Abstract
Brachial plexus birth palsy (BPBP) is a neurologic injury that can result in mild to full paralysis of the affected upper extremity. In severe cases, nerve surgery is often performed before age 1 year. Several studies report gains in elbow flexion with onabotulinum toxin type A (OBTT-A) injections to the triceps; however, its use in infants is not widely reported. The purpose of this study is to present our experience using these injections before 6 months of age to therapeutically unmask elbow flexion and diagnostically guide surgical decision making.
This is a retrospective observational cohort study. The cohort included infants with BPBP who received OBTT-A injection to the triceps before age 6 months. Indications for the injections include trace elbow flexion and palpable co-contraction of the biceps and triceps. Elbow flexion was evaluated using the Toronto Test score. Therapeutic success was defined as an increase in post-injection scores. These scores were then used diagnostically as an indication for surgery if the infant did not achieve full elbow flexion by 8 months. A treatment algorithm for OBTT-A triceps injection was developed based on all treatment options offered to infants with elbow flexion deficits seen in the clinic.
Of the 12 infants that received OBTT-A triceps injections, 10 (83%) had improved Toronto test elbow flexion scores post-injection. Gains in elbow flexion once attained were maintained. Of the 9 OBTT-A infants with at least 2 years follow-up, 4 achieved full elbow flexion without surgery; the remainder after surgery. No complications with OBTT-A injections were noted and patients were followed on average 6 years. The average age at time of injection was 4 months (range: 2–5 months). Compared to other treatments given, OBTT-A infants tended to present with more elbow flexion than the 4 infants requiring immediate surgical intervention and less elbow flexion than the 16 infants treated conservatively.
OBTT-A injection to the triceps in infants with BPBP before 6 months of age therapeutically improved elbow flexion and diagnostically guided surgical decisions when full elbow flexion was not achieved by 8 months of age with no known complications.
Keywords: bicep, BOTOX, brachial plexus birth palsy, neonatal brachial plexus, onabotulinum toxin A, Toronto test score
1. Introduction
Brachial plexus birth palsy (BPBP) is a relatively common injury to the brachial plexus that occurs at birth (incidence 1:1000).[1–6] The injury to the nerve can vary from a mild stretch presenting as transient neuropraxia to a complete avulsion resulting in non-recovering paralysis of the affected arm.[3,5,6] Initial treatment for BPBP includes conservative management with occupational or physical therapy.[7,8] Later, depending on recovery, invasive techniques such as plexus repair nerve surgery and various reconstructive orthopedic procedures may be needed.[1–16] If an affected infant has not shown meaningful return of biceps or shoulder function, nerve surgery is recommended by 1 year of age, although some authors advocate surgery as early as 3 months.[7–11,17] Recent literature suggests that nerve grafting is preferred over neurolysis.[18]
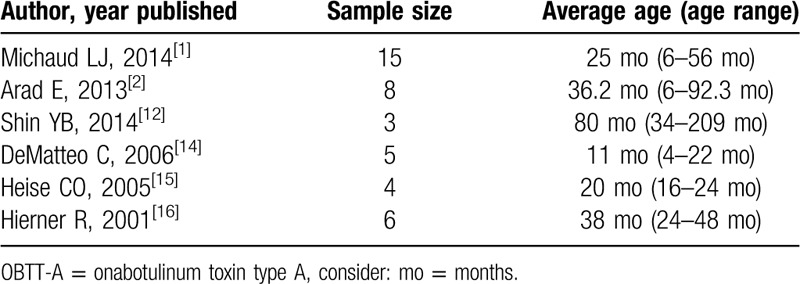
In recent years, off-label use of onabotulinum toxin type A (OBTT-A) injections have been reported as an effective treatment for BPBP.[1,2,12–16,19] OBTT-A injections function by inhibiting the release of acetylcholine from the presynaptic neuronal end plate causing a temporary weakening of the injected muscle. If injected into the antagonist muscle to which desired function is to be achieved, relaxation of the antagonist muscle will be attained.[20] Various studies suggest that it is most beneficial when co-contraction of agonist and antagonist is present.[1,2,13–16,19] A majority of the papers reporting OBTT-A as a BPBP treatment have focused on injections to the shoulder girdle while a limited number have reported injections to the triceps.[1,2,12–16,19–22] The majority of patients receiving OBTT-A injections to the triceps to unmask biceps function were performed on patients with ages ranging from age 4 months to 13 years, with very few under 1 year of age (Table 1).[1,2,12,14–16,21,22]
Table 1.
A summary of cases in the literature of OBTT-A injections to the triceps to unmask biceps activity.
The purpose of this study is to present our experience with OBTT-A injections to the triceps muscle in infants less than 6 months of age with BPBP to therapeutically unmask elbow flexion and diagnostically guide surgical decision making in the first year of life. It is not the intent of this paper to demonstrate that OBTT-A injections change the natural history of elbow flexion recovery.
2. Methods
2.1. Ethical statement
This study was approved with waivers of consent and assent by the institutional review board of the participating institution.
2.2. Study design and patient selection
This is a retrospective observational cohort study. Medical records from a single Brachial Plexus Clinic from September 2006 and September 2017 were reviewed to identify infants meeting inclusion/exclusion criteria. All infants with BPBP and elbow flexion weakness treated with OBTT-A triceps injections before 6 months of age were included, even those with incomplete chart data or limited follow-up. They were excluded if they were ≥6 months of age when first seen in the clinic. For comparison purposes, infants with a similar presentation that did not receive these injections were included if they had complete chart data and were followed until they were at least 1 year of age. Three treatment groups are compared as follows: OBTT-A triceps injection group, immediate surgical intervention group, and the conservative care only group.
2.3. Data acquired and outcomes measured
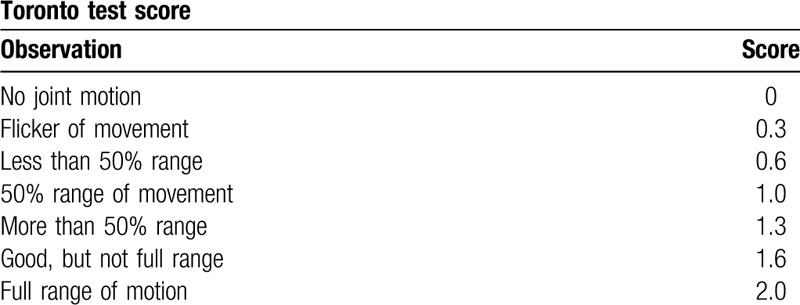
Medical record data acquired included the following: Toronto test scores for elbow flexion as a measure of biceps function and in total as an indication of global upper extremity function;[23] whether co-contraction of the biceps and triceps was palpable; treatment given; complications; age at follow-up, and; demographic information. The Toronto test score is a validated measure routinely used to evaluate and monitor infants with BPBP where 5 upper extremity joint motions (elbow, wrist, finger, and thumb extension and elbow flexion) are each scored between 0 and 2.0 based on clinical observation (Table 2), resulting in a maximum total score of 10.0.[23] In infants that received OBTT-A triceps injections, therapeutic success was defined as an increase in the Toronto elbow flexion score post-injection. Post-injection Toronto elbow flexion scores were also used diagnostically as an indication for surgery if the infant did not achieve full elbow flexion by 8 months. Nerve repair surgeries performed on infants included neurolysis, nerve grafting, and nerve transfers.
Table 2.
Summary of the Toronto test scoring system.[23] Five upper extremity joint motions, including elbow flexion, are scored based on clinical observation between 0 and 2.0 as documented below.
2.4. Data analyses
Toronto elbow flexion scores each visit by infant up to 1 year of age were compared by group. In addition, groups were compared by total Toronto score at first visit and ages at which a total Toronto score of 10 and an elbow flexion score of 2.0 were achieved.
2.5. Treatment indications
The type of treatment chosen was patient specific based on clinical presentation and the decision of the treatment team. The treatment team consisted of a pediatric orthopedic surgeon, a physiatrist, a neurosurgeon or plastic surgeon with nerve repair expertise, occupational and physical therapists, social work, and the infants’ parents. Current evidence-based practice guidelines regarding treatment of infants with BPBP were followed.[1–8,12–16] All infants were followed monthly for the first year by the BPBP team and received conservative therapy care. Infants who demonstrated severe injuries with no or only a flicker of movement in the 5 Toronto score areas (including elbow flexion) by 3 months, were offered surgical interventions. Remaining infants were monitored and continued conservative care until the Toronto test score for elbow flexion was 2.0. If at 3 or 4 months of age, the infant had an elbow flexion Toronto score of at least 0.3, palpable co-contraction of biceps and triceps, with little to no improvement in elbow flexion between visits, the family was offered triceps OBTT-A injections and told this was off-label use of the product. If the infant did not achieve full a Toronto elbow flexion score of 2.0 (full elbow flexion) by 8 months, the family was encouraged to pursue surgical intervention. Our treatment algorithm is outlined in Figure 1. It is important to remember that this is a retrospective review, so infants were not placed in specific treatment groups for research purposes. As always, parents could refuse treatment recommendations.
Figure 1.
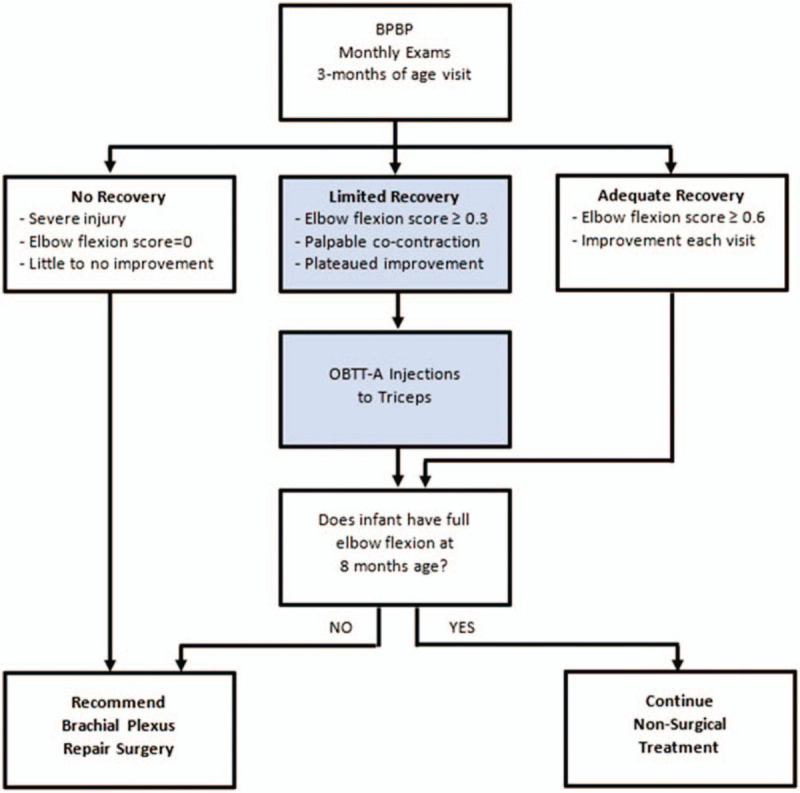
Treatment algorithm developed from this case series documenting indications for OBTT-A injection to the triceps. OBTT-A = onabotulinum toxin type A.
2.6. OBTT-A injection to the triceps
Before OBTT-A injection to the triceps, risks were discussed with families which included the risk of bruising, bleeding, infection, pain, flulike symptoms, muscle weakness, respiratory depression, dysphagia, and aspiration (PDR [package insert] [BOTOX Cosmetics] Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf. Accessed: December 20, 2019). Topical EMLA cream or a surface lidocaine patch was applied to the injection site. Infants were sedated if given injections at other sites as well (shoulder). Electromyography or electrical stimulation guidance was utilized for accurate muscle localization. OBTT-A was then injected at 3 to 4 sites throughout the triceps at 10 to 30 units of OBTT-A (100 units: 2 mL concentration). Patients were held for observation and returned home the same day.
3. Results
3.1. Demographics
The retrospective review identified 12 infants with BPBP who received OBTT-A injections to the triceps before 6 months of age. For comparison purposes, the retrospective review also identified 20 infants with BPBP that did not receive OBTT-A triceps injections; 4 with severe injuries that had surgery before 8 months of age and 16 treated with conservative care only. The average age at time of OBTT-A injection was 4 months (range: 2–5 months). The average age of OBTT-A patients at last visit was 6 years (range: 3–11 years). The average age at nerve repair surgery for infants after failed OBTT-A triceps injections was 11 months (range: 8–16 months); those taken directly to surgery 5 months (range: 1–7 months).
3.2. Outcomes and comparisons with other treatment groups
Of the 12 infants that received OBTT-A injections to the triceps, 10 (83%) had improved Toronto test elbow flexion scores and 8 (67%) achieved a score of 1.0 or higher (Fig. 2). Three of the 12 infants were followed less than 2 years and were not included in further analyses. Of the remaining 9 OBTT-A infants with at least 2 years follow-up, 4 achieved full elbow flexion without surgery and 5 after surgery. Full elbow flexion, once attained, was maintained through final follow-up except in 1 OBTT-A infant. This child achieved a Toronto elbow flexion score of 2.0, but had significant shoulder deficits requiring additional surgery and lost full active elbow flexion with age after the second surgery. All results were achieved with a single OBTT-A injection to the triceps. No complications occurred from the OBTT-A injections to date.
Figure 2.
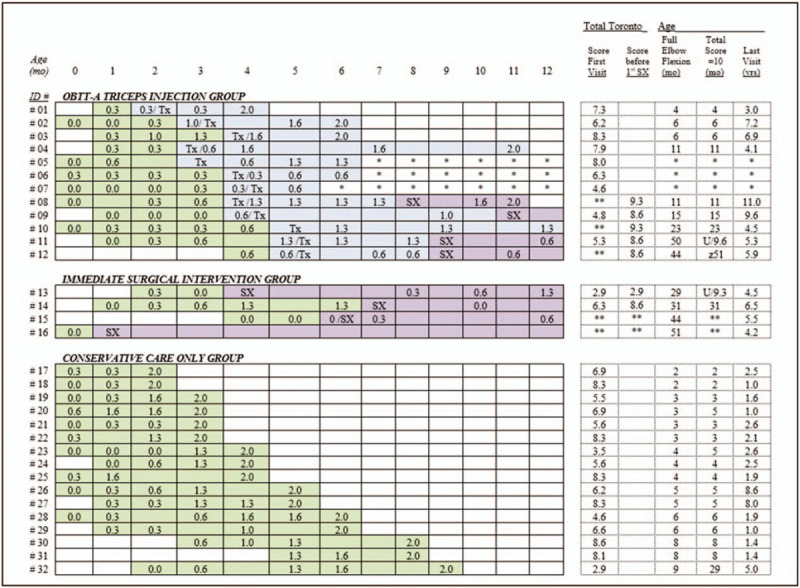
Toronto elbow flexion scores by patient over time until full elbow flexion is achieved or 12 mo. Treatment received is color coded to indicate the different levels of treatment received with green signifying conservative treatment alone, blue the time after OBTT-A triceps injection but before any surgery, and purple surgical treatment. Additional data presented includes the total Toronto score at first visit and age at last visit and when full elbow flexion and total Toronto score of 10 is achieved. mo = months, OBTT-A = onabotulinum toxin type-A, SX = nerve surgery, Tx = OBTT-A triceps injection, U/# = unattained/highest total Toronto score attained; yrs = years, z = did not maintain, ∗ = biceps deficits with less than 1-yr follow-up, ∗∗ = value not recorded.
By last visit, all children in all groups achieved total Toronto scores of 10.0, except for the child previously mentioned and 2 others. One of the remaining children required surgery after OBTT-A triceps injection, the other went directly to surgery. Both had significant shoulder and wrist active range of motion deficits at presentation.
Compared to the conservative group, OBTT-A infants tended to have lower elbow flexion scores at 3 months of age but had higher scores than the immediate surgical intervention group. Specifically, 9 of the 12 OBTT-A infants (75%) had 3-month Toronto elbow flexion scores of 0.6 or less, compared to 31% (5 of 16) in the conservative group and 100% (4 of 4) in the immediate surgical intervention group (Fig. 2). In addition, the OBTT-A infants tended to be older when they achieved full elbow flexion (median 18 months, range 4–50) when compared to the conservative group (median 4 months, range 2–9) and younger when compared to the immediate surgical intervention group (median 38 months, range 29–51). These findings were consistent with our treatment indications.
4. Discussion
This is the largest study documenting the success of OBTT-A injections to the triceps in infants with BPBP less than 6 months of age. In this case series, OBTT-A injection to the triceps was used therapeutically to unmask elbow flexion and diagnostically to guide surgical decision making in the first year of life when full elbow flexion was not achieved. The therapeutic benefits of injection are evidenced by the increase in elbow flexion scores in 10 of 12 infants (83%) post-injection. In addition, 4 of the 9 infants with 2-year follow-up achieved full elbow flexion, avoiding the need for surgery. Diagnostically, a post-injection Toronto elbow flexion score of less than 2.0 at 8 months of age was used as an indication for surgery, since full elbow flexion was typically gained 3 months post-injection. Interestingly, parents of the 5 OBTT-A infants that required surgery appeared less anxious during pre-surgical discussion knowing that they explored all non-surgical options. Since it was not the intent of the paper to demonstrate that OBTT-A injection changes the natural history of the of elbow flexion recovery, this remains unknown.
Although other studies have reported the use of OBTT-A in BPBP patients, very few have reported on its use in infants (Table 1).[1,2,12,14–16,21,22] Our results are similar to that in the literature, but our population tended to be younger (all <6 months of age), had injections before surgery, and had a relatively long follow-up. Arad et al demonstrated sustained clinically useful improvement in 8 children (age range: 6–92 months) after OBTT-A injections to the triceps; however, 75% of the children had prior surgery.[2] Michaud et al demonstrated 66.7% (10/15) improvement in 15 children (age range: 6–56 months), but only 2 were before surgery.[1] Demateo et al also reported improvement in elbow flexion in 2 infants ages 4 and 6 months after OBTT-A triceps injections, but since they failed to achieve full range of motion, surgery was performed at 9 months of age.[14] More recently, Garcia Ron et al demonstrated good results in children using ultrasound-guided injection of OBTT-A for muscle imbalance.[22]
Careful patient selection and family agreement to treatment was critical to success. OBTT-A is not appropriate for every infant with BPBP. In this case series, infants with less severe BPBP, with at least a flicker of elbow flexion, who demonstrated a seemingly plateaued recovery at 3 months, and had palpable co-contraction of the biceps and triceps, appeared to benefit most from OBTT-A. The authors felt that for OBTT-A to be effective at least some contraction of the biceps had to be present. Infants that did not have at least a flicker of elbow flexion and palpable co-contraction of the biceps and triceps were not offered the injections, nor were those that showed continued improvement with conservative care only.
The authors are uncertain why 2 OBTT-A cases failed to show improvement. Both infants presented mid-series, so inexperience with the injection is unlikely. Both had active elbow flexion (Toronto elbow flexion scores >0.3), negating reliance on palpation alone as a measure of biceps contraction. Since both infants were among the oldest to receive the injections (5 months of age), it is possible that they each reached their natural plateaus. In the conservative group, 11 of 16 (70%) infants had full elbow flexion by 5 months; the remainder demonstrated continued improvement. Given our prior success with the injections, it was our hope that the injection in the older infants would lead to continued improvement and eventually full elbow flexion. When that did not occur by 8 months of age, surgical intervention was recommended and more readily accepted by the parents.
An argument could be made that the gains in elbow flexion post-injection would be seen from natural history alone. Due to the diversity in initial presentation evidenced by the Toronto elbow flexion and total scores first visit (Fig. 2) and the small sample size, direct comparison to historical controls was not possible. As previously stated, it is not the intent of this paper to demonstrate that OBTT-A injection changes the natural history of the of elbow flexion recovery. However, we present it as another option to offer families at a young age when elbow flexion recovery appears plateaued.
Consistent with the literature, infants that did not have full recovery by the age of 3 months still had some residual impairment requiring ongoing treatment.[7,8] However, OBTT-A may have helped them achieve full elbow flexion earlier, allowing them more time to establish motor patterns that included elbow flexion.[14,24] Interestingly, despite having full elbow flexion, BPBP children with and without OBTT-A still tend to demonstrate impairment with complex functional tasks, such as trumpeting when eating and running with their elbow flexed.
Although there were no complications from OBTT-A injections in this series, the long-term effects of this treatment in infants remains unknown. However, a recent systematic review found no severe adverse events reported in the literature.[25] OBTT-A is also used repeatedly to treat spasticity in children with cerebral palsy with minimal complications.[24,25] Since cerebral palsy is an upper motor neuron disorder, OBTT-A to spastic muscles only temporarily alleviates symptoms.[26,27] BPBP is a lower motor neuron condition. Therefore, a single OBTT-A injection allows time for healing of the nerves to the desired muscle group while temporarily weakening their antagonists, negating the need for repeat injections and hopefully minimizing any potential long-term adverse events, if any. In this case series, all infants except 2, demonstrated improved elbow flexion with a single triceps injection.
4.1. Limitations
As in any observational retrospective review, missing data poses a concern. In this series, 3 OBTT-A patients were lost to follow-up, so despite an improvement in Toronto elbow flexion scores post-injection, final patient outcome remains unknown for these cases. The reliance on clinical assessment of biceps and triceps function by palpation is also problematic as it is dependent on a clinician's skill. However, electromyography in this patient population is often difficult to obtain. The treatment offered was based on clinical presentation, so groups used in this study do not reflect a randomized, controlled study and are presented for comparison purposes only. Statistical analysis was also limited by the small sample size and varying presentation of each case as evidenced in Figure 2. Despite these limitations, it is important to note that interesting trends are presented consistent with the limitations of any case series.
5. Conclusions
This is the largest study of OBTT-A injections to the triceps in infants with BPBP less than 6 months of age to therapeutically improve elbow flexion and diagnostically guide surgical decision making in the first year of life if full elbow flexion was not achieved by age 8 months with no known complications.
Author contributions
Conceptualization: Melanie A. Morscher, Mark J. Adamczyk.
Data curation: Melanie A. Morscher, Matthew D. Thomas, Suneet Sahgal, Mark J. Adamczyk.
Formal analysis: Melanie A. Morscher, Matthew D. Thomas, Mark J. Adamczyk.
Funding acquisition: Mark J. Adamczyk.
Investigation: Melanie A. Morscher, Matthew D. Thomas, Suneet Sahgal, Mark J. Adamczyk.
Methodology: Melanie A. Morscher, Mark J. Adamczyk.
Project administration: Melanie A. Morscher, Mark J. Adamczyk.
Resources: Melanie A. Morscher, Mark J. Adamczyk.
Software: Mark J. Adamczyk.
Supervision: Melanie A. Morscher, Mark J. Adamczyk.
Validation: Melanie A. Morscher, Matthew D. Thomas, Suneet Sahgal, Mark J. Adamczyk.
Visualization: Melanie A. Morscher, Matthew D. Thomas, Suneet Sahgal, Mark J. Adamczyk.
Writing – original draft: Melanie A. Morscher, Matthew D. Thomas, Suneet Sahgal, Mark J. Adamczyk.
Writing – review and editing: Melanie A. Morscher, Matthew D. Thomas, Suneet Sahgal, Mark J. Adamczyk.
Footnotes
Abbreviations: BPBP = brachial plexus birth palsy, OBTT-A = onabotulinum toxin type A.
How to cite this article: Morscher MA, Thomas MD, Sahgal S, Adamczyk MJ. Onabotulinum toxin type A injection into the triceps unmasks elbow flexion in infant brachial plexus birth palsy: a retrospective observational cohort study. Medicine. 2020;99:34(e21830).
Institution where work was performed at the Akron Children's Hospital, Akron, OH, USA.
This paper discusses off-label use of onabotulinum toxin type A.
The authors have no conflicts of interest to disclose.
No funding was received for this publication. No direct or indirect commercial financial incentive was associated with this publication.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Michaud LJ, Louden EJ, Lippert WC, et al. Use of botulinum toxin type A in the management of neonatal brachial plexus palsy. PM R 2014;6:1107–19. [DOI] [PubMed] [Google Scholar]
- [2].Arad E, Stephens D, Curtis CG, et al. Botulinum toxin for the treatment of motor imbalance in obstetrical brachial plexus palsy. Plast Reconstr Surg 2013;131:1307–15. [DOI] [PubMed] [Google Scholar]
- [3].Romaña MC, Rogier A. Obstetrical brachial plexus palsy. Handb Clin Neurol 2013;112:921–8. [DOI] [PubMed] [Google Scholar]
- [4].Smania N, Berto G, La Marchina E, et al. Rehabilitation of brachial plexus injuries in adults and children. Eur J Phys Rehabil Med 2012;48:483–506. [PubMed] [Google Scholar]
- [5].Thatte MR, Mehta R. Obstetric brachial plexus injury. Indian J Plast Surg 2011;44:380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Borschel GH, Clarke HM. Obstetrical brachial plexus palsy. Plast Reconstr Surg 2009;124: 1 Suppl: 144e–55e. [DOI] [PubMed] [Google Scholar]
- [7].Strömbeck C, Krumlinde-Sundholm L, Forssberg H. Functional outcome at 5 years in children with obstetrical brachial plexus palsy with and without microsurgical reconstruction. Dev Med Child Neurol 2000;42:148–57. [DOI] [PubMed] [Google Scholar]
- [8].Waters PM. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am 1999;81:649–59. [DOI] [PubMed] [Google Scholar]
- [9].Brauer CA, Waters PM. An economic analysis of the timing of microsurgical reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am 2007;89:970–8. [DOI] [PubMed] [Google Scholar]
- [10].Gilbert A. Repair of the brachial plexus in the obstetrical lesions of the newborn [French]. Arch Pediatr 2008;15:330–3. [DOI] [PubMed] [Google Scholar]
- [11].Gilbert A, Pivato G, Kheiralla T. Long-term results of primary repair of brachial plexus lesions in children. Microsurgery 2006;26:334–42. [DOI] [PubMed] [Google Scholar]
- [12].Shin YB, Shin MJ, Chang JH, et al. Effects of botulinum toxin on reducing the co-contraction of antagonists in birth brachial plexus palsy. Ann Rehabil Med 2014;38:127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gobets D, Beckerman H, de Groot V, et al. Indications and effects of botulinum toxin A for obstetric brachial plexus injury: a systematic literature review. Dev Med Child Neurol 2010;52:517–28. [DOI] [PubMed] [Google Scholar]
- [14].DeMatteo C, Bain JR, Galea V, et al. Botulinum toxin as an adjunct to motor learning therapy and surgery for obstetrical brachial plexus injury. Dev Med Child Neurol 2006;48:245–52. [DOI] [PubMed] [Google Scholar]
- [15].Heise CO, Gonçalves LR, Barbosa ER, et al. Botulinum toxin for treatment of cocontractions related to obstetrical brachial plexopathy. Arq Neuropsiquiatr 2005;63:588–91. [DOI] [PubMed] [Google Scholar]
- [16].Hierner R, Rollnik JD, Berger AC, et al. Botulinum toxin treatment of biceps/triceps co-contraction in obstetrical brachial plexus lesions. Eur J Plast Surg 2001;24:2–6. [Google Scholar]
- [17].Bauer AS, Kalish LA, Adamczyk MJ, et al. Microsurgery for brachial plexus injury before versus after 6 months of age: results of the multicenter treatment and outcomes of brachial plexus injury (TOBI) study. J Bone Joint Surg Am 2020;102:194–204. [DOI] [PubMed] [Google Scholar]
- [18].Lin JC, Schwentker-Colizza A, Curtis CG, et al. Final results of grafting versus neurolysis in obstetrical brachial plexus palsy. Plast Reconstr Surg 2009;123:939–48. [DOI] [PubMed] [Google Scholar]
- [19].Rollnik JD, Hierner R, Schubert M, et al. Botulinum toxin treatment of cocontractions after birth-related brachial plexus lesions. Neurology 2000;55:112–4. [DOI] [PubMed] [Google Scholar]
- [20].Ezaki M, Malungpaishrope K, Harrison RJ, et al. Onabotulinum toxinA injection as an adjunct in the treatment of posterior shoulder subluxation in neonatal brachial plexus palsy. J Bone Joint Surg Am 2010;92:2171–7. [DOI] [PubMed] [Google Scholar]
- [21].Buchanan PJ, Grossman JAI, Price AE, et al. The use of botulinum toxin injection for brachial plexus birth injuries: a systematic review of the literature. Hand (N Y) 2019;14:150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].García Ron A, Gallardo R, Huete Hernani B. Utility of ultrasound-guided injection of botulinum toxin type A for muscle imbalance in children with obstetric brachial plexus palsy: description of the procedure and action protocol. Neurologia 2019;34:215–23. [DOI] [PubMed] [Google Scholar]
- [23].Michelow BJ, Clarke HM, Curtis CG, et al. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg 1994;93:675–80. [PubMed] [Google Scholar]
- [24].Xi SD, Zhu YL, Chen C, et al. The plasticity of the corticospinal tract in children with obstetric brachial plexus palsy after Botulinum Toxin A treatment. J Neurol Sci 2018;394:19–25. [DOI] [PubMed] [Google Scholar]
- [25].Bourseul JS, Molina A, Lintanf M, et al. Early botulinum toxin injections in infants with musculoskeletal disorders: a systematic review of safety and effectiveness. Arch Phys Med Rehabil 2018;99:1160–76.e5. [DOI] [PubMed] [Google Scholar]
- [26].Sätilä H, Kotamäki A, Koivikko M, et al. Low- and high-dose botulinum toxin A treatment: a retrospective analysis. Pediatr Neurol 2006;34:285–90. [DOI] [PubMed] [Google Scholar]
- [27].Papavasiliou AS. Management of motor problems in cerebral palsy: a critical update for the clinician. Eur J Paediatr Neurol 2009;13:387–96. [DOI] [PubMed] [Google Scholar]


