We find that female mice display no allodynia 10 months after nerve injury, and pregabalin loses its effectiveness after that delay, and in middle-aged mice.
Keywords: aging, allodynia, pregabalin, morphine, amitryptiline, diclofenac, neuropathic
Abstract
Introduction:
Increasing attention is being paid to the effects of organismic factors like age on pain sensitivity. However, very little data exist on this topic using modern algesiometric assays and measures in laboratory rodents.
Objectives:
We investigated the effect of age and duration of nerve injury on baseline mechanical thresholds, neuropathic allodynia, and the antiallodynic and analgesic efficacy of 4 systemically administered analgesics: amitriptyline, diclofenac, morphine, and pregabalin.
Methods:
Mice of both sexes and 3 conditions were compared: Young-Young, in which baseline testing (von Frey thresholds), the injury producing neuropathic pain (spared nerve injury [SNI]) and subsequent drug testing occurred while mice were young (8–10 weeks); Young-Old, in which mice received the nerve injury while young but were tested for drug efficacy over 10 months later; and Old-Old, in which both the nerve injury and drug testing occurred at approximately 1 year of age.
Results:
Old-Old mice were found to display higher baseline mechanical sensitivity than other groups. No group differences were seen in SNI-induced allodynia in males; female Young-Old mice were found to display greatly reduced allodynia. With respect to drug efficacy, no differences among conditions were observed for amitriptyline, diclofenac, or morphine. For pregabalin, however, Young-Old mice displayed significantly reduced antiallodynia, and the drug was completely ineffective in Old-Old mice.
Conclusion:
Novel findings include the apparent remission of SNI-induced allodynia in female mice 10 months after injury and reduced pregabalin antiallodynic effects produced by both the passage of time after nerve injury and aging.
1. Introduction
Spurred on by the desire to personalize the treatment of pain, there has been a resurgence of interest in organismic factors—those inherent to the organism experiencing pain, including sex, genetic background, previous pain experience, and age—that modulate pain perception and response to analgesic manipulations. Of these, age has received the least attention, likely due to practical barriers (ie, time and cost) associated with its longitudinal study. Aging humans display declines in all sensory modalities, including nociception, although older adults paradoxically report more, and more severe chronic pain, likely due to their increased susceptibility to chronic diseases with pain as a symptom (eg, osteoarthritis). Meta-analyses have demonstrated decreased sensitivity (ie, increased thresholds) of aged adults to acute pain of multiple modalities but also decreased tolerance.8 In preclinical experiments in rodents, the effect of aging on pain sensitivity has largely been studied with acute, thermal assays (see Ref. 7). There is a paucity of evidence from more modern algesiometric assays and measures (see Ref. 17). With respect to response to analgesics, most of the available evidence in humans comes from studies of postoperative analgesic consumption (cf. Ref. 14), and in laboratory animals from studies of morphine inhibition of tail-flick or other reflexes (see Ref. 7). Thus, much of the existing data on the modulatory role of age on pain are mismatched to the clinical reality in terms of time (acute pain vs the more clinically relevant chronic pain) and pain modality (thermal vs the more clinically relevant mechanical pain). The preclinical literature is further mismatched with respect to subject age, with most experiments being performed on adolescent/young adult rodents, and time elapsed since the pain-causing injury, which is usually a mere days to weeks (see Ref. 17).
For the treatment of chronic neuropathic pain, even first-line analgesic treatments have unimpressive numbers needed-to-treat of 3 and above.4 By contrast, such treatments are highly effective in preclinical experiments. For example, a recent meta-analysis of all pregabalin experiments in rodents showed an average analgesic response of 71% of the maximum possible effect (such that the majority of animals demonstrated a >50% decrease in pain),3 whereas clinically pregabalin has a number needed-to-treat of 4.5 against neuropathic pain (such that less than 25% of patients who would not have responded to placebo would show a >50% decrease in pain).4 It has never been directly evaluated whether part of the reason for this arguably poor translation lies with organismic factors such as age or duration of injury before the analgesic is administered. In this study, we assess the influence of both of these factors on mechanical allodynia after nerve injury, and the antiallodynic (and frank analgesic) effect of 4 common analgesics: amitriptyline, diclofenac, morphine, and pregabalin. To tease apart the effects of age vs injury duration, we tested 3 groups of mice: one in which both the nerve injury to produce pain and subsequent analgesic administration and testing occurred in young animals, one in which both procedures occurred in older animals, and one in which the nerve injury occurred while young but the testing occurred later.
2. Materials and methods
2.1. Mice
Naïve adult mice of both sexes were used, in approximately equal numbers.18 Subjects were outbred CD-1 (ICR; Crl) mice bred in our laboratory from breeders obtained from Charles River Laboratories (St. Constant, QC). All mice were housed with their same-sex littermates (2–4 animals per cage) in standard shoebox cages, maintained in a temperature-controlled (20 ± 1°C) environment (14:10 hours light/dark cycle), and fed (Envigo Teklad 2920X, Lachine, QC, Canada) and watered ad libitum. All experiments were approved by a local animal care and use committee and conform to Canadian Council on Animal Care guidelines.
2.2. Surgery
Spared nerve injury (SNI) was performed under isoflurane/oxygen anesthesia as described previously,27 sparing the left sural nerve. Anesthesia, surgery, and recovery were identical between conditions. Postsurgical analgesia was provided using 20-mg/kg carprofen to all mice.
2.3. Conditions
Mice were assigned to 1 of 3 age/duration conditions: Young-Young, Young-Old, and Old-Old. Note that the use of the term “Old” here is meant in a relative sense only; the “Old” mice used here were middle-aged (see section 4.4 below). This assignment was made randomly in the case of the first 2 conditions; Old-Old mice were those who, although experimentally naïve, were already present in our vivarium at 18 weeks of age. In the Young-Young condition, mice were tested for baseline mechanical thresholds at 7 to 8 weeks of age, received SNI surgery within 2 to 3 days, and then were retested for SNI allodynia and its reversal by analgesics 2 weeks later, at approximately 10 weeks of age. In the Young-Old condition, mice were treated identically to the Young-Young group until SNI surgeries were performed but were not retested for SNI allodynia and its reversal by analgesics until 10 to 11 months had passed, at approximately 13 months (≈56 weeks) of age. In the Old-Old condition, mice were tested for baseline mechanical thresholds at 18 to 19 weeks of age, were retested for mechanical thresholds at 11 to 12 months of age, received SNI surgery within 2 to 3 days, and then were retested for SNI allodynia and its reversal by analgesics 2 weeks later, at approximately 12.5 months (≈54 weeks) of age. See Figure 1 for a timeline describing these conditions. A difference between the Old-Old and Young-Old groups would imply an effect of injury duration, since drug testing was performed at a similar age. By contrast, a difference between the Old-Old and Young-Young groups would imply an effect of age, since in both cases drug testing occurred 2 weeks after SNI.
Figure 1.
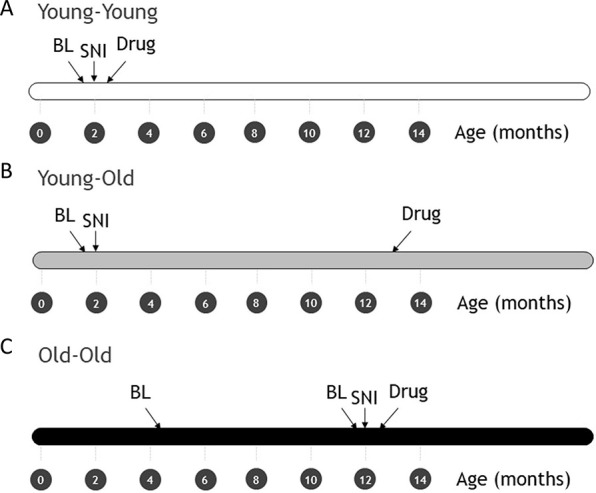
Timeline of procedures in Young-Young (A), Young-Old (B), and Old-Old (C) conditions. Note that time axes are in months. BL, baseline von Frey threshold measurement(s); Drug, measurement of SNI allodynia and its reversal by one of 4 drugs; SNI, spared nerve injury surgery.
2.4. Drugs
All mice received a systemic (subcutaneous) injection of 1 of 4 drugs immediately after post-SNI allodynia testing: amitriptyline (15 mg/kg), diclofenac (25 mg/kg), morphine (10 mg/kg), or pregabalin (30 mg/kg). Doses were chosen based on the results of recent studies using these analgesics to reverse SNI-induced allodynia in mice12,13,25 and confirmed by pilot studies in young mice in our laboratory. Diclofenac was used as a negative control. Mice were randomly assigned to drug condition, and the experimenter was blinded to the identity of the 4 drugs. Thresholds were tested at 30, 60, and 120 minutes after drug.
2.5. Behavioral testing
The up-down method of Dixon2 was used. Mice were placed on a perforated metal floor (with 5-mm-diameter holes placed 7 mm apart) within small Plexiglas cubicles as described above, and a set of 8 calibrated von Frey filaments (Stoelting Touch Test Sensory Evaluator Kit #2–#9, Wood Dale, IL; ranging from ≈0.015 to ≈1.3 g of force) was applied to the lateral aspect (sural nerve innervation territory) of the plantar surface of the hind paw until the fibers bowed, and then held for 3 seconds. Withdrawal of the hind paw within the 3-second period was counted as a positive response to the filament. Reported measurements represent the withdrawal threshold of the hind paw tested once (postdrug time points), or the averaged thresholds of hind paws tested twice (after surgery) or 3 times (baselines) in succession.
2.6. Data transformation
For purposes of presenting baseline mechanical thresholds, data from both the left and right hind paw were averaged. In all other cases, data from the left (ipsilateral) and right (contralateral) hind paw are considered separately. Percentage of maximum possible allodynia (% allodynia) of the ipsilateral hind paw was calculated as % allodynia = [(baseline threshold - post-SNI threshold)/baseline threshold] × 100] × 100. Drug antiallodynic (% antiallodynia) effect in the ipsilateral hind paw was calculated with respect to the threshold difference between the initial baseline threshold and the post-SNI (but pre-drug) threshold, and by considering the area under drug-treated threshold x time curve using the trapezoidal method. Thus, for a mouse to display 100% antiallodynia, it would need to revert from its post-SNI threshold to its most recent baseline threshold at all postdrug time points. % Antiallodynia scores exceeding 100% were possible (with no theoretical ceiling), and so they would not unduly influence the statistical analysis scores >150% (n = 4 in Young-Young, n = 2 in Young-Old, n = 5 in Old-Old) were set at 150%. To assess changes on the contralateral hind paw, the area under the drug-treated threshold × time curve (AUC) was calculated, using the post-SNI/predrug threshold as the floor.
2.7. Statistical analysis
Power analyses were not conducted because we had no way to anticipate effect sizes in this experiment, and instead were based on pilot data from young mice. Baseline threshold, % allodynia, % antiallodynia, and AUC data were analyzed by analysis of variance (ANOVA) followed by Tukey's post hoc test as appropriate. A criterion α = 0.05 was used in all cases. Mice were excluded from further analyses if they displayed baseline thresholds <0.30 g (n = 7), % allodynia scores <70% (n = 6), or % antiallodynia or AUC scores >3 standardized residuals from the condition/drug mean (n = 4). Outliers were found in all condition/drug groups in approximately equal numbers.
3. Results
3.1. Baseline sensitivity
Mice of all 3 conditions were tested for baseline von Frey withdrawal thresholds when they entered the study. In the case of the Young-Young and Young-Old groups, this was at 8 weeks of age. Mice in the Old-Old group, using a convenience sample in an attempt to reduce the overall duration of data collection, entered the study later, at 18 weeks of age. Mice in the (middle-aged) Old-Old group were retested for baseline thresholds at ≈12 months of age, immediately before surgery. Note that a second baseline measurement was not possible for the Young-Old group, since they had received SNI surgery already and were thus no longer at baseline. As shown in Figure 2, the Old-Old group displayed significantly lower baseline thresholds than the other groups (condition: F2,95 = 9.2, P < 0.001). However, in this group, thresholds were not significantly altered when mice were retested at 12 months of age (repeated measures: F1,34 = 3.3, P = 0.08). Mouse sex displayed no significant main effect on baseline thresholds (F1,92 = 0.01, P = 0.91) nor did it interact with condition (F2,92 = 1.0, P = 0.37). Also not interacting with condition was drug (F3,86 = 1.0, P = 0.40), suggesting that mice were appropriately randomized to drug groups.
Figure 2.
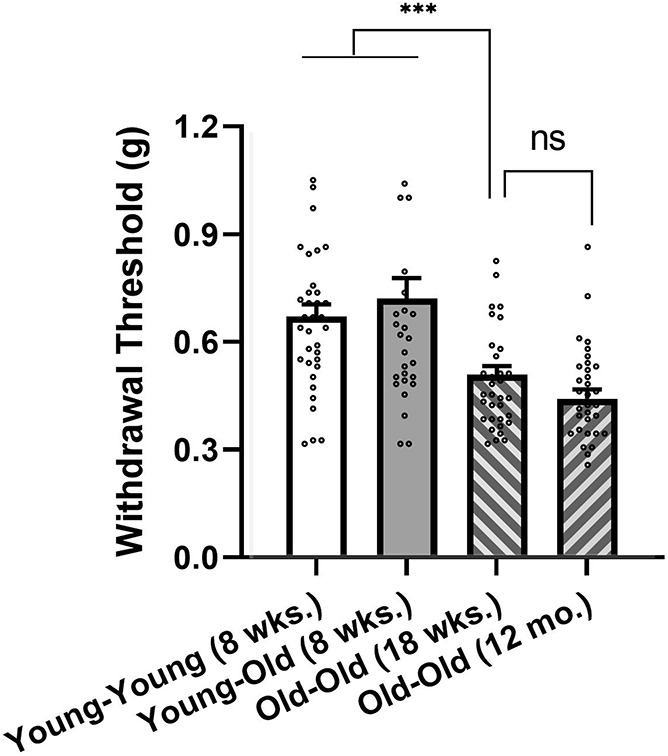
Baseline mechanical thresholds (all drug groups combined) of Young-Young (n = 34), Young-Old (n = 25), and Old-Old (n = 33) conditions. Old-Old mice were tested both while “young,” at 18 weeks (wks.) of age, and again at 12 months (mo.) of age. Bars represent mean ± SEM 50% withdrawal threshold (g) to the application of von Frey filaments; both hind paws are averaged. ***P < 0.001 (by ANOVA followed by Tukey's post hoc test; ns, not significantly different (by repeated-measures ANOVA). ANOVA, analysis of variance.
3.2. Spared nerve injury allodynia and sex differences
An ANOVA performed on % allodynia data unexpectedly revealed a highly significant interaction between condition and sex (F2,123 = 23.0, P < 0.001). As shown in Figure 3, this was due to the absence of significant allodynia in female Young-Old mice, suggestive of the loss of SNI allodynia over time in females. Thus, since in female Young-Old mice there was no allodynia for drugs to reverse, only male Young-Old mice were treated with analgesics. Because this dramatically reduced the effective sample size of this condition, a separate cohort of male Young-Old mice was tested subsequently, and their data were added to all subsequent analyses.
Figure 3.
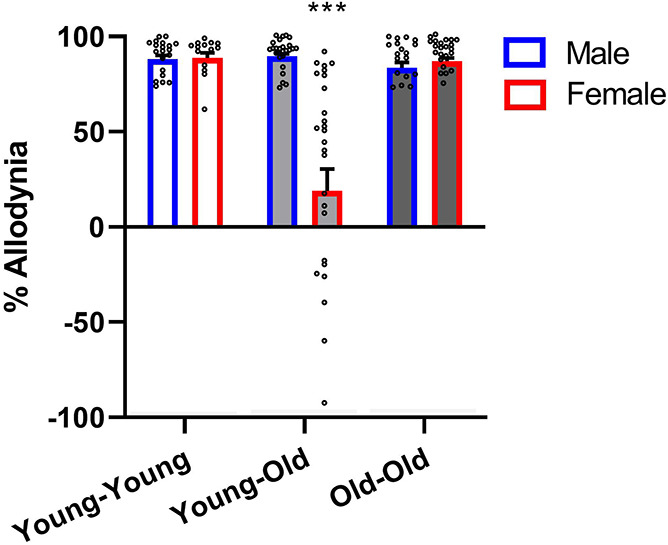
Female mice in the Young-Old condition (ie, >10 months after SNI) do not display significant SNI allodynia. Bars represent mean ± SEM % allodynia (see section 2.6); n = 14 to 28 mice/condition/sex. ***P < 0.001 compared to all other groups. A one-sample t test of Young-Old female mice compared with a reference value of zero was not significant (t27 = 1.6, P = 0.11). SNI, spared nerve injury.
3.3. Drug antiallodynia
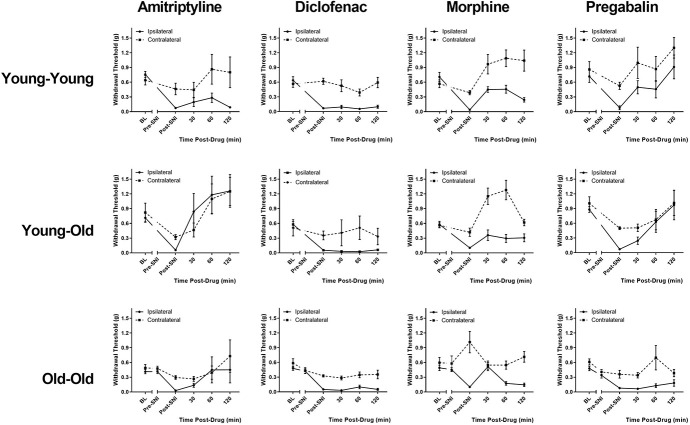
Mechanical thresholds at all time points for all condition/drug combinations are shown in Figure 4. As can be seen, reversal of SNI allodynia at postdrug time points was seen on the ipsilateral hind paw in most graphs, and frank analgesic effects on the contralateral hind paw were seen in some graphs. When analyzed as % antiallodynia as described in section 2.6, a significant condition × drug interaction was evinced (F6,80 = 3.6, P = 0.003). We then performed one-way ANOVAs for each drug separately. As shown in Figure 5A, no significant effects of condition were observed for amitriptyline (F2,19 = 2.1, P = 0.15), diclofenac (F2,23 = 0.5, P = 0.60), or morphine (F2,21 = 0.4, P = 0.68). By contrast, a highly significant effect of condition was observed for pregabalin (F2,23 = 12.1, P < 0.001). Pregabalin was highly effective in reversing SNI allodynia in Young-Young mice and significantly less so in Young-Old mice. Old-Old mice were, in turn, significantly less sensitive to pregabalin inhibition of SNI allodynia than were Young-Old mice. This effect of age and/or injury duration was seen in both male and female mice (condition × sex in Young-Young vs Old-Old mice: F1,14 = 0.2, P = 0.64).
Figure 4.
Mechanical thresholds of all condition-drug groups at all experimental time points. Symbols represent mean ± SEM 50% withdrawal thresholds (g); n = 6 to 9 mice/condition/drug. BL, baseline; Pre-SNI, second baseline measurement at 11 to 12 months in Old-Old mice only; Post-SNI, predrug assessment of mechanical allodynia; SNI, spared nerve injury.
Figure 5.
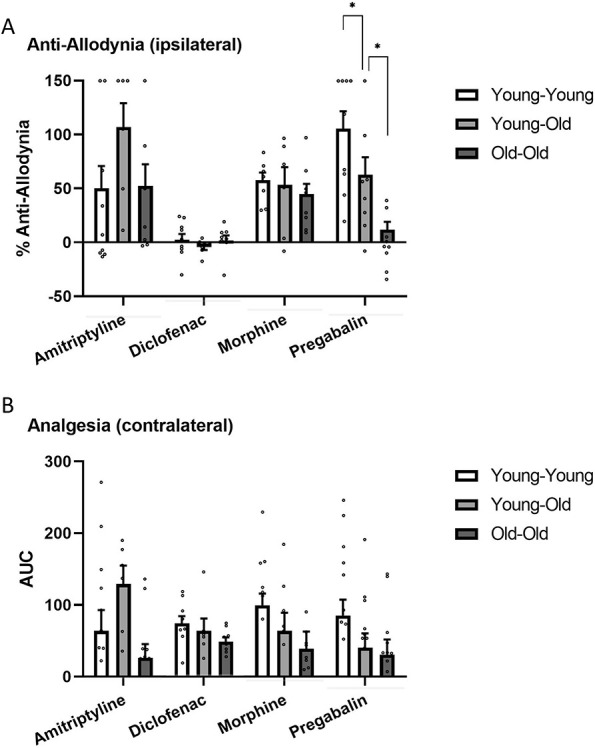
Drug effects on the ipsilateral (A, antiallodynia) and contralateral (B, analgesia) hind paws in all conditions. Bars represent mean ± SEM % antiallodynia (A) or AUC (B); see section 2.6. *P < 0.05 as indicated. AUC, area under the drug-treated threshold × time curve.
3.4. Drug contralateral analgesia
When analyzed through AUC as described in section 2.6, a significant main effect of condition (F2,80 = 3.4, P = 0.04) and drug was observed (F3,80 = 4.8, P = 0.006), but we observed no significant interaction between the two (F6,80 = 1.2, P = 0.32). Figure 5B shows contralateral AUC data broken down by drug; the Young-Young > Young-Old ≥ Old-Old pattern can also be seen for pregabalin.
4. Discussion
This study finds evidence for aging effects on mechanical thresholds and the efficacy of pregabalin (but not amitriptyline, diclofenac, or morphine) against SNI-induced mechanical allodynia. We also observe an extremely robust sex difference in SNI allodynia displayed by male and female mice 10 months after surgery.
4.1. Baseline mechanical thresholds
As shown in Figure 2, the Old-Old group displayed significantly decreased baseline mechanical thresholds (ie, increased sensitivity) compared with other groups. It is rather difficult to characterize this as an effect of “aging,” since the difference was apparent at 18 weeks of age (compared with 8 weeks of age in the Young-Young and Young-Old conditions), and did not become statistically greater (P = 0.08) when Old-Old mice were retested at 12 months of age. Thus, it is more likely a cohort effect.22 The pattern detected here has been shown previously, with Ruiz-Medina et al.23 observing significantly decreased von Frey thresholds in 12- to 16-week-old CD-1 mice compared with 31-day-old mice, with no further decreases in 12- to 13-month-old mice. However, other experiments comparing von Frey thresholds across similarly aged mice have reported no differences16 or reduced sensitivity with increasing age.24 Most experiments have used inbred C57BL/6 mice, rather than the outbred CD-1 mice used here. In the same manner as sex differences in pain and pain inhibition have been shown to interact thoroughly with genetic background,10,20 aging effects almost certainly do as well. For example, one study comparing 6- and 26-month-old rats showed that although young Brown Norway rats displayed lower von Frey thresholds than older ones, the pattern was reversed in Fischer 344 rats.1
4.2. Spared nerve injury–induced allodynia
Figure 3 robustly illustrates that female mice of the Young-Old group no longer display appreciable mechanical allodynia 10 to 11 months after SNI, whereas mechanical allodynia in males of this group is no different than in the Young-Young and Old-Old conditions. In other words, mechanical allodynia in a large subset of female mice resolves by 10 to 11 months after SNI but persists in male mice. That such a dramatic sex difference has never before been reported is due to both the overwhelming (and continuing) reliance on male rodents as research subjects in preclinical pain experiments19 and the paucity of experiments testing mice of either sex for pain at long time points after injury. We were able to identify less than 15 articles in which mechanical allodynia after a neuropathic injury (mostly SNI) was tested at any time point later than 3 months after surgery; in only 3 of these were female rodents used. Shepherd et al.26 observed unabated mechanical allodynia in female C57BL/6 mice at 120 days after surgery. Swartjes et al.28 observed maximal allodynia in female Sprague–Dawley rats at 140 days after SNI. Hubbard et al.9 observed SNI allodynia in female Sprague–Dawley rats at 19-week point after injury, although visual inspection (see Fig. 3A in Hubbard et al.) suggests that remission may have been in the process of occurring. Together, these observations suggest that SNI allodynia remission in females occurs after 4 to 5 months after nerve injury. Whether a similar sex difference in neuropathic pain duration occurs in humans is unknown and likely to remain so. Although women are more susceptible to developing neuropathic pain (cf. Ref. 19), to establish the average duration of chronic pain in humans would require the ability to distinguish between spontaneous remission of disease and successful response to treatment.
In male mice (and female mice of Young-Young vs Old-Old groups), there was no effect of age or injury duration on mechanical allodynia induced by SNI. Only a small number of studies have compared neuropathic mechanical allodynia in rodents of different ages; the 2 studies performed in mice observed no differences between young (2–4 months) and aging (12–24 month) animals.15,23
4.3. Analgesic efficacy
Our observations suggest that both duration of injury (Old-Old vs Young-Old) and aging itself (Old-Old vs Young-Young) affect pregabalin's antiallodynic efficacy, such that the drug becomes less effective as time passes from the injury and in older mice. No other significant differences were observed for either the antiallodynic or frank analgesic (contralateral hind paw) effects of any drug, although clear trends towards a potentiation of amitriptyline's effects at long time points after injury and decreases in morphine and pregabalin contralateral analgesia in Young-Old and Old-Old groups were seen. The pregabalin observations are, to the best of our knowledge, a novel finding. Perhaps the most relevant published finding to the current experiment is the report by Kimura et al.11 regarding changes in gabapentin efficacy over time after nerve injury in rats. They demonstrated that the peak of gabapentin's effectiveness in reversing spinal nerve ligation-induced mechanical sensitivities on the Randall–Selitto test was at 1 to 2 weeks after surgery, with significant reductions observed starting at 4 weeks after surgery and no differences from saline by 8 weeks after surgery. A similar diminution of efficacy was observed after intrathecal injection of clonidine. The mechanism underlying the decreasing analgesic efficacy was related to downregulation over time of glutamate transporter-1 (GLT-1) in the locus coeruleus and thus reduced spinal noradrenergic inhibition.11 It is unclear whether such a mechanism has direct relevance to the present observations because the 2 studies differed in their subjects (mice vs rats), drug (pregabalin vs gabapentin), injury (SNI vs spinal nerve ligation), and, most importantly, time after nerve injury (10 months vs 10 weeks).
4.4. Limitations and conclusions
The major limitation of the present work concerns the use of only a single dose of each drug. Ideally, full dose–response relationships would have been examined. The doses of amitriptyline, morphine, and pregabalin chosen were clearly appropriate; however, as both floor and ceiling effects were successfully avoided. The lack of efficacy of diclofenac against neuropathic mechanical allodynia was entirely consistent with the existing literature. Another limitation concerns the age of the “old” mice considered in this experiment. Given the mouse's life span of 2 to 3 years, the 12- to 14-month time point considered in this study corresponds only to middle age in humans, not old age.5 This decision was made on purpose because susceptibility to many forms of chronic pain may peak in middle age.6 Also complicating any generalizing to humans is the fact that the most common and important symptom of chronic pain in humans is spontaneous (ongoing) pain, not the static mechanical allodynia measured here.21
Despite these limitations, these observations suggest that a review of the clinical efficacy of pregabalin over the life span, and as a function of time since the precipitating injury, may be useful. We are unaware of any extant study that has performed this directly. More generally, we suggest that as the population ages, more attention ought to be paid to organismic issues of time and age despite the relatively high cost of such investigations.
Disclosures
The authors report no conflicts of interest.
This study was funded by a Canadian Institutes of Health Research Foundation grant, and the Louise and Alan Edwards Foundation (J.-S.M.). A. Muralidharan was supported by the Ronald Melzack Fellowship in Chronic Pain Research awarded by the Louise and Alan Edwards Foundation.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Chaloner A, Greenwood-Van Meerveld B. Genetic diversity contributes to abnormalities in pain behaviors between young and old rats. Age 2013;35:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia evoked by unilateral ligation of the fifth and sixth lumbar nerves in the rat. J Neurosci Meth 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [3].Federico CA, Mogil JS, Ramsay T, Fergusson DA, Kimmelman J. A systematic review and meta-analysis of pregabalin preclinical studies. PAIN 2020;161:684–93. [DOI] [PubMed] [Google Scholar]
- [4].Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. PAIN 2010;150:573–81. [DOI] [PubMed] [Google Scholar]
- [5].Flurkey K, Currer JM, Harrison DE. The mouse in aging research. In: Fox JG, editor. The mouse in biomedical research. 2nd ed Burlington: American College of Laboratory Animal Medicine (Elsevier), 2007. [Google Scholar]
- [6].Gagliese L, Melzack R. Chronic pain in elderly people. PAIN 1997;70:3–14. [DOI] [PubMed] [Google Scholar]
- [7].Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev 2000;24:843–54. [DOI] [PubMed] [Google Scholar]
- [8].Gibson SJ. Pain and aging: the pain experience over the adult life span. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Progress in pain research and management: Proceedings of the 10th world congress on pain, Vol. 24 Seattle: IASP Press, 2003. pp. 767–90. [Google Scholar]
- [9].Hubbard CS, Khan SA, Xu S, Cha M, Masri R, Seminowicz DA. Behavioral, metabolic and functional brain changes in a rat model of chronic neuropathic pain: a longitudinal MRI study. NeuroImage 2015;107:333–44. [DOI] [PubMed] [Google Scholar]
- [10].Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther 1999;289:1370–5. [PubMed] [Google Scholar]
- [11].Kimura M, Eisenach JC, Hayashida K. Gabapentin loses efficacy over time after nerve injury in rats: role of glutamate transporter-1 in the locus coeruleus. PAIN 2016;157:2024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kremer M, Yalcin I, Goumon Y, Wurtz X, Nexon L, Daniel D, Megat S, Ceredig RA, Ernst C, Turecki G, Chavant V, Theroux JF, Lacaud A, Joganah LE, Lelievre V, Massotte D, Lutz PE, Gilsbach R, Salvat E, Barrot M. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitripyline. J Neurosci 2018;38:9934–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lax NC, Chen R, Leep SR, Uhrich KE, Yu L, Kolber BJ. PolyMorphine provides extended analgesic-like effects in mice with spared nerve injury. Mol Pain 2017;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mecklenburg J, Patil MJ, Koek W, Akopian AN. Effects of local and spinal administrations of mu-opioids on postoperative pain in aged vs adult mice. Pain Rep 2017;2:e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Millecamps M, Shi XQ, Piltonen M, Echeverry S, Diatchenko L, Zhang J, Stone LS. The geriatric pain experience in mice: intact cutaneous thresholds but altered responses to tonic and chronic pain. Neurobiol Aging 2020;89:1–11. [DOI] [PubMed] [Google Scholar]
- [16].Millecamps M, Tajerian M, Sage EH, Stone LS. Behavioral signs of chronic back pain in the SPARC-null mouse. Spine 2011;36:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci 2009;10:283–94. [DOI] [PubMed] [Google Scholar]
- [18].Mogil JS. Equality need not be painful. Nature 2016;535:S7. [DOI] [PubMed] [Google Scholar]
- [19].Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 2020. doi: 10.1038/s41583-020-0310-6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [20].Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev 2000;24:375–89. [DOI] [PubMed] [Google Scholar]
- [21].Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? PAIN 2004;112:12–5. [DOI] [PubMed] [Google Scholar]
- [22].Mogil JS, Ritchie J, Sotocinal SG, Smith SB, Croteau S, Levitin DJ, Naumova AK. Screening for pain phenotypes: analysis of three congenic mouse strains on a battery of nine nociceptive assays. PAIN 2006;126:24–34. [DOI] [PubMed] [Google Scholar]
- [23].Ruiz-Medina J, Baulies A, Bura SA, Valverde O. Paclitaxel-induced neuropathic pain is age dependent and devolves on glial response. Eur J Pain 2013;17:75–85. [DOI] [PubMed] [Google Scholar]
- [24].Sadler KE, Gartland NM, Cavanaugh JE, Kolber BJ. Central amygdala activation of extracellular signal-regulated kinase 1 and age-dependent changes in inflammatory pain sensitivity in mice. Neurobiol Aging 2017;56:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sanna MD, Les F, Lopez V, Galeotti N. Lavender (Lavandula angustifolia Mill.) essential oil alleviates neuropathic pain in mice with spared nerve injury. Front Pharmacol 2019;10:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shepherd AJ, Cloud ME, Cao Y-Q, Mohapatra DP. Deficits in burrowing behaviors are associated with mouse models of neuropathic but not inflammatory pain or migraine. Front Behav Neurosci 2018;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shields SD, Eckert WA, III, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain 2003;4:465–70. [DOI] [PubMed] [Google Scholar]
- [28].Swartjes M, van Velzen M, Niesters M, Aarts L, Brines M, Dunne A, Cerami A, Dahan A. ARA 290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain coupled with suppression of the spinal microglia response. Mol Pain 2014;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]