Supplemental Digital Content is available in the text
Keywords: bariatric surgery, malnutrition, obesity, quality of life, type 2 diabetes mellitus
Abstract
The incidence of obesity and type 2 diabetes mellitus is growing, and bariatric surgery was applied as a new therapy in the past few decades. However, bariatric surgery started rather late in China, and the number of surgeries and the follow-up data is limited.
We assessed body weight, glucose, lipid levels, and blood pressure at baseline and 6-month, 1-year, 3-year in patients who underwent bariatric surgery. Vitamins and trace elements were investigated at 3-year after surgery. The quality of life was assessed at 3-year and compared with the control group.
In total 20 patients were recruited in the study, and all the 20 patients underwent surgery and completed all follow-ups. Results showed that the body weight, body mass index, glycated hemoglobin (HbA1C), glucose, and insulin level were decreased, and islet function improved significantly in 6-month and 1-year (P < .001), and the changes were more obvious in the first 6 months. However, all the indexes rebound significantly at the 3-year (P < .05), but still better than baseline (P < .05). Weight regain was 50% after 3 years, and the mean weight regain rate was 31.45%. Besides, blood pressure and lipid levels decreased significantly compared with baseline (P < .001). At the 3-year follow-up, we found that 100% of the patients showed vitamin D deficiency, 50% calcium deficiency, 20% vitamin B12 deficiency, 20% iron deficiency, and 15% suffered from anemia. Compared with the control group, the quality of life was better in patients who underwent surgery, especially in the physical health (P < .05).
The current study showed that the body weight, glucose and islet function improved significantly after bariatric surgery, and the indexes changed mainly in the first 6 months, but there seemed to be a rebound after 3 years. Furthermore, the surgery may improve the blood pressure, lipid profile, and the quality of life. However, some patients may suffer anemia, calcium deficiency, iron deficiency, vitamin D, and vitamin B12 deficiency after 3 years.
1. Introduction
In the past 30 years, the lifestyle of Chinese has changed greatly. People tend to have for more trans-fat, sugar, and less physical activities. As a result, the incidence of obesity and type 2 diabetes mellitus (T2DM) has increased significantly, but the awareness rate, diagnosis rate, and adequate control rate are relatively low.[1] In China, the prevalence of diabetes was 0.67% and 2.5% in 1980 and 1994, respectively.[2,3] While in 2000 to 2001, the incidence of a diabetes has increased to 5.5%, of which only 25.9% of diabetic patients were under well control (HbA1C < 6.5%).[4] In 2007, the prevalence of diabetes was 9.7%, indicating an estimated 92.4 million adults in China with diabetes.[5] The most recent national survey in 2010 reported that the incidence of diabetes has increased to 11.6%, only 25.8% of which received regular treatment, and only 39.7% were under well control.[1]
A large number of studies have shown that the proper treatment is not only to control body weight and blood glucose but also to reduce risks of complications and improve quality of life (QoL). Traditional treatments, such as a healthy diet, moderate exercise, antidiabetic medicines, and insulin, are effective, but the long-term effect is not ideal. Previous studies have suggested that bariatric surgery could not only reduce body weight and improve blood glucose but also improve obesity-related metabolic disorders and QoL.[6–10] QoL is a concept, which encompasses the concept of health, being composed of multiple domains: physical, psychological, among others. However, bariatric surgery also has limitations, such as recurrence, trace elements, and vitamin deficiency.[11–13] The limited data from randomized, controlled trials are available. In this study, we followed up Chinese obese patients with T2DM to determine the long-time changes in body weight, QoL, metabolism and nutritional condition to provide recommendations for patients.
2. Methods
2.1. Study design
We extracted 20 eligible patients’ clinical data who underwent Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) from March 2015 to May 2015. All patients signed medical informed consent form designed by the hospital ethics committee. Inclusion and exclusion criteria referred to the “Guidelines for Bariatric Surgical of Obesity and Type 2 Diabetes in China” issued by the Chinese Society for Metabolic & Bariatric Surgery in 2014.
It should be noted that Asians may have higher risk of cardiovascular diseases and metabolic disorders since the higher visceral fat content in the body,[14,15] and previous studies have shown that surgery is also effective for patients with BMI 25 to 27.5 kg/m2.[16–18] Therefore, we recruited patients with BMI 25 to 27.5 kg/m2 whose glucose level is not under well control even with medical therapy in this study.
2.2. Surgical treatment methods
Types of the treatments were selected according to the patients’ body weight, coexisting illnesses, diabetes complications, and treatments at baseline. Bariatric procedures were performed laparoscopically by a single surgeon team. Nutritional and psychological assessments were conducted both before and after the surgery. Patients were instructed to maintain healthy eating habits and take supplementations every day after surgery.
2.3. Data collection and assessment
Baseline characteristics including gender, age, occupation, marital status, birthplace, diabetes duration, diabetes complications, and medications were collected. Physical examinations including body weight, waist circumference, blood pressure were also performed. HbA1C, fasting plasma glucose (FPG) and 2-hour postprandial plasma glucose (2h-PG), fasting C-peptide (FCP) and 2-hour postprandial C-peptide (2h-CP), fasting, and 2-hour postprandial insulin were also collected at baseline, 6 months, and 1 and 3 years. BMI (the weight in kilograms divided by the square of the height in meters), percentage excess weight loss (EWL%, the loss weight divided by the difference between initial weight and standard weight), percentage excess BMI loss (EBMIL%, the loss BMI divided by the difference between initial BMI and standard BMI), C2/C0 (the 2h-CP divided by FCP), and weight regaining rate (the regained weight from 1 year divided by weight change from baseline to the minimum weight after surgery) were calculated. Weight regain was determined based on: 25% increase in lost weight from the first year after operation or weight regain more than 10 kg from the weight at 1 year after surgery.[19]
Nutritional assessment of the patients was conducted in 3 years after surgery. We collected trace elements, folic acid, vitamin A, B12, D (25-OH-D), and E. QoL questionnaire MOS 36-Item Short Form Health Survey (SF-36) were given to the patients. We chose 20 patients in our hospital as the control group based on the age, gender, and diabetes duration of the patients who underwent surgery. Recruitment criteria were an age of 30 to 60 years, a history of T2DM, a BMI of 25.0 kg/m2 or more, and an ability to understand the study protocol. Exclusion criteria were a history of type 1 diabetes, previous bariatric surgery, pregnancy, and severe diabetes complications.
2.4. Study end points
The primary end point of the study was HbA1c. According to the guideline, we defined HbA1c≤7.5% with fewer medicines as effective. Secondary end points are expressed in points below: glucose that includes FPG and 2h-PG; body weight that includes waist circumference, EWL% (100 × [baseline weight − follow-up weight]/[baseline weight – (height − 105)]), and EBMIL% (100 × [baseline BMI – follow-up BMI]/[baseline BMI – 24]), and we defined EWL%≥30% as effective; islet function, including fasting and 2-hour postprandial insulin, FCP, 2h-CP, and C2/C0; lipid levels and blood pressure; QoL assessed by SF-36; vitamins and micronutrients levels. To understand the similarity and difference between the 2 types of surgery, we made a comparison between SG and RYGB in blood glucose, body weight, islet function, lipid levels and blood pressure, QoL, vitamins, and micronutrients levels.
2.5. Statistical analysis
SPSS19.0 (IBM, USA) statistical software was used for data analysis, and all the data were tested for normality. Comparisons of variables between time points within groups were made with the paired-sample t test. Between-group comparisons were made with the independent-samples t test, and the non-normal distribution data were made with rank sum test. Continuous variables are expressed as means ±SD and categorical variables as numbers and percentages. Statistical significance was set at P < .05 for all analyses.
3. Results
3.1. Primary end point
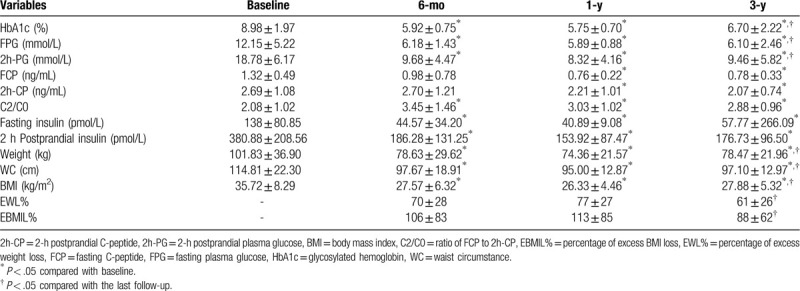
The baseline characteristics of the patients are shown in Table 1. HbA1c decreased significantly in 6 months after operation (P < .001), and kept dropping slowly in 1 year. However, HbA1c increased in 3-year compared with 1-year (P < .05), but still lower than baseline (P < .05). Besides, 12 patients took medication, 7 patients used insulin, and 1 patient took the combined treatment at baseline. At the 3-year, 4 patients took medications, 1 patient took the combined treatment, and 15 patients take no medications.
Table 1.
Baseline characteristics of the patients.

3.2. Glucose and islet function
After 6-month, FPG, 2h-PG, fasting, and 2-hour postprandial insulin decreased, and C2/C0 increased significantly compared with baseline (P < .05). Then FPG, 2h-PG, fasting, and 2-hour postprandial insulin continued to decrease but not significant in 1-year from 6-month (P > .05). FCP and 2h-CP also decreased in 6-month, but there is no statistical significance until 1-year (P < .05). After 3 years, FPG, 2h-PG, fasting and 2-hour postprandial insulin, FCP, and 2h-CP all increased compared with 1 year (P < .05), but still below the baseline (P < .05). Similarly, C2/C0 declined, but still higher than baseline (P < .05) (Table 2).
Table 2.
Weight, glucose and islet function of the patients.
3.3. Weight and waist circumference
The change of body weight, waist circumference, and BMI were similar to the glucose, which decreased significantly in 6 months, and continued to decrease slowly to 1-year. Similarly, there was a rebound in 3-year, but was still better than baseline. The EWL% and EBMIL% were (70 ± 28) and (106 ± 83) in 6-month, and increased to (77 ± 27) and (113 ± 85) in 1-year. Similarly, they decreased to (61 ± 26) and (88 ± 62) in 3-year (Table 2). Weight regain was 50% after 3 years, and the mean weight regain rate in 3-year was 31.45% of maximum weight loss.
3.4. Blood pressure and lipids
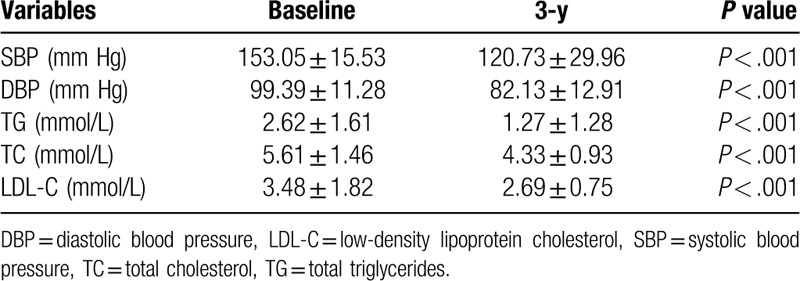
At baseline, 13 patients (65%) had hypertension and were on medications, and 11 of whom had poor control. The average systolic blood pressure (SBP) was (153.05 ± 15.53) mm Hg, and diastolic blood pressure (SDP) was (99.39 ± 11.28) mm Hg at baseline. In 3-year, the average SBP and DBP decreased significantly (P < .001), 5 of 13 patients (38.46%) had achieved successful blood pressure control and discontinued medications. Similarly, 14 patients (70%) had dyslipidemia at baseline, and the mean triglyceride, total cholesterol, and low-density lipoprotein cholesterol decreased significantly after 3 years (P < .001) (Table 3).
Table 3.
Blood pressure and lipid levels at baseline and 3 y.
3.5. Vitamins and minerals
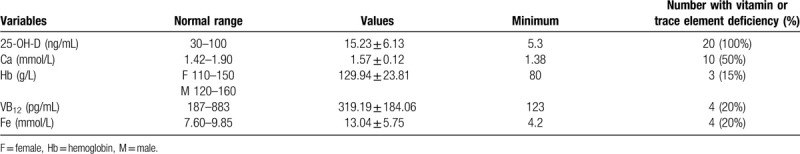
Table 4 showed vitamins and mineral content of the patients. All of the 20 patients had vitamin D deficiency, and the mean 25 (OH) D was (15.23 ± 6.14) ng/mL. Ten patients (50%) had calcium deficiency, and the average calcium level was (1.57 ± 0.12) mmol/L. Most patients with vitamin D and calcium deficiency reported back and leg pain. Besides, 3 females (15%) had anemia (Hb≤110 g/L), with hemoglobin of 80 g/L, 90 g/L, and 101 g/L, of which 2 were megaloblastic anemia and 1 hypoferric anemia, with vitamin B12 and serum iron deficiency. In total 4 patients (20%) had vitamin B12 deficiency, of whom 2 had anemia. 4 patients (20%) had serum iron deficiency, of whom 3 female patients had anemia. The average vitamin A, vitamin E, folic acid, zinc, magnesium, copper, and albumin were within normal range.
Table 4.
Vitamins and trace elements of the patients in 3-y after surgery.
3.6. Quality of life
There were no significant differences in age (49.05 ± 11.01 y vs. 47.50 ± 9.99 y), gender (male 45% vs male 50%), or T2DM duration (6.75 ± 4.1 y vs. 5.35 ± 2.6 y) between control and surgery group (P > .05). There are 8 domains in the SF-36, including physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). Scores in each domain of the SF-36 range from 0 to 100, and the higher scores indicated better health. Patients in control group had higher score in role emotional (P = .829) and lower score in the other 7 aspects, and the differences were significant in physical functioning, role physical, and general health (P < .05) (Table 5, Fig. 1).
Table 5.
Quality of life in surgery and control group.
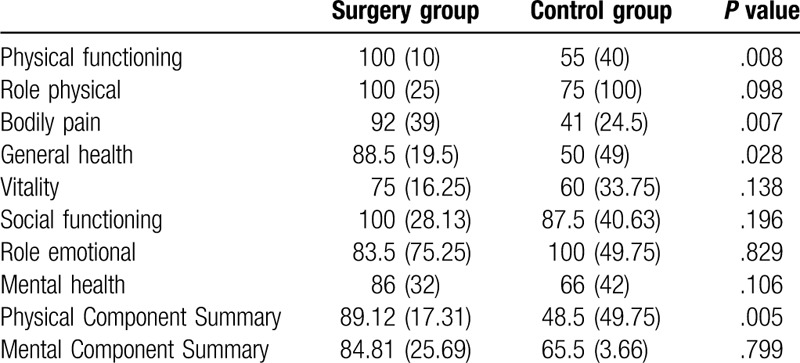
Figure 1.
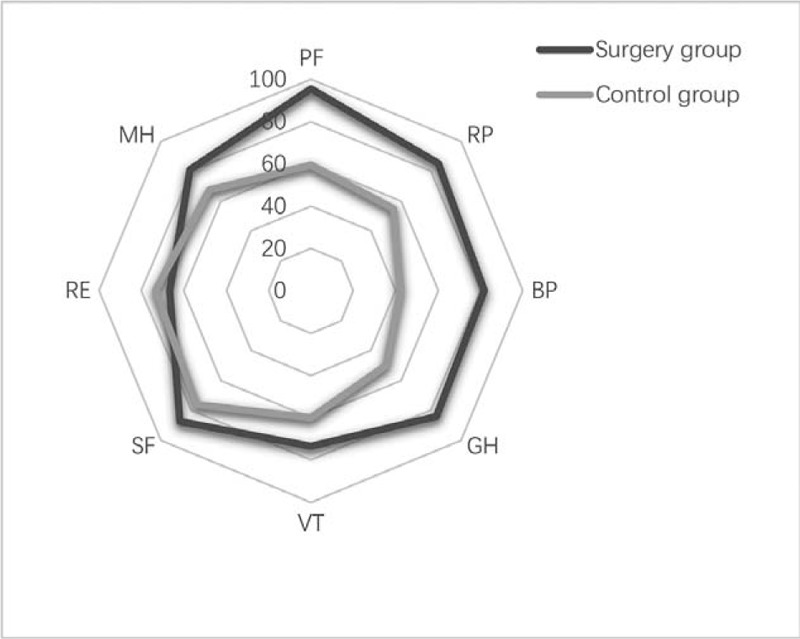
Quality of life in surgery and control group patients.
3.7. Comparison of SG and RYGB
There were no statistical differences in blood glucose, body weight, islet function, lipid levels, blood pressure, and QoL between RYGB and SG (P > .05). In addition, there were no statistical differences in vitamins and minerals between RYGB and SG (see Supplemental Digital Content (Table S1), which compares SG and RYGB in vitamins and minerals). However, we found some different features between them. All of the 20 patients had vitamin D deficiency, and the mean vitamin D and calcium levels were similar in 2 cohorts. Three females with anemia are all performed RYGB, and the mean hemoglobin, folic acid, vitamin b12, and iron are lower in RYGB cohort. There were no statistical differences in vitamins and micronutrients levels.
4. Discussion
Obesity has been a field of intense research in the last decades.[20] Development of bariatric surgery in China is rather late and limited, especially in the northeast region. Understanding and identifying long-term efficacy and adverse events may provide evidence to choose the proper treatment and predict the prognosis.
The results of this study showed that body weight and glucose improved significantly after bariatric surgery. According to previous studies,[7,21,22] the efficiency of bariatric surgery is better than lifestyle interventions and medications, which is consistent with the conclusions of this study. Weight regain is a great concern for endocrinology doctors and bariatric surgeons. It has been reported that 10% to 35% of the patients will experience weight regain after surgery.[23] In this study, 50% of the patients had weight regain 3 years after the operation, the mean of weight regain after surgery was 31.45% of maximum weight loss. The possible reasons can be summarized as follows: patients had less food intake for a short period of time after surgery, but they might recover gradually; since the absorption of nutrients is greatly reduced after surgery, the residual stomach and small intestine may automatically increase digestion and absorption capacity due to the compensatory mechanism in the human body; most of the patients with obesity and diabetes hold an unhealthy life style such as higher sugar intake and less physical activities, which may lead to weight regain. It is reported patients who regained weight after surgery had poor diet quality, characterized by excessive intake of calories, snacks, sweets, fatty foods, and higher intake of high glycemic index carbohydrates.[24] Therefore, physicians should provide more educations and supervisions, emphasizing the importance of lifestyle interventions such as diet and exercise, and pursue patients to develop a healthy life style. It can be foreseen that if there is no lifestyle intervention, the rebound of weight would be more obvious.
This study showed that islet function also improved significantly. Possible mechanism could be the decrease of body weight and glucose leads to less burden of β cells, alleviates the vicious circle of hyperglycemia-β cell failure, thus promoting the islets function.
In this study, 1 patient has HbA1C above 7.5% and used metformin combined with insulin during the follow-up. This 57-year-old female patient has a poor islet function and regained 10 kg in 3-year after surgery. Therefore, it is necessary to make a golden inclusion criterion and exclude the patients with old age and poor islet function.
The RYGB and SG are most commonly performed operations. In the past, RYGB was the most recorded operation,[25,26] but RYGB removed most of the stomach and small intestines, which affected the absorption of vitamins and minerals.[27] Contrarily, due to SG is technically simpler and has fewer gastrointestinal anatomy alterations and perioperative complications, the number of SG is increasing greatly in the recent years.[28,29] Previous studies had found that there were no statistically significant differences between SG and RYGB in weight loss, T2DM remission and QoL improvement.[30,31]
In previous studies, the incidence of nutrient deficiencies was different due to different surgical methods, nutrient supplementations, personal compliances, and eating habits.[32–34] In this study, all patients had vitamin D deficiency, which is much higher than previous studies,[35,36] and the possible reasons are as follows: stomach and small intestines are the main area that absorbs vitamin D and calcium; lipid malabsorption leads to the lack of fat-soluble vitamins; northeastern people have less outdoor activities and less exposure to ultraviolet radiation due to cold weather, which affects the endogenous synthesis of vitamin D. Three females (15%) had anemia, of whom 2 cases are megaloblastic anemia, 1 case is iron deficiency anemia, with vitamin B12 and serum iron deficiency, respectively. In addition, all these 3 patients performed RYGB, and the mean hemoglobin, folic acid, vitamin b12, iron are lower in RYGB cohort. The reason of iron and vitamin B12 deficiency is not only the reduction of the absorption area, but also the reduction of gastric acid secretion that leads to a decrease in the bioavailability of vitamin B12 and iron. Furthermore, some patients have bacterial overgrowth in the small intestine that leads to vitamin deficiencies after RYGB.[37] Previous studies showed that vitamin B12 and iron deficiency occurs much more often in RYGB after surgery, which is similarly to our results.[27,38]
Nutrient deficiencies were common in obesity or T2DM patients, so nutrition assessment and malnutrition treatment are necessary before operation. More importantly, physicians should provide supplementation immediately after surgery. Generally, all the patients should receive lifelong vitamin and mineral screening and supplementation, and a higher dose is necessary in the RYGB patients. Various organizations recommended that vitamin D supplement doses of 3000 IU/d to 50,000 IU 1 to 3 times per week are needed.[39] Additionally, the efficiency of supplementation is different according to patients’ compliance. In this study, only 50% of patients follow the doctor's advice to take supplement regularly. Therefore, long-term follow-up and education are needed to adjust supplementation according to systematic periodical assessment of patients.
Quality of life covers many aspects, including physical health and mental health. Obesity and T2DM affect the QoL significantly, and even lead to depression and suicide. In this study, patients in the surgical group scored higher than control group, especially in terms of physical functioning, role physical, and general health, which is consistent with previous studies.[40,41] The possible reasons are as follows: bariatric surgery could reduce body weight, waist circumference, and improve various restrictions caused by obesity in daily activities, including climbing stairs, running, bending, and etc.; surgery could decrease glucose, reduce medications and diabetes complications, then improve patients’ judgment on self-health; we found that the main causes of body pain in patients are gout, knee pain, waist and back pain, and the symptoms could be improved with weight loss. In terms of mental health, the scores of patients in the surgical group were also higher than control group, but the difference was not statistically different. The possible reasons are as follows: Psychology status is affected by many aspects, including the individual's temperament and psychological quality, and these would not change greatly after surgery; due to the small number of cases in this study, the results of statistical analysis may have some deviation.
There are some limitations in this study: The quality of life and nutrients were not collected at baseline, so the conclusions may have bias; The data collection of body nutrition content is insufficiency, and there is a lack of data on hyperparathyroidism and osteoporosis; Sample size is small and follow-up time is short, so we need more clinical cases and longer follow-up to further clarify the curative effect and complication.
5. Conclusions
Our study showed that bariatric surgery is an effective approach to treat obesity and T2DM. The body weight, glucose, and islet function of the patients improved significantly after surgery, but there seemed to be a rebound in 3-year. Furthermore, the surgery could improve the blood pressure, lipids, and the quality of life, especially physical health significantly. However, some patients may suffer anemia, calcium deficiency, iron deficiency, vitamin D and vitamin B12 deficiency after 3-year. Further studies with larger sample size and more sufficient data are needed to confirm these findings.
Acknowledgments
The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version. The authors gratefully acknowledge all volunteers who participated in their research.
Author contributions
Didi Zuo: Investigation, Data extraction, Methodology, Software, Formal analysis, Writing-original draft
Guixia Wang & Guang Ning: Project administration, Supervision
Shuo Yang: Methodology, Writing-original draft
Xianchao Xiao: Data extraction, Software, Validation
Yuan Gao: Investigation
Supplementary Material
Footnotes
Abbreviations: 2h-CP = 2-hour postprandial C-peptide, 2h-PG = 2-hour postprandial plasma glucose, BMI = body mass index, DBP = diastolic blood pressure, EBMIL% = percentage of excess BMI loss, EWL% = percentage of excess weight loss, FCP = fasting C-peptide, FPG = fasting plasma glucose, HbA1c = glycated hemoglobin, QoL = quality of life, RYGB = Roux-en-Y Gastric Bypass, SBP = systolic blood pressure, SF-36 = The MOS 36 Item Short Form Health Survey, SG = sleeve gastrectomy, T2DM = type 2 diabetes mellitus.
How to cite this article: Zuo D, Xiao X, Yang S, Gao Y, Wang G, Ning G. Effects of bariatric surgery in Chinese with obesity and type 2 diabetes mellitus: A 3-year follow-up. Medicine. 2020;99:34(e21673).
The data used to support the findings of this study are included within the article, which are available from the corresponding author upon request.
This work was supported by the Nature Science Foundation of the Jilin Science and Technology Department (NO. 20160101126JC), and the Innovative Capability Project of Jilin Province Development and Reform Commission (NO. 2017C019).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59. [DOI] [PubMed] [Google Scholar]
- [2].Group NDR. A mass survey of diabetes mellitus in a population of 300,000 in 14 provinces and municipalities in China (author's transl). Zhonghua Nei Ke Za Zhi 1981;20:678–83. [PubMed] [Google Scholar]
- [3].Pan XR, Yang WY, Li GW, et al. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care 1997;20:1664–9. [DOI] [PubMed] [Google Scholar]
- [4].Gu D, Reynolds K, Duan X, et al. Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). Diabetologia 2003;46:1190–8. [DOI] [PubMed] [Google Scholar]
- [5].Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:2425–6. [PubMed] [Google Scholar]
- [6].Dixon JB, Blazeby JM. Quality of life after bariatric surgery. Lancet Diabetes Endocrinol 2014;2:100–2. [DOI] [PubMed] [Google Scholar]
- [7].Simonson DC, Halperin F, Foster K, et al. Clinical and patient-centered outcomes in obese patients with Type 2 diabetes 3 years after randomization to Roux-en-Y gastric bypass surgery versus intensive lifestyle management: the SLIMM-T2D study. Diabetes Care 2018;41:670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA 2018;319:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Salminen P, Helmio M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA 2018;319:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yan Y, Sha Y, Yao G, et al. Roux-en-Y gastric bypass versus medical treatment for type 2 diabetes mellitus in obese patients: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Freeland-Graves JH, Lee JJ, Mousa TY, et al. Patients at risk for trace element deficiencies: bariatric surgery. J Trace Elem Med Biol 2014;28:495–503. [DOI] [PubMed] [Google Scholar]
- [12].Billeter AT, Probst P, Fischer L, et al. Risk of malnutrition, trace metal, and vitamin deficiency post Roux-en-Y gastric bypass—a prospective study of 20 patients with BMI < 35 kg/m(2). Obes Surg 2015;25:2125–34. [DOI] [PubMed] [Google Scholar]
- [13].Khiyani N, Tulchinsky M, Hava S, et al. Gastric emptying scintigraphy results may influence the selection of the type of bariatric surgery: a cohort study. Medicine (Baltimore) 2019;98:e17205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681–8. [DOI] [PubMed] [Google Scholar]
- [15].Bragg F, Tang K, Guo Y, et al. Associations of general and central adiposity with incident diabetes in Chinese men and women. Diabetes Care 2018;41:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liang H, Guan W, Yang Y, et al. Roux-en-Y gastric bypass for Chinese type 2 diabetes mellitus patients with a BMI < 28 kg/m(2): a multi-institutional study. J Biomed Res 2015;29:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pak J, Kwon Y, Lo ME, et al. Impact of gastrointestinal bypass on nonmorbidly obese type 2 diabetes mellitus patients after gastrectomy. Surg Obes Relat Dis 2015;11:1266–72. [DOI] [PubMed] [Google Scholar]
- [18].Panunzi S, De Gaetano A, Carnicelli A, et al. Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: do BMI or procedure choice matter? A meta-analysis. Ann Surg 2015;261:459–67. [DOI] [PubMed] [Google Scholar]
- [19].Baig SJ, Priya P, Mahawar KK, et al. Weight regain after bariatric surgery-a multicentre study of 9617 patients from Indian Bariatric Surgery Outcome Reporting Group. Obes Surg 2019;29:1583–92. [DOI] [PubMed] [Google Scholar]
- [20].Zhao N, Tao K, Wang G, et al. Global obesity research trends during 1999 to 2017: a bibliometric analysis. Medicine (Baltimore) 2019;98:e14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Inge TH, Laffel LM, Jenkins TM, et al. Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr 2018;172:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 2017;376:641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jirapinyo P, Abu DB, Thompson CC. Weight regain after Roux-en-Y gastric bypass has a large negative impact on the Bariatric Quality of Life Index. BMJ Open Gastroenterol 2017;4:e000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alvarez V, Carrasco F, Cuevas A, et al. Mechanisms of long-term weight regain in patients undergoing sleeve gastrectomy. Nutrition 2016;32:303–8. [DOI] [PubMed] [Google Scholar]
- [25].Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822–32. [DOI] [PubMed] [Google Scholar]
- [26].Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg 2018;28:3783–94. [DOI] [PubMed] [Google Scholar]
- [27].Bailly L, Schiavo L, Sebastianelli L, et al. Anemia and bariatric surgery: results of a national French survey on administrative data of 306,298 consecutive patients between 2008 and 2016. Obes Surg 2018;28:2313–20. [DOI] [PubMed] [Google Scholar]
- [28].Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg 2019;29:782–95. [DOI] [PubMed] [Google Scholar]
- [29].Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS Randomized Clinical Trial. JAMA 2018;319:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ignat M, Vix M, Imad I, et al. Randomized trial of Roux-en-Y gastric bypass versus sleeve gastrectomy in achieving excess weight loss. Br J Surg 2017;104:248–56. [DOI] [PubMed] [Google Scholar]
- [31].Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012;308:1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bazuin I, Pouwels S, Houterman S, et al. Improved and more effective algorithms to screen for nutrient deficiencies after bariatric surgery. Eur J Clin Nutr 2017;71:198–202. [DOI] [PubMed] [Google Scholar]
- [33].Caron M, Hould FS, Lescelleur O, et al. Long-term nutritional impact of sleeve gastrectomy. Surg Obes Relat Dis 2017;13:1664–73. [DOI] [PubMed] [Google Scholar]
- [34].Li L, Yu H, Liang J, et al. Meta-analysis of the effectiveness of laparoscopic adjustable gastric banding versus laparoscopic sleeve gastrectomy for obesity. Medicine (Baltimore) 2019;98:e14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mihmanli M, Isil RG, Isil CT, et al. Effects of laparoscopic sleeve gastrectomy on parathyroid hormone, vitamin D, calcium, phosphorus, and albumin levels. Obes Surg 2017;27:3149–55. [DOI] [PubMed] [Google Scholar]
- [36].Shah M, Sharma A, Wermers RA, et al. Hypocalcemia after bariatric surgery: prevalence and associated risk factors. Obes Surg 2017;27:2905–11. [DOI] [PubMed] [Google Scholar]
- [37].Majumder S, Soriano J, Louie CA, et al. Vitamin B12 deficiency in patients undergoing bariatric surgery: preventive strategies and key recommendations. Surg Obes Relat Dis 2013;9:1013–9. [DOI] [PubMed] [Google Scholar]
- [38].Antoniewicz A, Kalinowski P, Kotulecka KJ, et al. Nutritional deficiencies in patients after Roux-en-Y gastric bypass and sleeve gastrectomy during 12-month follow-up. Obes Surg 2019;29:3277–84. [DOI] [PubMed] [Google Scholar]
- [39].Versteegden D, Van Himbeeck M, Nienhuijs SW. Improvement in quality of life after bariatric surgery: sleeve versus bypass. Surg Obes Relat Dis 2018;14:170–4. [DOI] [PubMed] [Google Scholar]
- [40].Sarwer DB, Wadden TA, Spitzer JC, et al. 4-Year changes in sex hormones, sexual functioning, and psychosocial status in women who underwent bariatric surgery. Obes Surg 2018;28:892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thereaux J, Lesuffleur T, Czernichow S, et al. Long-term adverse events after sleeve gastrectomy or gastric bypass: a 7-year nationwide, observational, population-based, cohort study. Lancet Diabetes Endocrinol 2019;7:786–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


