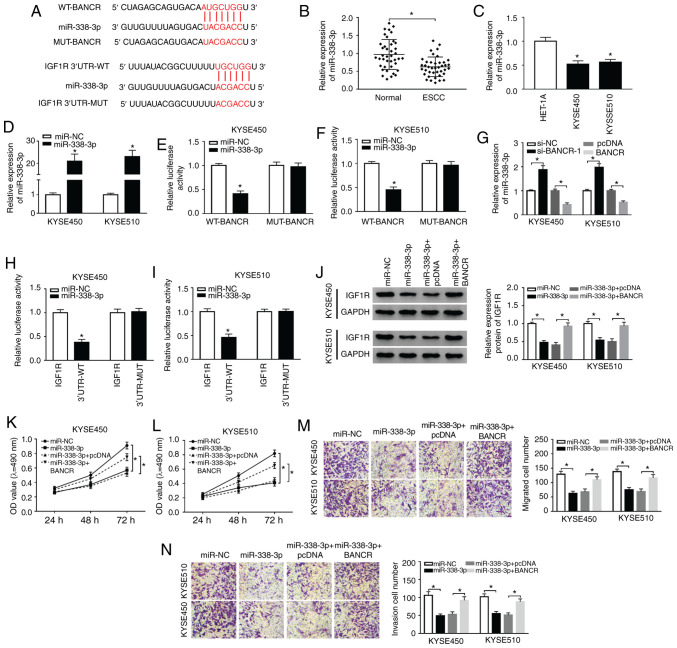
In view of the above-mentioned results, the present study further explored the latent molecular mechanisms between BANCR and IGF1R. It was discovered that miR-338-3p had complementary bases pairing with BANCR and IGF1R through starBase2.0 (Fig. 5A). Moreover, miR-338-3p expression was markedly decreased in ESCC tissues and cells (Fig. 5B and C). In comparison to miR-NC, the expression of miR-338-3p was overtly increased in the KYSE450 and KYSE510 cells following transfection with miR-338-3p mimics (Fig. 5D). The results of dual-luciferase reporter assay revealed that the luciferase activity of the luciferase reporters containing WT-BANCR was markedly decreased in the miR-338-3p-overexpressing KYSE450 and KYSE510 cells, whereas the luciferase reporters containing MUT-BANCR were not affected (Fig. 5E and F). Furthermore, miR-338-3p expression was markedly elevated by BANCR silencing and was overtly reduced by BANCR over-expression in the KYSE450 and KYSE510 cells (Fig. 5G). The overexpression of miR-338-3p also suppressed the luciferase intensity of the luciferase reporters with IGF1R 3′UTR-WT in both KYSE450 and KYSE510 cells, while there was no marked difference in the luciferase reporters with IGF1R 3′UTR-MUT (Fig. 5H and I). Furthermore, it was observed that miR-338-3p overexpression abnormally suppressed the protein levels of IGF1R in the KYSE450 and KYSE510 cells, while this reduction was abrogated by BANCR overexpression (Fig. 5J). In addition, the suppressive effects of miR-338-3p overexpression on the proliferation of the KYSE450 and KYSE510 cells were attenuated by BANCR overexpression (Fig. 5K and L). Transwell assay also revealed that BANCR elevation reversed the inhibitory effects of miR-338-3p upregulation on the migration and invasion of KYSE450 and KYSE510 cells (Fig. 5M and N). Taken together, these results indicated that BANCR regulated the expression of IGF1R by targeting miR-338-3p in ESCC cells.
Figure 5.
BANCR modulates IGF1R expression via sponging miR-338-3p. (A) The potential binding sites between miR-338-3p and BANCR or IGF1R were predicted using starBase2.0. (B and C) The expression of miR-338-3p in ESCC tissues and adjacent normal tissues, as well as ESCC cells and HET-1A cells, was detected by RT-qPCR. (D) Following miR-NC or miR-338-3p transfection, the levels of miR-338-3p in KYSE450 and KYSE510 cells were detected by RT-qPCR. (E and F) Dual-luciferase reporter assay was conducted for the determination of the luciferase activity in KYSE450 and KYSE510 cells co-trans-fected luciferase reporters containing WT-BANCR or MUT-BANCR with miR-NC or miR-338-3p. (G) Effects of BANCR knockdown or overexpression on the expression of miR-338-3p were determined by RT-qPCR. (H and I) The luciferase activity of luciferase reporters containing IGF1R 3′UTR-WT or IGF1R 3′UTR-MUT in KYSE450 and KYSE510 cells transfected with miR-NC or miR-338-3p was detected using dual-luciferase reporter assay. (J-N) KYSE450 and KYSE510 cells were transfected with miR-NC, miR-338-3p, miR-338-3p+cDNA, or miR-338-3p+BANCR. (J) The levels of IGF1R protein in KYSE450 and KYSE510 cells were measured via using western blotting. (K-N) MTT or Transwell assays was carried out to analyze the proliferation, migration, and invasion of KYSE450 and KYSE510 cells. Data are presented as the means ± standard error of 3 experimental results. The Student's t-test or one-way ANOVA were applied to compare differences between groups where appropriate. *P<0.05. BANCR, BRAF activated non-coding RNA; IGF1R, insulin-like growth factor 1 receptor; ESCC, esophageal squamous cell carcinoma.
BANCR regulates the Raf/MEK/ERK pathway by regulating IGF1R expression
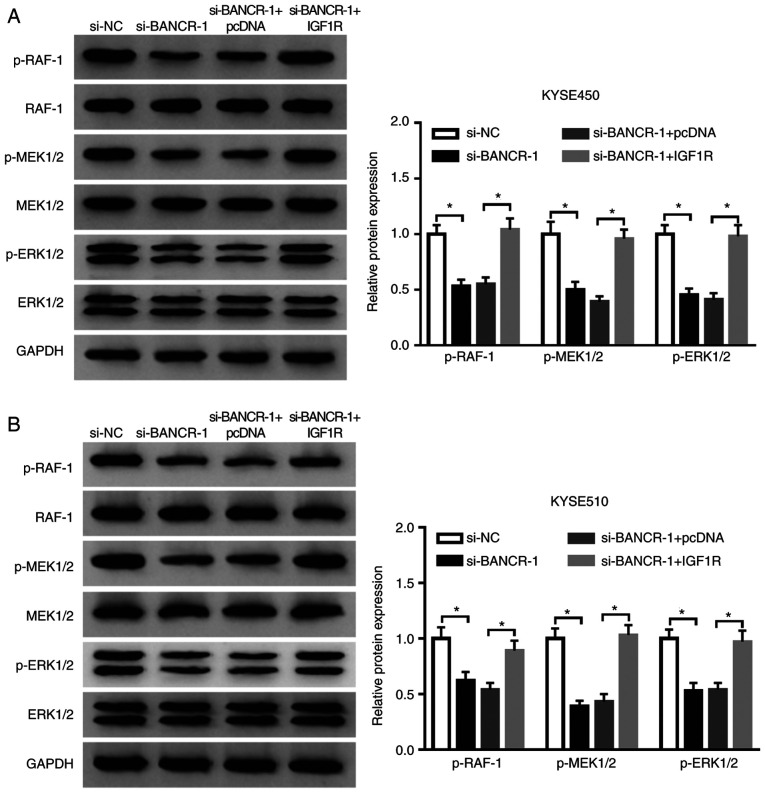
Considering that IGF1R was involved in the Raf/MEK/ERK pathway (36,37), the present study further examined whether BANCR regulated the Raf/MEK/ERK pathway through IGF1R in ESCC cells. The results indicated that the inhibition of BANCR prominently reduced the levels of p-Raf-1, p-MEK1/2 and p-ERK1/2 in both the KYSE450 and KYSE510 cells. However, this decrease was attenuated by IGF1R overexpression in the KYSE450 and KYSE510 cells (Fig. 6A and B). These results indicated that BANCR regulated the Raf/MEK/ERK pathway by modulating IGF1R expression.
Figure 6.
BANCR regulates the Raf/MEK/ERK pathway by regulating IGF1R expression. (A and B) The levels of p-Raf-1, p-MEK1/2, and p-ERK1/2 in KYSE450 and KYSE510 cells transfected with si-NC, si-BANCR, si-BANCR+pcDNA, or si-BANCR+IGF1R were measured by western blot analysis. Data are presented as the means ± standard error of 3 experimental results. One-way ANOVA was applied to compare differences between groups. *P<0.05. BANCR, BRAF activated non-coding RNA; IGF1R, insulin-like growth factor 1 receptor; ESCC, esophageal squamous cell carcinoma.
Knockdown of BANCR inhibits tumor growth in vivo
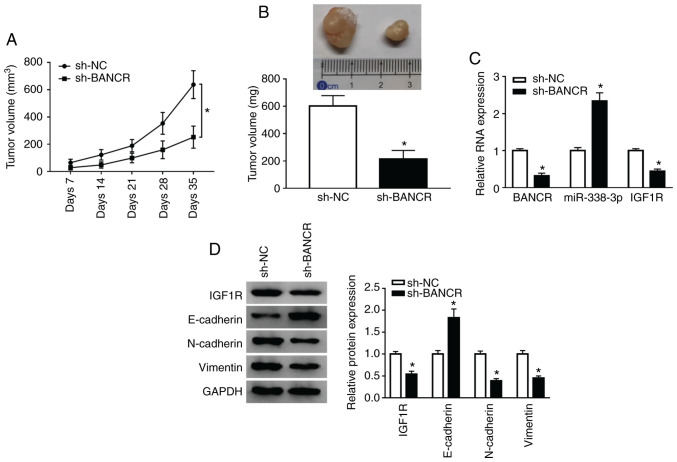
To confirm the role of BANCR in vivo, KYSE450 cells stably transfected with lentivirus-mediated sh-BANCR or sh-NC were subcutaneously injected into nude mice. BANCR knockdown markedly inhibited tumor volume when compared with the control group (Fig. 7A). In addition, tumor weight was evidently decreased in the sh-BANCR group when compared to the control group (Fig. 7B). Moreover, BANCR silencing prominently reduced the BANCR and IGF1R levels, and enhanced the miR-338-3p levels in the tumor tissues of mice (Fig. 7C). Moreover, the levels of IGF1R, N-cadherin and Vimentin were downregulated in the tumor tissues of mice in the sh-BANCR group, whereas the E-cadherin levels were upregulated (Fig. 7D). These results indicated that BANCR inhibition suppressed tumor growth in vivo.
Figure 7.
Knockdown of BANCR inhibited tumor growth in vivo. (A) The growth curves of tumor in the sh-BANCR and sh-NC groups were recorded. (B) The weight of tumor in the sh-BANCR and sh-NC groups was presented. (C) The expression of BANCR, miR-338-3p, and IGF1R mRNA was detected by qRT-PCR. (D) The levels of IGF1R protein, N-cadherin, Vimentin, and E-cadherin in the sh-BANCR and sh-NC groups were determined by western blot analysis. The Student's t-test was applied to compare differences between groups. *P<0.05. BANCR, BRAF activated non-coding RNA; IGF1R, insulin-like growth factor 1 receptor; ESCC, esophageal squamous cell carcinoma.
Discussion
ESCC is a gastrointestinal malignant tumor, which is often invasive and recurrent, has a poor prospect of survival in patients with advanced disease (38). A substantial amount of evidence has indicated that lncRNAs play vital roles in the progression of ESCC (39,40). BANCR has been reported to play a carcinogenic role in various types of cancer. For instance, Shen et al indicated that a high BANCR expression was associated with lymph node metastasis and a poor prognosis in colorectal cancer (12). Another study also reported that BANCR upregulation promoted the initiation and progression of hepatocellular carcinoma (13). In the present study, BANCR expression was elevated in ESCC tissues and cells. Moreover, BANCR silencing suppressed tumor growth in vivo, and suppressed the proliferation, migration, invasion and EMT of ESCC cells in vitro. For in vivo experiments, only one cell line (KYSE450) was selected for verification, which is a limitation of the present study. A previous study indicated that BANCR enhancement was associated with the development and a poor prognosis in ESCC (14). Chen et al demonstrated that BANCR silencing blocked cell invasion and migration via inactivating the Wnt/β-catenin pathway in ESCC (15). In addition, the study of Sadeghpour and Ghorbian demonstrated that the BANCR expression levels were positively associated with tumor stage, tumor differentiation and lymph node metastasis (41). However, BANCR plays an antitumor role in bladder cancer and non-small cell lung cancer, which may be related to the differences in the tissues and microenvironment (42,43).
Recently, increasing evidence has indicated that lncRNAs can function as sponges for miRNAs (44). For example, lncRNA ZEB2-AS1 depletion has been shown to suppress HMGB1 expression via the enhancement of miR-204 expression, which inhibits the invasion and growth of pancreatic cancer cells (45). In addition, lncRNA SNHG20 downregulation has been shown to suppress cervical cancer advancement by regulating ADAM10 expression through the targeting of miR-140-5p (46). Herein, BANCR regulated IGF1R expression via targeting miR-338-3p. Moreover, miR-338-3p expression was decreased in ESCC tissues and cells.
EMT is the transformation of epithelial cells into active mesenchymal cells, which leads to invasion and migration (47). Li et al reported that miR-338-3p mimic suppressed the growth of tumor in ESCC in vitro and in vivo (24), and the results of the present study are consistent with this finding. The study by Cao et al demonstrated that miR-338-3p overexpression suppressed cell EMT, migration, proliferation and invasion in osteosarcoma in vitro (48). Moreover, miR-338-3p has also been shown to play an antitumor role in in non-small cell lung cancer (49), gastric cancer (50) and prostate cancer (22).
The signaling pathway of Ras/Raf/MEK/ERK is usually related to the development and progression of diverse types of cancer (51). It has been revealed that the RAS/Raf/MEK/ERK signaling pathway is a vital downstream intracellular signal of IGF1R (36). A previous study demonstrated that IGF1R played vital roles in cell apoptosis, angiogenesis, proliferation and invasion in cancers (52). There is a growing body of evidence to suggest that IGF1R expression is increased in various types of tumor. For instance, Chen et al demonstrated that IGF1R expression was markedly enhanced in renal cell cancer cells, and the silencing of IGF1R suppressed cancer cell proliferation, migration and invasion (53). In the present study, it was found that IGF1R expression was augmented in ESCC tissues and cells. Furthermore, the silencing of IGF1R expression suppressed the proliferation, migration, invasion and EMT of ESCC cells. Moreover, BANCR regulated the Raf/MEK/ERK pathway by regulating IGF1R expression. Mei et al indicted that IGF1R knockdown blocked the proliferation, migration, invasion and slug-induced EMT of ESCC cells (33). Another study also indicated that miR-98 elevation inhibited retinoblastoma cell growth and invasion by targeting IGF1R/k-RAS/Raf/MEK/ERK pathway (37). Therefore, it was concluded that BANCR silencing suppressed cell proliferation, migration, invasion and EMT in ESCC through the inactivation of the IGF1R/Raf/MEK/ERK pathway via sponging miR-338-3p. However, the present study only confirmed that IGF1R was the target of miR-338-3p, and whether miR-338-3p can target RAF, MEK or ERK in ESCC remains to be further investigated in the future.
In conclusion, the present study demonstrated that BANCR downregulation suppressed ESCC progression through the inactivation of the IGF1R/Raf/MEK/ERK pathway by sponging miR-338-3p, which may provide a possible target for the treatment of ESCC.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KW and XY were involved in the conceptualization and methodology of the study. XY, WD and ZF were involved in formal analysis and data curation. WS, KW and WD were involved in data validation and investigation. WS, KW and XY were involved in the writing and preparation of the original draft, and in the reviewing and editing of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Review Committee of The Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical University. Written informed consent was obtained from all enrolled subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Kang X, Chen K, Li Y, Li J, D'Amico TA, Chen X. Personalized targeted therapy for esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:7648–7658. doi: 10.3748/wjg.v21.i25.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;68:7–30. doi: 10.3322/caac.21442. [DOI] [Google Scholar]
- 4.Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Wang J, Chen Z, Gao Y, He J. Immunohistochemical prognostic markers of esophageal squamous cell carcinoma: A systematic review. Chin J Cancer. 2017;36:65. doi: 10.1186/s40880-017-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Qi Z, Hu YP, Wang YX. Possible biomarkers for predicting lymph node metastasis of esophageal squamous cell carcinoma: A review. J Int Med Res. 2019;47:544–556. doi: 10.1177/0300060518819606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salehi S, Taheri MN, Azarpira N, Zare A, Behzad-Behbahani A1. State of the art technologies to explore long non-coding RNAs in cancer. J Cell Mol Med. 2017;21:3120–3140. doi: 10.1111/jcmm.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Wu Z, Fu X, Han W. lncRNAs: Insights into their function and mechanics in underlying disorders. Mutat Res Rev Mutat Res. 2014;762:1–21. doi: 10.1016/j.mrrev.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Forrest ME, Khalil AM. Review: Regulation of the cancer epigenome by long non-coding RNAs. Cancer Lett. 2017;407:106–112. doi: 10.1016/j.canlet.2017.03.040. [DOI] [PubMed] [Google Scholar]
- 10.Flockhart RJ, Webster DE, Qu K, Mascarenhas N, Kovalski J, Kretz M, Khavari PA. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res. 2012;22:1006–1014. doi: 10.1101/gr.140061.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Lin X, Fu X, Yan W, Lin F, Kuang P, Luo Y, Lin E, Hong X, Wu G. Long non-coding RNA BANCR regulates cancer stem cell markers in papillary thyroid cancer via the RAF/MEK/ERK signaling pathway. Oncol Rep. 2018;40:859–866. doi: 10.3892/or.2018.6502. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, Bai Y, Luo B, Zhou X. Upregulation of lncRNA BANCR associated with the lymph node metastasis and poor prognosis in colorectal cancer. Biol Res. 2017;50:32. doi: 10.1186/s40659-017-0136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou T, Gao Y. Increased expression of lncRNA BANCR and its prognostic significance in human hepatocellular carcinoma. World J Surg Oncol. 2016;14:8. doi: 10.1186/s12957-015-0757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Yang T, Xu Z, Cao X. Upregulation of the long non-coding RNA BANCR correlates with tumor progression and poor prognosis in esophageal squamous cell carcinoma. Biomed Pharmacother. 2016;82:406–412. doi: 10.1016/j.biopha.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Zheng Y, Wu B, Chen X, Sun F, Ge P, Wang P. BANCR regulates the cell invasion and migration in esophageal squamous cell carcinoma through Wnt/β-catenin signaling pathway. Onco Targets Ther. 2019;12:9319–9327. doi: 10.2147/OTT.S227220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anfossi S, Fu X, Nagvekar R, Calin GA. MicroRNAs, regulatory messengers inside and outside cancer cells. Adv Exp Med Biol. 2018;1056:87–108. doi: 10.1007/978-3-319-74470-4_6. [DOI] [PubMed] [Google Scholar]
- 17.Abba ML, Patil N, Leupold JH, Moniuszko M, Utikal J, Niklinski J, Allgayer H. MicroRNAs as novel targets and tools in cancer therapy. Cancer Lett. 2017;387:84–94. doi: 10.1016/j.canlet.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Wu X, Gu L, Shen Q, Luo W, Deng C, Zhou Q, Chen X, Li Y, Lim Z, et al. Long non-coding RNA ATB promotes malignancy of esophageal squamous cell carcinoma by regulating miR-200b/Kindlin-2 axis. Cell Death Dis. 2017;8:e2888. doi: 10.1038/cddis.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Deng H, Zhao Y, Li C, Liang Y. lncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J Exp Clin Cancer Res. 2018;37:279. doi: 10.1186/s13046-018-0950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Wei Z, Wu K, Dai W, Zhang C, Peng J, He Y. Long noncoding RNA B3GALT5-AS1 suppresses colon cancer liver metastasis via repressing microRNA-203. Aging (Albany NY) 2018;10:3662–3682. doi: 10.18632/aging.101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang R, Shi H, Ren F, Liu Z, Ji P, Zhang W, Wang W. Down-regulation of miR-338-3p and Up-regulation of MACC1 indicated poor prognosis of epithelial ovarian cancer patients. J Cancer. 2019;10:1385–1392. doi: 10.7150/jca.29502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhang D, Lv J, Wang S, Zhang Q. lncRNA SNHG15 acts as an oncogene in prostate cancer by regulating miR-338-3p/FKBP1A axis. Gene. 2019;705:44–50. doi: 10.1016/j.gene.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Luan X, Wang Y. lncRNA XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis as a ceRNA against miR-331-3p and miR-338-3p. J Gynecol Oncol. 2018;29:e95. doi: 10.3802/jgo.2018.29.e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Li Z, Yang G, Pan Z. MicroRNA-338-3p suppresses tumor growth of esophageal squamous cell carcinoma in vitro and in vivo. Mol Med Rep. 2015;12:3951–3957. doi: 10.3892/mmr.2015.3820. [DOI] [PubMed] [Google Scholar]
- 25.Sharmila G, Bhat FA, Arunkumar R, Elumalai P, Raja Singh P, Senthilkumar K, Arunakaran J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin Nutr. 2014;33:718–726. doi: 10.1016/j.clnu.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Ma W, Zhang T, Pan J, Shi N, Fan Q, Wang L, Lu SH. Assessment of insulin-like growth factor 1 receptor as an oncogene in esophageal squamous cell carcinoma and its potential implication in chemotherapy. Oncol Rep. 2014;32:1601–1609. doi: 10.3892/or.2014.3348. [DOI] [PubMed] [Google Scholar]
- 27.Kong KL, Kwong DL, Chan TH, Law SY, Chen L, Li Y, Qin YR, Guan XY. MicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut. 2012;61:33–42. doi: 10.1136/gutjnl-2011-300178. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Chen D, Gao X, Li X, Shi G. lncRNA NEAT1 regulates cell viability and invasion in esophageal squamous cell carcinoma through the miR-129/CTBP2 axis. Dis Markers. 2017;2017:5314649. doi: 10.1155/2017/5314649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Liu P, Chen Y, Chen Z, Shen M, Liu X, Li X, Li A, Lin Y, Yang R, et al. Long noncoding RNA FAM201A mediates the radiosensitivity of esophageal squamous cell cancer by regulating ATM and mTOR expression via miR-101. Front Genet. 2018;9:611. doi: 10.3389/fgene.2018.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang ZW, Jia YX, Zhang WJ, Song LJ, Gao M, Li MJ, Zhao RH, Li J, Zhong YL, Sun QZ, et al. lncRNA-TUSC7/miR-224 affected chemotherapy resistance of esophageal squamous cell carcinoma by competitively regulating DESC1. J Exp Clin Cancer Res. 2018;37:56. doi: 10.1186/s13046-018-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Hu L, Liang Y, Li J, Wang K, Chen X, Meng H, Guan X, Yang K, Bai Y. Up-regulation of lncRNA CASC9 promotes esophageal squamous cell carcinoma growth by negatively regulating PDCD4 expression through EZH2. Mol Cancer. 2017;16:150. doi: 10.1186/s12943-017-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mei LL, Qiu YT, Huang MB, Wang WJ, Bai J, Shi ZZ. miR-99a suppresses proliferation, migration and invasion of esophageal squamous cell carcinoma cells through inhibiting the IGF1R signaling pathway. Cancer Biomark. 2017;20:527–537. doi: 10.3233/CBM-170345. [DOI] [PubMed] [Google Scholar]
- 34.Ke K, Sun Z, Wang Z. Downregulation of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion in esophageal squamous cell carcinoma. Oncol Lett. 2018;16:1801–1808. doi: 10.3892/ol.2018.8797. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Zhang Q, Zhao X, Zhang C, Wang W, Li F, Liu D, Wu K, Zhu D, Liu S, Shen C, et al. Overexpressed PKMYT1 promotes tumor progression and associates with poor survival in esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:7813–7824. doi: 10.2147/CMAR.S214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertrand FE, Steelman LS, Chappell WH, Abrams SL, Shelton JG, White ER, Ludwig DL, McCubrey JA. Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia. 2006;20:1254–1260. doi: 10.1038/sj.leu.2404217. [DOI] [PubMed] [Google Scholar]
- 37.Guo L, Bai Y, Ji S, Ma H. MicroRNA98 suppresses cell growth and invasion of retinoblastoma via targeting the IGF1R/k-Ras/Raf/MEK/ERK signaling pathway. Int J Oncol. 2019;54:807–820. doi: 10.3892/ijo.2019.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundaram GM, Veera Bramhachari P. Molecular interplay of pro-inflammatory transcription factors and non-coding RNAs in esophageal squamous cell carcinoma. Tumour Biol. 2017;39:1010428317705760. doi: 10.1177/1010428317705760. [DOI] [PubMed] [Google Scholar]
- 39.Tan D, Wu Y, Hu L, He P, Xiong G, Bai Y, Yang K. Long noncoding RNA H19 is up-regulated in esophageal squamous cell carcinoma and promotes cell proliferation and metastasis. Dis Esophagus. 2017;30:1–9. doi: 10.1111/dote.12481. [DOI] [PubMed] [Google Scholar]
- 40.Bao J, Zhou C, Zhang J, Mo J, Ye Q, He J, Diao J. Upregulation of the long noncoding RNA FOXD2-AS1 predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Biomark. 2018;21:527–533. doi: 10.3233/CBM-170260. [DOI] [PubMed] [Google Scholar]
- 41.Sadeghpour S, Ghorbian S. Evaluation of the potential clinical prognostic value of lncRNA-BANCR gene in esophageal squamous cell carcinoma. Mol Biol Rep. 2019;46:991–995. doi: 10.1007/s11033-018-4556-2. [DOI] [PubMed] [Google Scholar]
- 42.He A, Liu Y, Chen Z, Li J, Chen M, Liu L, Liao X, Lv Z, Zhan Y, Zhuang C, et al. Over-expression of long noncoding RNA BANCR inhibits malignant phenotypes of human bladder cancer. J Exp Clin Cancer Res. 2016;35:125. doi: 10.1186/s13046-016-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Liu G. lncRNA BANCR suppresses cell viability and invasion and promotes apoptosis in non-small-cell lung cancer cells in vitro and in vivo. Cancer Manag Res. 2019;11:3565–3574. doi: 10.2147/CMAR.S194848. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao H, Gong N, Ma Z, Miao X, Chen J, Cao Y, Zhang G. lncRNA ZEB2-AS1 promotes pancreatic cancer cell growth and invasion through regulating the miR-204/HMGB1 axis. Int J Biol Macromol. 2018;116:545–551. doi: 10.1016/j.ijbiomac.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 46.Guo H, Yang S, Li S, Yan M, Li L, Zhang H. lncRNA SNHG20 promotes cell proliferation and invasion via miR-140-5p-ADAM10 axis in cervical cancer. Biomed Pharmacother. 2018;102:749–757. doi: 10.1016/j.biopha.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Heery R, Finn SP, Cuffe S, Gray SG. Long non-coding RNAs: Key regulators of epithelial-mesenchymal transition, tumour drug resistance and cancer stem cells. Cancers (Basel) 2017;9:38. doi: 10.3390/cancers9040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao R, Shao J, Hu Y, Wang L, Li Z, Sun G, Gao X. MicroRNA-338-3p inhibits proliferation, migration, invasion, and EMT in osteosarcoma cells by targeting activator of 90 kDa heat shock protein ATPase homolog 1. Cancer Cell Int. 2018;18:49. doi: 10.1186/s12935-018-0551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding Z, Zhu J, Zeng Y, Du W, Zhang Y, Tang H, Zheng Y, Qin H, Liu Z, Huang JA. The regulation of Neuropilin 1 expression by miR-338-3p promotes non-small cell lung cancer via changes in EGFR signaling. Mol Carcinog. 2019;58:1019–1032. doi: 10.1002/mc.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S, Suo J, Wang C, Sun X, Wang D, He L, Zhang Y, Li W. Downregulation of tissue miR-338-3p predicts unfavorable prognosis of gastric cancer. Cancer Biomark. 2017;21:117–122. doi: 10.3233/CBM-170339. [DOI] [PubMed] [Google Scholar]
- 51.Liu F, Yang X, Geng M, Huang M. Targeting ERK, an Achilles' Heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8:552–562. doi: 10.1016/j.apsb.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Deng T, Li X, Cai W. miR-193b inhibits the growth and metastasis of renal cell carcinoma by targeting IGF1R. Artif Cells Nanomed Biotechnol. 2019;47:2058–2064. doi: 10.1080/21691401.2019.1620251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.