Abstract
Background:
A meta-analysis was performed to evaluate the relationship between chronic obstructive pulmonary disease (COPD) and occupational dust exposure, and to provide a scientific basis for the prevention and treatment of COPD caused by occupational factors.
Methods:
PubMed and Embase databases were used to search for original epidemiological literature related to theme. Both random and fixed effects models were used to calculate pooled odds ratios and their corresponding 95% confidence intervals. Review Manager was used to perform data analysis.
Results:
Nine studies were included in the meta-analysis in accordance with the inclusion criteria. There was a significantly obvious correlation between occupational dust exposure and COPD of the population-based studies assessed in this article. The risk of developing COPD for workers exposed to dust was 1.51 times higher than for controls (I2 = 40%, 95% confidence interval: 1.27-1.79). The presence of publication bias was not found.
Conclusion:
The study provided evidence supporting the association between occupational dust exposure and the risk of developing COPD.
Keywords: inorganic dust, organic dust, occupational exposure, chronic obstructive pulmonary disease
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common and preventable disease characterized by extensive exposure to toxic particles or gases, resulting in persistent airway symptoms and airflow limitation caused by airway and alveolar abnormalities. COPD is a public health challenge worldwide and is currently the third leading cause of death from cardiovascular and cerebrovascular diseases.[1] Smoking is the most common risk factor for COPD. Exposure to occupational and environmental risk factors and indoor material fuel combustion can also affect the occurrence and progression of the disease.[2] In 2003, the American Thoracic Society conducted an evaluation of the accumulated evidence related to the role of occupational factors in the pathogenesis of obstructive airway disease. This systematic review concluded that about 15% of cases of COPD could be attributed to occupational exposure.[3] Recently published studies of COPD estimated that between 25% and 45% of patients with COPD were non-smokers.[4] In addition, many systematic evaluations have concluded that there was indeed a causal relationship between occupational exposure and COPD.[5–9]
Productive dust refers to solid microparticles generated by human production activities that can float in the production environment for a long period of time. Productive dust comes from industrial and agricultural production industries, such as mining, machinery processing, smelting, construction materials, textiles, road construction, hydropower, and food industries. According to the nature of dust, it can be classified as inorganic dust, organic dust, and mixed dust. Inorganic dust includes mineral dust, metal dust, and artificial inorganic dust; organic dust includes biological dust, plant dust, and animal dust. It is a major harmful occupational hazard that pollutes the working environment and harms the health of workers, leading to the development of various occupational lung diseases. In 1985, Margaret Becklake stated that occupational dust exposure may be causally associated with the pathogenesis of COPD.[10,11] Coggon and Newman Taylor[12] analyzed the literature on airflow obstruction of coal miners, and concluded that there was an obvious correlation between exposure to coal dust and the development of chronic airflow obstruction.
Therefore, in-depth research on the role of occupational dust exposure in the occurrence and development of COPD is of great significance for reducing its incidence and alleviating its disease burden. The correlation between occupational dust exposure and COPD risk was further evaluated based on prior analyses and using new data from published literature. In this meta-analysis, epidemiologic studies were assessed to identify new information on the correlation between occupational dust exposure and COPD.
2. Methods
2.1. Literature search
A systemic search of PubMed and Embase was performed of the COPD and occupational dust exposure literature for articles published between 2009 and 2019. Medical subject headings used as search terms were: [“COPD” or “chronic obstructive pulmonary disease”] and [“occupational exposure”, “dust”]. Only English language publications were included in the analysis. In addition, the literature traceability method was used to assist in the search as much as possible to find detailed information. First, a preliminary screening was carried out to assess literature titles and abstracts. For those studies whose titles and abstracts were not clear and those for which there was uncertainty about whether the exclusion criteria were met, the full text was screened and the unqualified literature were excluded according to the inclusion and exclusion criteria. If more than 2 articles were published reporting on the same study, a more comprehensive literature was selected for reporting. Eligible articles were reviewed by 2 researchers (CP, YC), and the following information was extracted independently: first author; year of publication; study region; study type; age range; sample size; type of exposure evaluation; type of dust; diagnostic criteria; adjustments made based on age and smoking status; and outcome indicators. Two reviewers (CP, YC) evaluated the validity of each study for inclusion; if there was no mutual agreement, a third reviewer (ZL) was used.
2.2. Selection criteria
A study was included if it met all of the following criteria: an epidemiological study that examined the correlation between COPD and occupational dust exposure, and reported outcome indicators adjusted for odds ratios (ORs) based on age and smoking status, with 95% confidence intervals (CIs).
The exclusion criteria were:
-
(1)
no external or internal control group;
-
(2)
inadequate analysis of the correlation between occupational dust exposure and lung function definition results;
-
(3)
no analysis of subject age or smoking exposure;
-
(4)
no recorded measurements of lung function, with only recorded forced expiratory volume in 1 second (FEV1) data.
Many studies evaluate COPD related to occupational risk factors differently. Measurements based on the following criteria should be considered optimal: the Global Initiative for COPD diagnostic criteria should be used rather than other criteria; objective assessments should be performed based on expert assessment or a job exposure matrix (JEM) rather than subjective assessments, such as self-reports and summary questionnaires.
2.3. Quality evaluation of included studies
Methodological quality evaluations of the included case-control and cross-sectional studies were performed based on the Newcastle-Ottawa Scale.[13] The items of the Newcastle-Ottawa scale to assess cohort studies were modified to allow for cross-sectional evaluation.
2.4. Ethical review
As this was a systematic review and meta-analysis, the ethical approval was waived and not necessary in this study.
2.5. Statistical analysis
RevMan 5.2 software was used for data analysis; it reported the pooled effects as ORs. To determine the heterogeneity of the study, the Higgins I2 was calculated. I2 represents the percentage of total variation in the study, with 0%, 25%, 50%, and 75% of the I2 values indicating no, low, medium, and high heterogeneity, respectively.[14] If the I2 value was greater than 50%, then there was considerable heterogeneity for a given study. When there was large heterogeneity, a random effects model was selected to compute the pooled OR. When there was no obvious heterogeneity, a fixed effects model was selected to calculate the pooled OR.[15]
RevMan 5.2 software was also used for sensitivity analysis and subgroup analysis to assess the stability of the literature and the credibility of the results. Begg funnel plots were applied to check whether there was a publication bias across the studies. There was a publication bias when the funnel plot was asymmetrical. In such cases, we used the trim method to check if the effect changed before and after the trimming to determine whether the results were stable.
3. Results
3.1. Characteristic of participants
Figure 1 shows the selection process for studies suitable for meta-analysis, which was performed based on PRISMA guidelines. A total of 505 articles were obtained from the initial search, including 334 from the PubMed database and 171 from the Embase database. After removing duplicate studies, based on screening topics and abstracts, 36 articles were eligible for inclusion. Of these, 27 were excluded based on the inclusion/exclusion criteria; finally nine were included for the analysis.[16–24]
Figure 1.
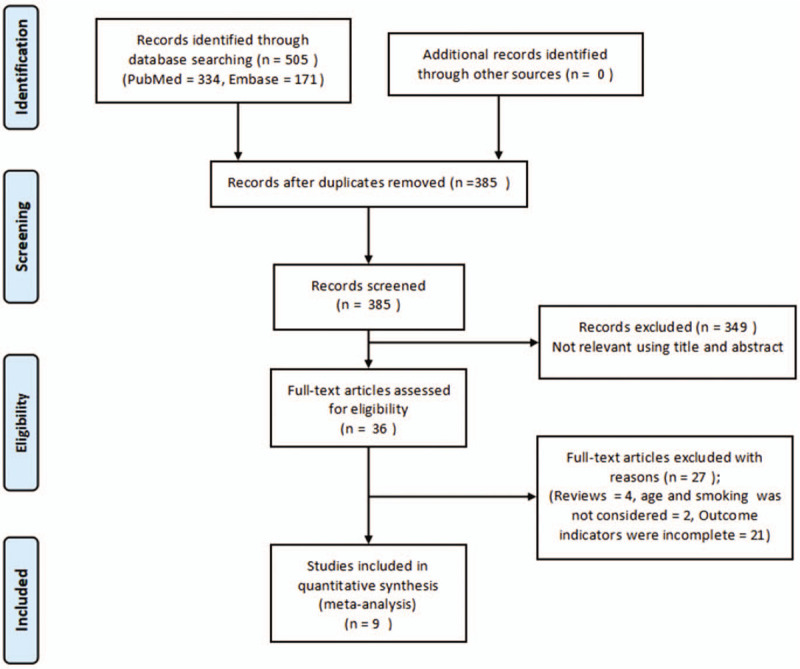
Select strategy flow chart for article quantity at different stages.
The characteristics of the included trials are summarized in Table 1. In the included studies, there were 7 cross-sectional and 2 case-control studies. For the cross-sectional studies, the quality score ranged from 6 to 8 (Table 2); for the case-control studies, the scores ranged from 7 to 8 (Table 3).
Table 1.
The characteristics of included studies.
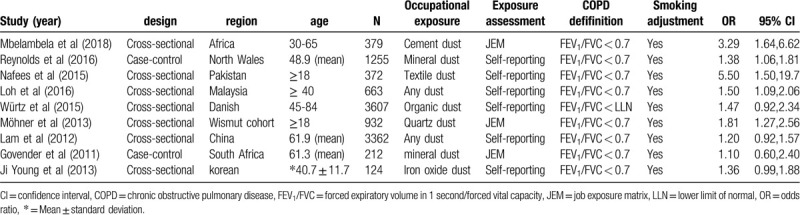
Table 2.
Methodological quality of included cross-sectional studies based on the modified NOS for cohort study.
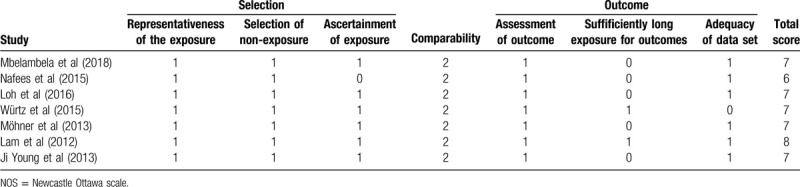
Table 3.
Methodological quality of included case-control studies based on NOS.

3.2. Meta-analysis
Studies have shown that occupational exposure to dust was obviously correlated with COPD. The pooled OR of the 9 studies calculated using the random effects model was 1.51 (95% CI: 1.27-1.79) (Fig. 2). The heterogeneity of the included studies was moderate (I2 = 40%). In this meta-analysis, publication bias was not observed (Fig. 3).
Figure 2.
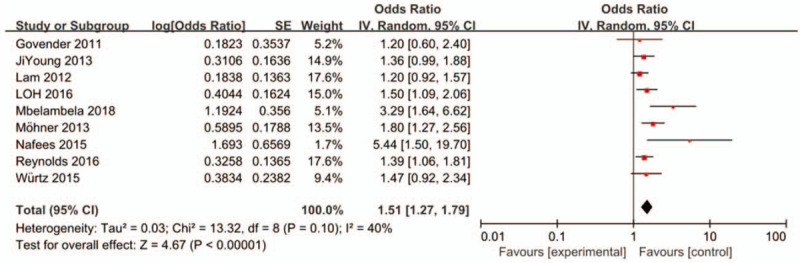
Forrest plot of the association between occupational dust exposure and COPD in meta-analysis. The random effects model was used to analyze the data of 9 articles. CI = confidence interval, df = degrees of freedom, IV = inverse variance; SE = standard error.
Figure 3.
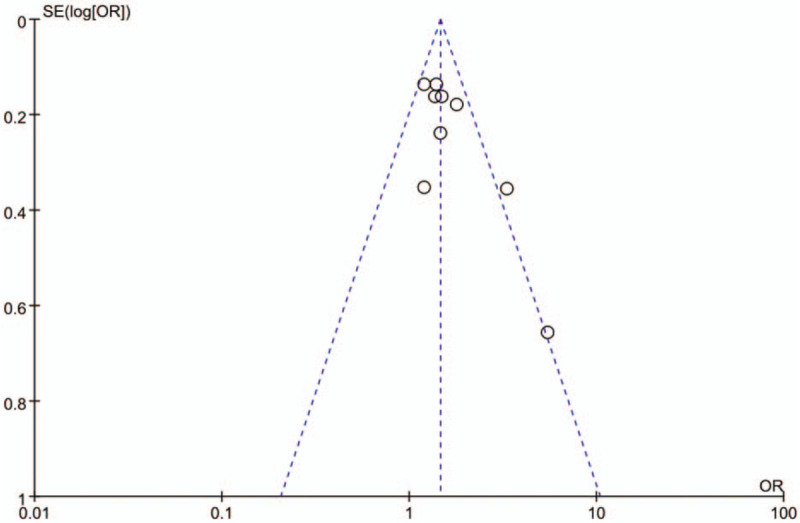
Funnel plot for identifying publication bias.
3.3. Sensitivity analysis and subgroup analysis
In order to evaluate the stability of the results of this study, the included studies were excluded in turn and the pooled OR was re-evaluated. After excluding data from studies by Mbelambela[16] and Nafees[18] from our analysis, the heterogeneity score of the meta-analysis was reduced (I2 = 0%) (Fig. 4). The result suggests that Mbelambela and Nafees's research was sensitive and unstable. This also indicated that the results from the 7 other studies were consistent; calculated using the fixed effects model, the OR was 1.40 (95% CI: 1.24-1.59)
Figure 4.
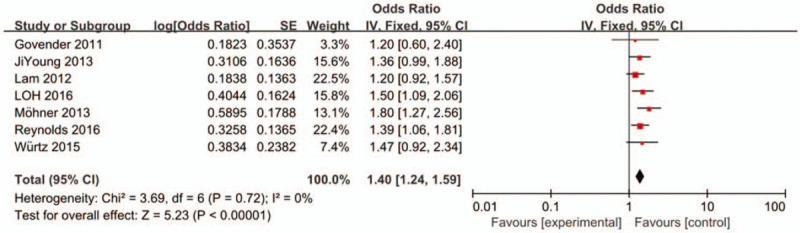
Association between occupational dust exposure and COPD in fixed effects model after excluding Mbelambela and Nafees's study. CI = confidence interval, df = degrees of freedom, IV = inverse variance; SE = standard error.
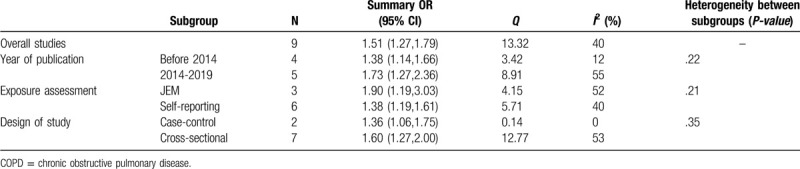
In order to explore the source of heterogeneity, the data were analyzed and compared according to the studies’ characteristics. Based on publication year, exposure evaluation type, and design of study, each subgroup demonstrated an obvious positive correlation, but the discrepancy of correlation between groups was not statistically significant (P > .05) (Table 4).
Table 4.
Subgroup analysis of the association between occupational dust exposure and COPD.
3.4. Analysis of different exposure concentrations
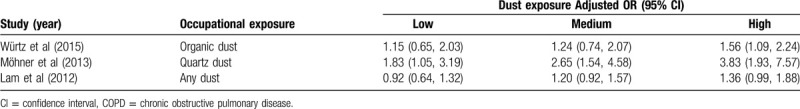
Among the 9 studies, 3 listed the dust exposure concentration; these were divided into 3 groups with low, medium, and high exposure levels. It can be seen from the results that the higher the dust exposure concentration, the higher the sensitivity to COPD (Table 5).
Table 5.
Association between occupational dust exposure and COPD at different exposure concentration.
4. Discussion
Meta-analyses differ from traditional systematic reviews in that they use quantitative data to merge results, thereby increasing the confidence that can be placed in the conclusion. This meta-analysis combined survey data from multiple studies and merged them using statistical methods to improve the statistical analysis of the original data. The reliable conclusion of this meta-analysis is that the risk of COPD due to occupational dust exposure is much greater than that of the unexposed control group.
The degree of decrease in lung function may be related to the time of occupational dust exposure. After excluding 2 studies from the sensitivity analysis, the results were not heterogeneous. Therefore, after re-reading the articles of Mbelambela and Nafees, it was found that the outcome measures selected by these 2 studies required participants to have worked for more than 5 years; the remaining 7 studies did not require participants to have been exposed for a long period of time. From the results, the OR value of the 2 studies was much greater than the overall OR value. In addition, Iftikhar[25] conducted a cross-sectional study of 160 workers who worked for more than 5 years and were exposed to inhalable free silica dust. The severity of the symptoms was related to the duration of exposure. Workers who have been exposed to silica dust for 5 to 10 years, 11 to 15 years, and 16 to 20 years account for 26.0, 31.5, and 49.0% of cases, respectively, so it can be inferred that the longer the working hours, the more likely 1 is to suffer from COPD.
In addition, the degree of lung function decline depends not only on the exposure time, but also on the intensity of dust exposure. Three studies that classified and analyzed the dust concentrations were separately extracted. These 3 studies divided the dust exposure concentrations into 3 levels: low, medium, and high. It can be seen from Table 5 that the OR value increases with increasing dust exposure concentration. Monsó[26] conducted research on 105 non-smoking animal breeders working in enclosed buildings, showing that higher organic dust concentrations in enclosed buildings may be the main factor that causes breeders to suffer from COPD. The OR (95% CI) value was 5.38 (1.17 - 24.74) when exposed to high concentrations of organic dust. After adjusting for covariates such as temperature, humidity, area, and endotoxin presence in indoor pollutants in an enclosed building, the OR (95% CI) value was 6.60 (1.10 - 39.54), which was still statistically significant. Therefore, it can be inferred that the higher the dust concentration, the greater the probability of developing COPD.
Most COPD patients had a history of past or current smoking; that is, their COPD was attributed to smoking, ignoring the pathogenic effects of occupational exposure. At the same time, age is also a risk factor for COPD. Therefore, adjustments were made for confounding factors such as smoking and age. After excluding such confounding factors, a significant positive correlation between dust and COPD could still be seen. Studies have shown that smoking and occupational dust exposure have a synergistic effect on respiratory symptoms, but the synergistic effect of smoking and occupational dust exposure on COPD was not statistically significant, which may be related to the small sample size.[27] The specific reason needs to be further studied with larger samples.
For subgroup analyses based on publication year, exposure evaluation type, and design of study, each subgroup demonstrated an obvious positive correlation, but no significant discrepancies were observed in association with these elements. In 9 studies, 3 used JEM and 6 used self-reporting to evaluate occupational dust exposure. It can be argued that using JEM for assessment was less subjective than self-reporting because self-reporting is prone to recall bias and classification errors.[28] However, self-reporting can also be used as 1 of the assessment tools.[29,30] There was no difference in association between the self-reporting and the JEM groups. The meta-analysis consisted of 2 case-control studies and 7 cross-sectional studies. Some studies have found that the correlation between occupational dust exposure and COPD risk was influenced by study design.[31] However, subgroup analysis showed no statistically significant differences between the 2, which may be related to the differences in sample sizes among different studies.
There are some limitations with this meta-analysis. First, there was moderate heterogeneity. The possible reasons include the differences in sampling methods, age ranges, and sample sizes among the studies. Second, many studies only assessed workers’dust exposure, and the included studies could not assess the influence on workers leaving the working environment with severe COPD. The duration of exposure was not considered and the true risk exposure of COPD was underestimated. Third, lung function was the most objective measurement method, which can improve the diagnostic rate of patients with early stage COPD, but the actual application of FEV1/FVC (forced vital capacity) is also flawed. Although expiratory velocity has a good sensitivity, its specificity is weak.[32,33] The use of the fixed FEV1/FVC ratio to define airflow limitation may result in more frequent diagnosis of COPD in the elderly, and less frequent diagnosis in adults <45 years, especially in mild disease cases, compared to using a cut-off based on the lower limit of normal values for FEV1/FVC.[34,35] Therefore, the measurement of expiratory peak velocity alone cannot be reliably used as the only diagnostic indicator. The age of onset of COPD associated by occupational risk factors is earlier than the age of onset of general COPD, and missed diagnosis is more likely to occur. Therefore, the correlation between dust and COPD is underestimated. Finally, most of the included studies were cross-sectional studies; there were no high-quality prospective cohort studies, so the highest level of evidence supporting causal inference was not strong. Confirming the causes of occupational dust exposure as they relate to COPD identified by epidemiological studies requires a prospective cohort study with a large sample size.
5. Conclusion
In conclusion, there was a consistent correlation between occupational dust exposure and COPD, which was independent of smoking status and age. Since the pathogenic relationship between occupational dust and COPD is still unclear, more large-scale epidemiological and experimental studies are needed to confirm the causal relationship between the 2 variables. In addition, because the exposure concentration, exposure time, and nature of dust are difficult to accurately assess, the personal protection of dust workers is particularly important for the prevention and treatment of COPD.
Acknowledgment
We thank the financial support from the Key Research and Development Plan of Shandong Province [grant numbers 2019GSF111025].
Author contributions
Conceptualization: Yongjian Yan.
Data curation: Cong Peng, Yu Cai, Zhen Li.
Formal analysis: Cong Peng, Yongjian Yan.
Funding acquisition: Yongjian Yan.
Investigation: Yuxin Jiang.
Methodology: Cong Peng, Zhen Li, Yongjian Yan.
Project administration: Yongjian Yan.
Supervision: Yongjian Yan.
Writing – original draft: Cong Peng.
Writing – review & editing: Cong Peng, Yongjian Yan.
Footnotes
Abbreviations: CI = confidence interval, COPD = Chronic obstructive pulmonary disease, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, JEM = job exposure matrix, LLN = lower limit of normal, NOS = Newcastle Ottawa scale, OR = odds ratio.
How to cite this article: Peng C, Yan Y, Li Z, Jiang Y, Cai Y. Chronic obstructive pulmonary disease caused by inhalation of dust: a meta-analysis. Medicine. 2020;99:34(e21908).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest 2011;139:752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Paulin LM, Diette GB, Blanc PD, et al. Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir CritCare Med 2015;191:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Balmes J, Becklake M, Blanc P, et al. American thoracic society statement: occupational contribution to the burden of airway disease. Am J Respir Crit Care Med 2003;167:787–97. [DOI] [PubMed] [Google Scholar]
- [4].Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009;374:733–43. [DOI] [PubMed] [Google Scholar]
- [5].Blanc PD, Torén K. Occupation in chronic obstructive pulmonary disease and chronic bronchitis: an update. Int J Tuberc Lung Dis 2007;11:251–7. [PubMed] [Google Scholar]
- [6].Hendrick DJ. Occupational and chronic obstructive pulmonary disease (COPD). Thorax 1996;51:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burge PS. Occupation and COPD. Eur Respir Rev 2002;12:293–4. [Google Scholar]
- [8].Viegi G, Di Pede C. Chronic obstructive lung diseases and occupational exposure. Curr Opin Allergy Clin Immunol 2002;2:115–21. [DOI] [PubMed] [Google Scholar]
- [9].The Norwegian Medical Association Yrkesbetinget kronisk obstruktiv lungesykdom (KOLS) [Occupational COPD]. Oslo: Norwegian Medical Association; 2007. [Google Scholar]
- [10].Becklake MR. Chronic airflow limitation: Its relationship to work in dusty occupations. Chest 1985;88:608–17. [DOI] [PubMed] [Google Scholar]
- [11].Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989;140(3 Pt 2):S85–91. [DOI] [PubMed] [Google Scholar]
- [12].Coggon D, Newman Taylor A. Coal mining and chronic obstructive pulmonary disease: a review of the evidence. Thorax 1998;53:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wells GA, Shea B, O’ Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 29 January 2014). [Google Scholar]
- [14].Higgins JP, Th ompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [16].Mbelambela EP, Eitoku M, Muchanga SMJ, et al. Prevalence of chronic obstructive pulmonary disease (COPD) among Congolese cement workers exposed to cement dust, in Kongo Central Province. Environ Sci Pollut Res Int 2018;25:35074–83. [DOI] [PubMed] [Google Scholar]
- [17].Reynolds CJ, MacNeill SJ, Williams J, et al. Chronic obstructive pulmonary disease in Welsh slate miners. Occup Med (Lond) 2017;67:20–5. [DOI] [PubMed] [Google Scholar]
- [18].Nafees AA, Fatmi Z, Kadir MM, et al. Chronic bronchitis and chronic obstructive pulmonary disease (COPD) among textile workers in Karachi, Pakistan. J Coll Physicians Surg Pak 2016;26:384–9. [PubMed] [Google Scholar]
- [19].Loh LC, Rashid A, Sholehah S, et al. Low prevalence of obstructive lung disease in a suburban population of Malaysia: a BOLD collaborative study. Respirology 2016;21:1055–61. [DOI] [PubMed] [Google Scholar]
- [20].Würtz ET, Schlünssen V, Malling TH, et al. Occupational chronic obstructive pulmonary disease in a Danish population-based study. COPD 2015;12:435–43. [DOI] [PubMed] [Google Scholar]
- [21].Möhner M, Kersten N, Gellissen J. Chronic obstructive pulmonary disease and longitudinal changes in pulmonary function due to occupational exposure to respirable quartz. Occup Environ Med 2013;70:9–14. [DOI] [PubMed] [Google Scholar]
- [22].Lam KB, Yin P, Jiang CQ, et al. Past dust and GAS/FUME exposure and COPD in Chinese: the Guangzhou Biobank Cohort Study. Respir Med 2012;106:1421–8. [DOI] [PubMed] [Google Scholar]
- [23].Govender N, Lalloo UG, Naidoo RN. Occupational exposures and chronic obstructive pulmonary disease: a hospital based case-control study. Thorax 2011;66:597–601. [DOI] [PubMed] [Google Scholar]
- [24].Young J, Yoon S, Hwan D. Obstructive pulmonary function impairment among Korean male workers exposed to organic solvents, iron oxide dust and welding fumes. Ind health 2013;51:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iftikhar B, Khan HM, Hussain H, et al. Relationship between silica dust expose and chronic obstrustive pulmonary disease in workers of dust generating industries of district peshawar. GJMS 2009;7:46–50. [Google Scholar]
- [26].Monsó E, Riu E, Radon K, et al. Chronic obstructive pulmonary disease in never smoking animal farmers working inside confinement buildings. Am J Ind Med 2004;46:357–62. [DOI] [PubMed] [Google Scholar]
- [27].Zhou Yumin, Wang Chen, Yao Wanzhen, et al. Effects of occupational exposure to dust and smoke on chronic obstructive pulmonary disease and respiratory symptoms. Chin J Respir CritCare Med 2009;8:6–11. [Google Scholar]
- [28].Cullinan P. Occupation and chronic obstructive pulmonary disease (COPD). Br Med Bull 2012;104:143–61. [DOI] [PubMed] [Google Scholar]
- [29].Blanc PD, Eisner MD, Balmes JR, et al. Exposure to vapors, gas, dust, or fumes: assessment by a single survey item compared to a detailed exposure battery and a job exposure matrix. Amer J Indust Med 2005;48:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Quinlan PJ, Earnest G, Eisner MD, et al. Performance of self-reported occupational exposure compared to a job-exposure matrix approach in asthma and chronic rhinitis. Occup Environ Med 2009;66:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ryu JY, Sunwoo YE, Lee SY, et al. Chronic obstructive pulmonary disease (COPD) and vapors, gases, dusts, or fumes (VGDF): a meta-analysis. COPD 2015;12:374–80. [DOI] [PubMed] [Google Scholar]
- [32].Global Strategy for the Diagnosis, Management, and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease(GOLD)2019[DB/OL]. Available at: http://goldcopd.org/. [Google Scholar]
- [33].Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax 2015;70:482–9. [DOI] [PubMed] [Google Scholar]
- [34].Zaman M, Mahmood S, Altayeh A. Low inspiratory capacity to total lung capacity ratio is a risk factor for chronic obstructive pulmonary disease exacerbation. Am J MedSci 2010;339:411. [DOI] [PubMed] [Google Scholar]
- [35].Vandijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med 2015;13:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


