Abstract
Although a strong association between idiopathic inflammatory myositis (IIM) and malignancy has been widely reported, few studies have solely focused on the concurrence of dermatomyositis (DM) and malignancies (DM-malignancy).
We conducted a retrospective analysis of 37 DM-malignancy cases among 363 DM patients admitted to our hospital between January 2012 and December 2017.
(1) The mean age at DM diagnosis was higher for DM-malignancy patients than for DM-non-malignancy patients [(54.76 ± 9.77) years vs (48.57 ± 12.82) years, t = 2.84, P = .005]. (2) Gynecological malignancies (35.90%/14 cases) were the most common malignancies. Malignancies were diagnosed before DM for 7 DM-malignancy patients. The interval between the DM and malignancy diagnoses for the remaining 32 DM-malignancy patients was less than 6 months for 18 patients (46.15%), less than 1 years for 23 patients (58.9%), and less than 2 years for 29 patients (74.26%). (3) There was no significant difference either in antinuclear antibody or anti-Ro-52 positivity between the 2 groups (P > .05). (4) Multivariate analysis demonstrated that DM onset age ≥50 years and concurrence with ILD increased the risk of death for DM patients [hazard ratio (HR): 1.62 and 2.72; 95% confidence interval (CI): (1.08–2.43) and (1.47–5.02); P = .02 and 0.001, respectively], and male gender decreased the risk of death [HR 0.66, 95% CI (0.44–0.98), P = .04]. DM-malignancy patients were older than DM-non-malignancy patients. Gynecological malignancies were the most common malignancies among these patients. A DM onset age ≥50 years, female sex and the presence of ILD were independent risk factors for death.
Keywords: dermatomyositis, malignancy, prognosis, serum biomarkers
1. Introduction
The strong association between idiopathic inflammatory myositis (IIM) and malignancy has been widely reported since the first case report in 1916.[1–9] Dermatomyositis (DM) patients were found to be more likely to have a malignancy than polymyositis (PM) patients.[10] Malignancy is diagnosed more frequently within the first year of a DM diagnosis than at other time points.[3,6,11,12] Serum autoantibodies might be related to the risk of malignancy in DM patients (DM-malignancy). Hoesly et al reported that negative antinuclear antibody (ANA) results were associated with an underlying malignancy risk among DM patients.[13] Furthermore, with the widespread use of myositis antibody profiles in recent years, anti-transcriptional intermediary factor (anti-TIF1-γ) has been suggested to be a risk factor for malignancy in DM patients.[14–17] There were some differences in the clinical manifestations and prognosis of patients with malignancy and PM (PM-malignancy) and DM-malignancy.[9] Although some of these studies separately described the PM and DM groups, the relevant information and discussions on DM-malignancy and PM-malignancy patients were combined in most studies.[1,4,6,7,11,18–27] Interstitial lung disease (ILD) has been regarded as a common and important complication among DM patients,[28] and ILD and malignancy have seldomly been simultaneously discussed in regard to the prognostic analysis of DM patients.[29] We conducted this retrospective analysis of patients with DM-malignancy among 363 DM patients who were admitted to our hospital between January 2012 and December 2017, focusing on serum biomarkers and whether or not these conditions were combined with ILD.
2. Methods
2.1. Patients enrollment for the study
The electronic medical records from Peking Union Medical College Hospital (PUMCH) from January 2012 to December 2017 were searched for “dermatomyositis”. Three hundred sixty-three DM cases were identified according to the 1975 Bohan and Peter criteria,[30,31] including symmetrical proximal muscle weakness, typical DM cutaneous lesions (heliotrope rash, Gottron's macules/papules), elevated serum skeletal muscle enzymes, myopathic electromyography pattern, and muscle biopsy with characteristic pathological changes. All patients had typical DM cutaneous lesions.
The complete medical records, including follow-up information, radiological imaging and pathological reports, of all 363 enrolled patients were retrospectively reviewed in this study. Follow-up information was obtained from outpatient medical records or telephone conversations with the patients and/or their emergency contacts. The final follow-up date was June 30, 2018. Survival time was defined as the time from the diagnosis of DM to death or to June 30, 2018. ILD was defined as the presence of the hallmark manifestations on chest high-resolution computed tomography (CT) (HRCT).[32] All patients underwent chest HRCT scanning. Malignancy was identified with a definite pathological diagnosis.
2.2. Associated autoantibodies and serum tumor markers in the study
The ANA profiles were analyzed in the clinical laboratory department of PUMCH. The myositis autoantibody profiles were measured at Dean Diagnostic Technology Co., Ltd. (Beijing, China). Both ANA and myositis autoantibody profiles were assessed by an immunoblotting assay using a EUROIMMUN AG kit (Euroline ANA profile assay and myositis antigens profile, Germany).
Detailed ANA profiles were analyzed for all 363 DM patients. The ANA profiles included an ANA, anti-double stranded DNA (anti-dsDNA) antibody, anti-Sm antibody, anti-SSA antibody, anti-SSB antibody, anti-Scl-70 antibody, anti-histidyl-tRNA synthetase (anti-Jo-1) antibody, anti-ribosomal RNA-protein (anti-rRNP) antibody, anti-proliferating cell nuclear Ag (anti-PCNA) antibody, anti-histone antibody (AHA), anti-Ro-52 antibody, PM-Scl, anti-u1 small-nuclear antibody (ANuA), a circulating anti-centromere antibody B (anti-CENP B), and an anti-mitochondrial antibody (AMA)-M2.
Myositis autoantibody profiles, that is, 16 anti-autoimmune inflammatory myopathy Ags, as assessed by “EUROIMMUN AG”, have been used widely in our hospital since 2015. Therefore, the myositis autoantibody profiles were tested for only 174 patients. Anti-Jo-1, anti-threonyl-tRNA synthetase (anti-PL7), anti-alanyl-tRNA synthetase (anti-PL12), anti-glycyl tRNA synthetase (anti-EJ), anti-isoleucyl-tRNA synthetase (anti-OJ), anti-melanoma differentiated-associated protein 5 (anti-MDA-5), anti-Mi-2α, anti-Mi-β, anti-TIF1 γ, anti-NXP2, anti-SAE1, anti-signal recognition particle (SRP), anti-RO-52, anti-PM-SCL-75, anti-PM-SCL100 and anti-Ku were included in the myositis autoantibody profiles.
Detailed serum tumor marker screening profiles were analyzed in the clinical laboratory department at PUMCH. A peripheral blood sample was taken within two months (m) after the diagnosis of DM/PM. Serum tumor marker screening profiles included carcinoembryonic Ag (CEA), carbohydrate Ag-125 (CA125), CA153, CA242, CA724, CA19-9, α-fetoprotein (AFP), cytokeratin-19 fragment (CYFRA21-1), neuron-specific enolase (NSE), squamous-cell cancer Ag (SCCAg), and tissue polypeptide specific (TPS) Ag. The normal ranges for the tumor markers were CEA < 5.0 ng/ml, CA125 < 35.0 U/ml, CA15-3 < 25 U/ml, CA242 < 20 U/ml, CA724 < 9.8 U/ml, CA19-9 < 34.0 U/ml, AFP < 12 ng/ml, CYFRA21-1 < 3.5 ng/ml, NSE < 16.3 ng/ml, SCCAg < 2.7 ng/ml, and TPS Ag < 80 U/l. All patients except for 16 patients with DM-non-malignancy underwent serum carbohydrate Ag (CA) profile screening.
This study was approved by the ethics committee of PUMCH (S-K870) and was performed in accordance with the principles of the Declaration of Helsinki. All patients and/or their relatives provided written informed consent.
2.3. Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 23.0 software package. Quantitative variables (age) are presented as the mean ± the standard deviation (SD), and categorical data are presented as frequencies and percentages. A t test or rank sum test was used for measurement data, a chi-square test was used for the count data, and a difference was defined as statistically significant when P < .05. Cox regression models were used to identify risk factors associated with mortality for DM patients. The log-rank test was used to compare the survival rates of different subgroups, and Kaplan-Meier survival curves were plotted.
3. Results
3.1. Demographic and general characteristics of the enrolled DM-malignancy patients
A total of 37 cases (10.19%) of malignancy developed among the 363 DM patients. The general characteristics of the enrolled cases were described in Table 1. Two patients suffered two different malignancies: breast cancer combined with vaginal cancer in 1 patient and colon cancer combined with thyroid cancer in another patient. Gynecological malignancies accounted for 35.90% (14 cases) of malignancies and were the most common malignancies in our study. A detailed description of the malignancies in our study is shown in Figure 1A.
Table 1.
General characteristics of the enrolled dermatomyositis (DM) cases.

Figure 1.
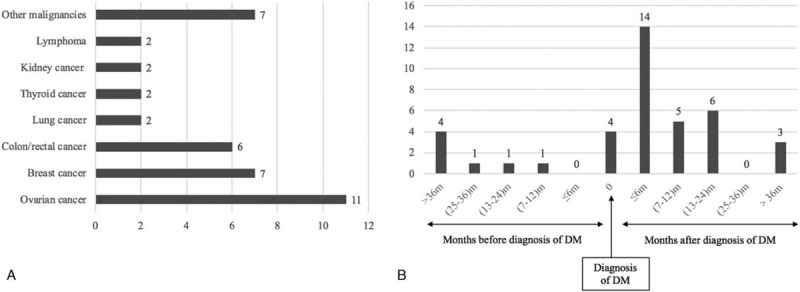
(A) Types of malignancies in the enrolled dermatomyositis patients. (B) The temporal association between the diagnosis of malignancies and dermatomyositis.
The temporal association between DM and malignancy is summarized in Figure 1B. There were only 7 DM-malignancy patients whose malignancies were diagnosed before DM. The remaining 32 malignancies were diagnosed after or at the same time as the DM diagnosis. The interval between these diagnoses was less than 6 m for 18 patients (46.15%), less than 1 year for 23 patients (58.9%), and less than 2 years for 29 patients (74.26%).
There were 9 males among the 37 DM-malignancy patients and 96 males among the 326 DM-non-malignancy patients. The gender ratio between these two groups was not significantly different (P > .05). The mean age at the diagnosis of DM for DM-malignancy patients was higher than that for DM-non-malignancy patients [(54.76 ± 9.77) years vs (48.57 ± 12.82) years, t = 2.84, P = .005].
3.2. Predictive value of serum tumor markers for DM-malignancy patients
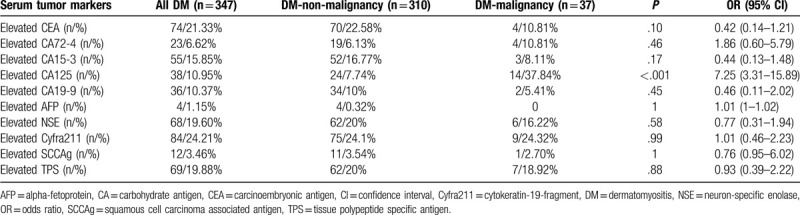
It is well reported that the risk of malignancy is strongly associated with IIM, so cancer screening is performed for almost all admitted IIM patients in our hospital. All patients except for 16 DM-non-malignancy patients underwent serum CA profile screening. These 16 patients were all diagnosed with rapidly progressive ILD (RP-ILD) and died of respiratory failure in less than 1 month. The serum CA profile results for the remaining 347 patients are summarized in Table 2. Serum CA125 was the only serum tumor marker that was significantly elevated in DM-malignancy patients (P < .001).
Table 2.
Diagnostic value of serum tumor markers for DM-malignancy cases.
3.3. Autoantibody analysis for DM-malignancy patients
3.3.1. Serum ANA profiles for the enrolled patients
All DM patients underwent ANA profile analysis. When an ANA titration ≥1:80 was defined as ANA positivity, the ANA positivity rate was 51.4% (19 patients) in the DM-malignancy group (n = 37) and 53.4% (174 patients) in the DM-non-malignancy group (n = 326). There was no significant difference in ANA positivity between the 2 groups (P > .05). If the definition of ANA positivity complied with the criteria from the 2015 Interstitial Pneumonia with Autoimmune Features (IPAF) statement,[33] the ANA positivity rate was 8.1% (3 patients) in the DM-malignancy group (n = 37) and 15.9% (52 patients) in the DM-non-malignancy group (n = 326). There was no difference in the ANA positivity rate between these 2 groups (P > .05).
Anti-RO-52 positivity is common among DM patients. Anti-RO-52 positivity was defined as “++” to “+++” among the anti-RO-52 results. The anti-RO-52 positivity rate was 29.7% (11 patients) in the DM-malignancy group (n = 37) and 44.5% (145 patients) in the DM-non-malignancy group (n = 326). There was no significant difference between the 2 groups (P > .05).
3.3.2. Myositis autoantibody profiles for the enrolled patients
The myositis autoantibody profiles were analyzed for 174 of the 363 DM patients. Three DM patients among the 17 patients with anti-TIF-1-γ positivity developed a malignancy. None of 9 patients with positive anti-NXP2 results and none of 5 patients with positive anti-SRP results developed a malignancy. None of the patients had anti-SAE-1 positivity. There were 68 patients with anti-MDA-5 positivity, and none of these patients had a malignancy.
3.3.3. Concurrence with ILD
ILD is a common complication of DM, and ILD is a poor prognostic factor for DM patients. There were 267 patients (77.84%) who were diagnosed with DM-associated ILD (DM-ILD) in our cohort. The incidence of malignancy in the DM-ILD group (12 patients/4.49%) was lower than that in the DM-non-ILD group (25 patients/26%) (χ2 = 35.81, P < .001).
3.3.4. Prognostic analysis of DM patients
The mean follow-up period was 27.1 months, ranging from 1 to 77 months. There were 33 patients (9.09%) who were lost to follow-up, 104 patients (28.65%) died, and 226 patients (62.26%) were stable or improved. Among the dead IIM cases, 11 cases were combined with malignancies. Eight cases were died of the progression of malignancies, and 3 cases were died of respiratory failure because of the progression of basic ILD.
The age of patients who survived was lower than that of the patients who died [(47.78 ± 12.83) years vs (52.58 ± 11.22) years, t = 3.29, P < .01]. In our DM group, the mean age at the diagnosis of DM for DM-malignancy group was (54.76 ± 9.77) years and for DM-non-malignancy group was (48.57 ± 12.82) years. Age and sex difference might be related to the prognosis or DM,[13] and combination of ILD and/or malignancy are poor prognostic factors for DM. So, a DM onset age ≥50 years, different sex ratio and the presence of ILD or malignancy were selected as likely prognostic factors for our enrolled IIM cases.
The univariate analysis and Cox proportional hazards regression analysis showed that a DM onset age ≥50 years, female sex and the presence of ILD were independently associated with the risk of death among DM patients (Tables 3 and 4). The Kaplan-Meier survival curve (Fig. 2) showed that the prognosis of patients with DM-malignancy and ILD was worse than that of DM patients without ILD, whether or not DM was combined with malignancies (with malignancies, P = .009; without malignancies, P < .001). However, there was no difference between patients with DM-malignancy and ILD and patients with DM-non-malignancy and ILD (χ2 = 3.32, P = .07).
Table 3.
Univariate analysis of death risk factors for dermatomyositis cases.
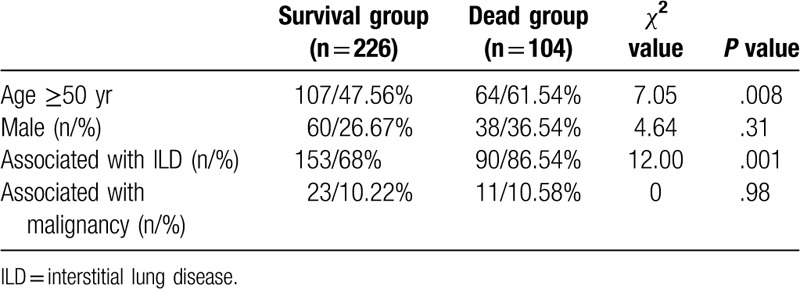
Table 4.
Multivariate analysis of death risk factors for dermatomyositis cases.
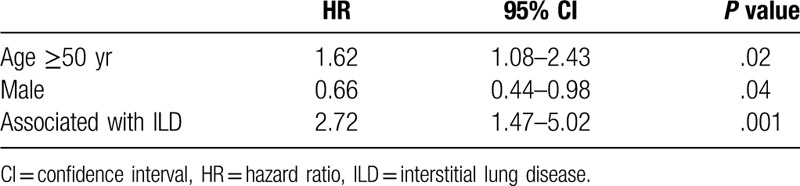
Figure 2.
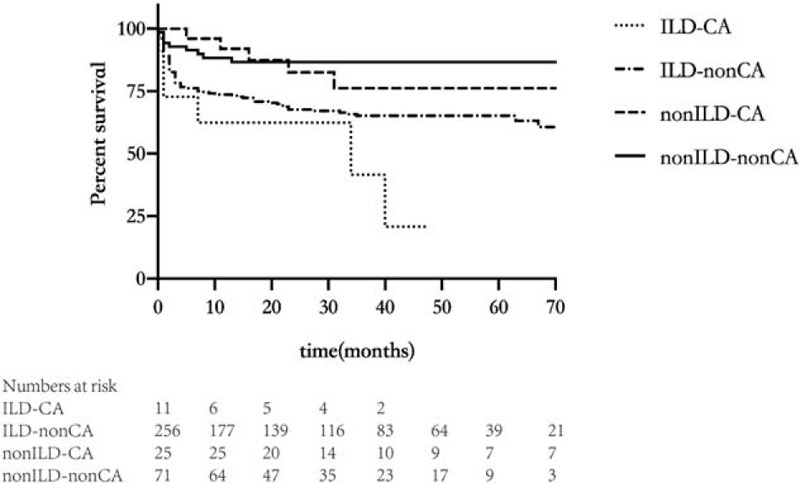
Kaplan-Meier survival analysis for the enrolled dermatomyositis patients: the prognosis of patients with DM-malignancy and ILD (ILD-CA) was worse than that of DM patients without ILD, whether (non-ILD-CA) or not DM (non-ILD-non-CA) was combined with malignancies (with malignancies, P = .009; without malignancies, P < .001). However, there was no difference between patients with DM-malignancy and ILD (ILD-CA) and patients with DM-non-malignancy and ILD (ILD-non-CA) (χ2 = 3.32, P = .07).
4. Discussion
The incidence of malignancy among DM patients varied among patients from different countries, and the reported incidence ranged from 11.7% to 39.4%.[3,12,18,20,23,29,34] The rate of malignancy in our DM group was 10.19%, which is similar to the previously reported incidence in a Chinese cohort, which ranged from 12.8% to 24.4%.[3,5,23,34] Older age might be related to the concurrence of malignancy and DM. In a Scottish cohort, no cancers were reported for DM patients who were younger than 15 years, and the standardized incidence ratio (SIR) was 3.6 for DM patients who were 45 to 74 years old.[18] Qiang's meta-analysis showed that the relative risk of malignancy was 2.79 (95% CI 1.73–4.5) and 3.13 (95% CI 2.65–3.19) for those younger or older than 45, respectively.[11]. Although malignancy was more common for DM patients who were younger than 50 years in a DM cohort from Taiwan,[23] the malignancy risk increased with age in most previous studies (>40 or >45).[2,3,12,18,29] Our DM-malignancy patients were older than our DM-non-malignancy patients, and DM patients over 50 years old had a worse prognosis than DM patients under 50 years old.
On the other hand, the reported malignancy predisposition for different genders was varied in different studies. Qiang's meta-analysis showed that the relative risk of malignancy was 5.29 (95% CI 3.48–8.04) and 4.56 (95% CI 2.97–7.02) for males and females, respectively, among DM patients.[11] Although Sigurgeirsson et al showed that female DM patients were susceptible to malignancy,[1] Antiochos et al showed that male DM patients were more prone to malignancy.[12] Both Stockton's and Yun's studies reported that there was no difference in the incidence of malignancies among male and female DM patients.[18,29] In our study, the incidence of malignancy was higher for older DM patients. However, in the survival analysis, male DM patients seemed to have a better prognosis.
Though the interval between the diagnosis of malignancies and DM varied in different studies, most malignancies were diagnosed within 1 year of the diagnosis of DM, and a large majority of malignancies were diagnosed within 2 years of the diagnosis of DM.[3,12,18,20,34] Stockton's meta-analysis showed that there was a 17.3-fold increase in the risk of malignancy within 1 year of the diagnosis of DM, which decreased to 1.37-fold (95% CI 1.27–1.48) at an interval of >5 years between the diagnosis of DM and malignancy.[18] In a Scottish cohort, most of the malignancies were diagnosed within 3 m of the diagnosis of DM, and the majority of malignancies were diagnosed within 2 years of the diagnosis of DM.[18] In Guangzhou's study, the DM diagnosis was made before the diagnosis of malignancy in 101 patients (87.8%), and there were 77 patients (76.5%) with an interval between the diagnosis of DM and malignancy of 1 year or less.[3] In a report from northern New England in the US, the interval between the diagnosis of DM and malignancy was within 1 year in 75% of patients and was within 2 years in 87.5% of DM-malignancy patients.[12] In our group, most of the malignancies were diagnosed after or at the same time as the DM diagnosis: 46.15% of patients were diagnosed within 6 m, 58.97% patients within 1 year and 74.36% patients within 2 years.
The type of malignancy that was combined with DM varied in different studies, including among different regions, races and genders. Nasopharyngeal cancer was more commonly reported in Hong Kong, Taiwan and Guangzhou,[3,23] especially among male DM patients. Gastric cancer was more common in a DM-malignancy cohort from South Korea.[29] In a Scottish report and in Liu's report, lung cancer was the most commonly reported malignancy.[18,34] In a Swedish cohort, ovarian cancer, breast cancer and colon cancer were the most common malignancies among female DM-malignancy patients; however, lung cancer, colon cancer and pancreatic cancer were more common for male DM-malignancy patients.[1] Breast cancer was the most commonly reported malignancy for female DM patients in Antiochos’ and Liu's studies.[12,34] However, ovarian cancer was the most commonly reported malignancy for female European DM patients.[1,20] Most of the ovarian cancers were at an advanced stage when they were detected through screening examinations after the diagnosis of DM.[35] Ovarian cancer (11 patients; 28.2%) was the most common malignancy in our group, and all except for 2 of the patients were diagnosed because of an elevation of serum CA125 during the screening tests. These patients underwent surgery and subsequent chemotherapy. The mean follow-up duration was 47.91 ± 28.86 months, ranging from 13 to 77 months. Three of these patients died due to cancer progression, and the other patients are still alive. Whitmore et al suggested that serial serum CA125 and endovaginal ultrasound examinations might be efficient screening tests for ovarian cancer in DM patients.[36] If the screening tests are abnormal, gynecological consultations are suggested.
There are few studies on the role of serum CA profile screening for the prediction of malignancy in DM patients. CA125, CA199 and CA153 might have predictive value for screening solid tumors in DM patients; the predictive value of serum CA125 was particularly high for ovarian cancer.[2,36,37] However, Ponyi et al suggested that serum tumor markers seemed to be useless for detecting malignancy in DM patients.[2,38] Serum CA profiles were performed for the majority of our enrolled DM patients, and serum CA125 was the only significantly elevated serum tumor marker. Serum CA125 seemed to have a high predictive value for ovarian cancer in our DM patients.
Serum ANA profiles are normally analyzed for DM patients. Ponyi et al reported that the extractable nuclear Ag (ENA) profile positivity rate was significantly higher in DM patients without malignancy.[2] Hoesly et al's study showed that ANA negativity was associated with an increased likelihood of a malignancy diagnosis within 3 years of a DM diagnosis (43% vs 11%, P < .001).[13] However, there was no significant difference between the positivity rate of ANA and ENA for the DM patients with or without malignancies in Antiochos et al's and Yun et al's studies.[12,29] The detailed serum ANA profiles were analyzed for all of our DM patients, and there was no significant association between the positivity of anti-ANA or anti-Ro-52 antibodies between the DM patients with or without malignancies.
Myositis specific antibodies (MSA) have been recently associated with different clinical phenotypes and/or prognoses of DM,[39] and the positivity of some specific MSAs, including anti-SAE1, anti-TIF1-γ and anti-NXP2 antibodies, has predictive value for malignancies in DM patients.[14,15,17,22,24,26,40] Although anti-SAE1, anti-TIF1-γ and anti-NXP2 antibodies might be associated with an increased cancer risk among IIM patients,[26,41] data concerning the associations between anti-NXP-2 and anti-SAE with cancer are controversia.[42–44] Myositis autoantibody profiles have been widely used in our hospital since 2015, so nearly half of the enrolled DM patients (47.9%) underwent myositis autoantibody profile analysis. There were 17 patients with anti-TIF-1γ autoantibody positivity, and 3 of these patients (17.6%) developed malignancies. In addition, there was no DM patients with positive anti-NXP2 results who had malignancies, and none of our DM patients had positive anti-SAE1 results. With the more widespread use of serum myositis autoantibody profiles for DM patients over much longer follow-up periods, an association between DM-malignancy and positivity for specific MSAs might be found.
ILD is a common complication among DM patients and is also an important prognostic factor for these patients[28] On the other hand, ILD patients have a predisposition to have concurrent malignancies. Patients who were less than 60 years old with connective tissue disease-associated ILD (CTD-ILD) were more likely to have concurrent malignancies than CTD-only patients.[45] However, studies on the association between ILD and malignancy in DM patients have shown that the presence of a malignancy occurs less frequently in the DM-ILD group than in the non-DM-ILD group.[2,12,29,34] In our cohort, 77.84% of DM patients had ILD. The incidence rate of malignancies in these DM-ILD patients was lower than that in non-DM-ILD patients (4.49% vs 26%, P < .001).
Malignancy is an important poor prognostic factor for DM patients and is also the main cause of death among these patients.[2,29,34] However, few studies have examined the association of ILD and malignancies with the prognosis of DM patients. Both RP-ILD and malignancy seemed to be risk factors for death in DM patients in Woo et al's study.[29] In our cohort, a DM onset age ≥50 years, female sex and the presence of ILD were independently associated with the risk of death for DM patients, but the presence of a malignancy was not an independent prognostic factor for DM patients. A larger cohort with a longer follow-up is needed for the prognostic analysis of DM patients.
There are several limitations in our study. Firstly, all enrolled patients had a definite diagnosis of malignancy and DM. And all had complete clinical records, radiological images and pathological specimen, which could cause a selection bias. Secondly, the treatment strategies might be varied for DM cases who were admitted in different departments, including respiratory department, rheumatology department, and/or general internal department. Thirdly, not all enrolled cases were analyzed for myositis autoantibody profiles. The association between MSA and DM-malignancy couldn’t be analyzed well in our study.
5. Conclusions
DM-malignancy patients were older than DM-non-malignancy patients. Gynecological malignancies were the most common malignancy among our DM patients. And the incidence of malignancies in DM-ILD patients was lower than that in DM-non-ILD patients. However, a DM onset age of ≥50 years of age, female sex and the presence of ILD were independent risk factors for death.
Acknowledgments
Thanking Professor Weijiang Hu (Chinese Center for Disease Control and Prevention, Beijing, China) for the statistical consultation.
Author contributions
H.H takes responsibility for the content of the manuscript, including the data and analysis. H.H, C.S. and S.L conceived and designed the study; YX.S, S.L, C.S, K.X. Y.Z. and X.Z. performed the study; H.H, C.S. and S.L. analyzed the data and wrote the paper.
Footnotes
Abbreviations: AFP = α-fetoprotein, AHA = anti-histone antibody, ANA = antinuclear antibody, anti-AMA = anti-mitochondrial antibody, anti-CENP B = anti-centromere antibody B, anti-dsDNA = anti-double stranded DNA, anti-EJ = anti-glycyl tRNA synthetase, anti-Jo-1 = anti-histidyl-tRNA synthetase antibody, anti-MDA-5 = anti-melanoma differentiated-associated protein 5, anti-OJ = anti-isoleucyl-tRNA synthetase, anti-PCNA = anti-proliferating cell nuclear Ag antibody, anti-PL-12 = anti-alanyl-tRNA synthetase, anti-PL7 = anti-threonyl-tRNA synthetase, anti-rRNP = anti-ribosomal RNA-protein antibody, anti-TIF1-γ = anti-transcriptional intermediary factor, ANuA = anti-u1 small-nuclear antibody, CA = carbohydrate Ag, CEA = carcinoembryonic Ag, CTD-ILD = connective tissue disease-associated ILD, CYFRA21-1 = cytokeratin-19 fragment, DM = dermatomyositis, ENA = extractable nuclear Ag, HRCT = high-resolution computed tomography, IIM = idiopathic inflammatory myositis, ILD = interstitial lung disease, IPAF = interstitial pneumonia with autoimmune features, MSA = myositis specific antibodies, NSE = neuron-specific enolase, PM = polymyositis, PUMCH = Peking Union Medical College Hospital, RP-ILD = rapidly progressive ILD, SCCAg = squamous-cell cancer Ag, SD = standard deviation, SIR = standardized incidence ratio, SPSS = Statistical Package for the Social Sciences, TPS = tissue polypeptide specific Ag.
How to cite this article: Shao C, Li S, Sun Y, Zhang Y, Xu K, Zhang X, Huang H. Clinical characteristics and prognostic analysis of Chinese dermatomyositis patients with malignancies. Medicine. 2020;99:34(e21899).
CS and SL contributed equally to this work.
This work was supported by the Chinese National Natural Science Fund Youth Fund project [grant number 81600050], and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences [grant number 2019XK320037].
The authors have no conflicts of interest to disclose
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med 1992;326:363–7. [DOI] [PubMed] [Google Scholar]
- [2].Ponyi A, Contantin T, Garami M, et al. Cancer-associated myositis. Ann N Y Acad Sci 2005;1051:64–71. [DOI] [PubMed] [Google Scholar]
- [3].Zhang W, Jiang SP, Huang L. Dermatomyositis and malignancy: a retrospective study of 115 cases. Eur Rev Med Pharmaco Sci 2009;13:77–80. [PubMed] [Google Scholar]
- [4].Ungprasert P, Leeaphorn N, Hosiriluck N, et al. Clinical features of inflammatory myopathies and their association with malignancy: a systematic review in Asian population. ISRN Rheumatol 2013;2013:509354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen D, Yuan S, Wu X, et al. Incidence and predictive factors for malignancies with dermatomyositis: a cohort from southern China. Clin Exp Rheumatol 2014;32:615–21. [PubMed] [Google Scholar]
- [6].Yang Z, Lin F, Qin B, et al. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol 2015;42:282–91. [DOI] [PubMed] [Google Scholar]
- [7].Lu X, Yang H, Shu X, et al. Factors predicting malignancy in patients with polymyositis and dermatomyositis: a systematic review and meta-analysis. PLoS One 2014;9:e94128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Olazagasti JM, Baez PJ, Wetter DA, et al. Cancer risk in dermatomyositis: a meta-analysis of cohort studies. Am J Clin Dermatol 2015;16:89–98. [DOI] [PubMed] [Google Scholar]
- [9].Tiniakou E, Mammen AL. Idiopathic inflammatory myopathies and malignancy: a comprehensive review. Clin Rev Allergy Immunol 2017;52:20–33. [DOI] [PubMed] [Google Scholar]
- [10].Callen JP, Hyla JF, Bole GG, Jr, et al. The relationship of dermatomyositis and polymyositis to internal malignancy. Arch Dermatol 1980;116:295–8. [PubMed] [Google Scholar]
- [11].Qiang JK, Kim WB, Baibergenova A, et al. Risk of malignancy in dermatomyositis and polymyositis. J Cutan Med Surg 2017;21:131–6. [DOI] [PubMed] [Google Scholar]
- [12].Antiochos BB, Brown LA, Li Z, et al. Malignancy is associated with dermatomyositis but not polymyositis in Northern New England, USA. J Rheumatol 2009;36:2704–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoesly PM, Sluzevich JC, Jambusaria-Pahlajani A, et al. Association of antinuclear antibody status with clinical features and malignancy risk in adult-onset dermatomyositis. J Am Acad Dermatol 2019;80:1364–70. [DOI] [PubMed] [Google Scholar]
- [14].Ogawa-Momohara M, Muro Y, Mitsuma T, et al. Strong correlation between cancer progression and anti-transcription intermediary factor 1γ antibodies in dermatomyositis patients. Clin Exp Rheumatol 2018;36:990–5. [PubMed] [Google Scholar]
- [15].Best M, Molinari N, Chasset F, et al. Use of anti-transcriptional intermediary actor-1gamma autoantibody in identifying adult dermatomyositis patients with cancer: a systematic review and meta-analysis. Acta Derm Venereol 2019;99:256–62. [DOI] [PubMed] [Google Scholar]
- [16].Aussy A, Fréret M, Gallay L, et al. The IgG2 isotype of anti-transcription intermediary factor 1γ antibodies is a biomarker of cancer and mortality in adult dermatomyositis. Arthritis Rheumatol 2019;71:1360–70. [DOI] [PubMed] [Google Scholar]
- [17].Trallero-Araguás E, Rodrigo-Pendás JÁ, Selva-O’Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum 2012;64:523–32. [DOI] [PubMed] [Google Scholar]
- [18].Stockton D, Doherty VR, Brewster DH. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: a Scottish population-based cohort study. Br J Cancer 2001;85:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med 2001;134:1087–95. [DOI] [PubMed] [Google Scholar]
- [20].Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 2001;357:96–100. [DOI] [PubMed] [Google Scholar]
- [21].Wakata N, Kurihara T, Saito E, et al. Polymyositis and dermatomyositis associated with malignancy: a 30-year retrospective study. Int J Dermatol 2002;41:729–34. [DOI] [PubMed] [Google Scholar]
- [22].Chinoy H, Fertig N, Oddis CV, et al. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis 2007;66:1345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang YL, Chen YJ, Lin MW, et al. Malignancies associated with dermatomyositis and polymyositis in Taiwan: a nationwide population-based study. Br J Dermatol 2009;161:854–60. [DOI] [PubMed] [Google Scholar]
- [24].Ichimura Y, Matsushita T, Hamaguchi Y, et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann Rheum Dis 2012;71:710–3. [DOI] [PubMed] [Google Scholar]
- [25].Kang EH, Lee SJ, Ascherman DP, et al. Temporal relationship between cancer and myositis identifies two distinctive subgroups of cancers: impact on cancer risk and survival in patients with myositis. Rheumatology (Oxford) 2016;55:1631–41. [DOI] [PubMed] [Google Scholar]
- [26].Yang H, Peng Q, Yin L, et al. Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: a large longitudinal cohort study. Arthritis Res Ther 2017;19:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev 2017;16:1049–57. [DOI] [PubMed] [Google Scholar]
- [28].Kamiya H, Panlaqui OM, Izumi S, et al. Systematic review and meta-analysis of prognostic factors for idiopathic inflammatory myopathy-associated interstitial lung disease. BMJ Open 2018;8:e023998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Woo JH, Kim YJ, Kim JJ, et al. Mortality factors in idiopathic inflammatory myopathy: focusing on malignancy and interstitial lung disease. Mod Rheumatol Mod Rheumatol 2013;23:503–8. [DOI] [PubMed] [Google Scholar]
- [30].Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. [DOI] [PubMed] [Google Scholar]
- [31].Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–7. [DOI] [PubMed] [Google Scholar]
- [32].American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplineary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- [33].Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976–87. [DOI] [PubMed] [Google Scholar]
- [34].Liu Y, Xu L, Wu H, et al. Characteristics and predictors of malignancy in dermatomyositis: analysis of 239 patients from northern China. Oncol Lett 2018;16:5960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Davis MD, Ahmed I. Ovarian malignancy in patients with dermatomyositis and polymyositis: a retrospective analysis of fourteen cases. J Am Acad Dermatol 1997;37:730–3. [DOI] [PubMed] [Google Scholar]
- [36].Whitmore SE, Anhalt GJ, Provost TT, et al. Serum CA-125 screening for ovarian cancer in patients with dermatomyositis. Gynecol Oncol 1997;65:241–4. [DOI] [PubMed] [Google Scholar]
- [37].Amoura Z, Duhaut P, Huong DL, et al. Tumor antigen markers for the detection of solid cancers in inflammatory myopathies. Cancer Epidemiol Biomarkers Prev 2005;14:1279–82. [DOI] [PubMed] [Google Scholar]
- [38].Lim CH, Tseng CW, Lin CT, et al. The clinical application of tumor markers in the screening of malignancies and interstitial lung disease of dermatomyositis/polymyositis patients: a retrospective study. SAGE Open Med 2018;6:2050312118781895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McHugh NJ, Tansley SL. Autoantibodies in myositis. Nat Rev Rheumatol 2018;14:290–302. [DOI] [PubMed] [Google Scholar]
- [40].Oldroyd A, Sergeant JC, New P, et al. The temporal relationship between cancer and adult onset anti-transcriptional intermediary factor 1 antibody-positive dermatomyositis. Rheumatology (Oxford) 2019;58:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lu X, Peng Q, Wang G. The role of cancer-associated autoantibodies as biomarkers in paraneoplastic myositis syndrome. Curr Opin Rheumatol 2019;31:643–9. [DOI] [PubMed] [Google Scholar]
- [42].Zhong L, Yu Z, Song H. Association of anti-nuclear matrix protein 2 antibody with complications in patients with idiopathic inflammatory myopathies: a meta-analysis of 20 cohorts. Clin Immunol 2019;198:11–8. [DOI] [PubMed] [Google Scholar]
- [43].Cassius C, Le Buanec H, Bouaziz JD, et al. Biomarkers in adult dermatomyositis: tools to help the diagnosis and predict the clinical outcomes. J Immunol Res 2019;2019:9141420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gunawardena H, Wedderburn LR, Chinoy H, et al. Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum 2009;60:1807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Choi WI, Lee DY, Choi HG, et al. Lung cancer development and mortality in interstitial lung disease with and without connective tissue disease: a five-year Nationwide population-based study. Respir Res 2019;20:117. [DOI] [PMC free article] [PubMed] [Google Scholar]


