Abstract
In December 2019, a cluster of coronavirus Disease 2019 (COVID-19) occurred in Wuhan, Hubei Province, China. The present study was conducted to report the clinical characteristics of 201 COVID-19 patients in Changsha, China, a city outside of Wuhan. All of the patients with confirmed COVID-19 were admitted to the First Hospital of Changsha City, the designated hospital for COVID-19 assigned by the Changsha City Government. The clinical and epidemiological characteristics, data of laboratory, radiological picture, treatment, and outcomes records of 201 COVID-19 patients were collected using electronic medical records. This study population consisted of 201 hospitalized patients with laboratory-confirmed COVID-19 in Changsha by April 28, 2020. The median age of the patients was 45 years (IQR 34–59). About half (50.7%) of the patients were male, and most of the infected patients were staff (96 [47.8%]). Concerning the epidemiologic history, the number of patients linked to Wuhan was 92 (45.8%). The most common symptoms were fever (125 [62.2%]), dry cough (118 [58.7%]), fatigue (65 [32.3%]), and pharyngalgia (31 [15.4%]). One hundred and forty-four (71.6%) enrolled patients showed bilateral pneumonia. Fifty-four (26.9%) patients showed unilateral involvement, and three (1.5%) patients showed no abnormal signs or symptoms. The laboratory findings differed significantly between the Intensive Care Unit (ICU) and non-ICU groups. Compared with non-ICU patients, ICU patients had depressed white blood cell (WBC), neutrocytes, lymphocytes, and prolonged prothrombin time (PT). Moreover, higher plasma levels of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin (PCT), alanine aminotransferase (ALA), aspartate aminotransferase (AST), creatine kinase (CK), creatine kinase-MB (CK-MB), creatinine (CREA), and lactate dehydrogenase (LDH) were detected in the ICU group. In this single-center study of 201 COVID-19 patients in Changsha, China, 22.4% of patients were admitted to ICU. Based on our findings, we propose that the risk of cellular immune deficiency, hepatic injury, and kidney injury should be monitored. Previous reports focused on the clinical features of patients from Wuhan, China. With the global epidemic of COVID-19, we should pay more attention to the clinical and epidemiological characteristics of patients outside of Wuhan.
Keywords: Clinical features, COVID-19, Epidemiological characteristics, SARS-CoV-2
1. Introduction
In December 2019, a series of COVID-19 occurred in Wuhan, Hubei Province, China,[1–4] which was caused by SARS-CoV-2 infection. Most of the COVID-19 patients were concentrated in Wuhan, and their exposure history related them to the Huanan Seafood Wholesale Market at the beginning.[5] However, the infection rapidly spread from Wuhan to all over the country because of the population movement during the Spring Festival.[6,7] Acute respiratory infection symptoms, including high temperature, dry cough, fatigue, and breathing difficulty, are the main early symptoms of the disease.[8] Along with disease progression, some patients rapidly develop acute respiratory distress syndrome (ARDS), acute respiratory failure, and other several complications, especially for older patients or those with immunodeficiency.
In the past month, several studies[9–13] reported the epidemiological, demographic, clinical, laboratory, and radiological characteristics of COVID-19 patients in Wuhan city. In the present study, we performed a comprehensive analysis to describe the clinical features, epidemiologic characteristics, treatment, and outcomes of 201 COVID-19 patients in Changsha, China, a city outside of Wuhan, and the differences of clinical features between ICU and non-ICU patients were analyzed. Our study findings provide information about COVID-19 patients outside of Wuhan.
2. Methods
2.1. Patients
Related data were collected from the First Hospital of Changsha city, the designated hospital for COVID-19 assigned by the Changsha city government. All of the patients enrolled in this report were admitted from January 1, 2020 to April 28, 2020. During the entire outbreak, a total of 242 patients received treatment in the First Hospital of Changsha City. Forty-one patients were excluded because of plenty of data missing and 201 COVID-19 patients were involved in the present study. Criteria for patients admitted into ICU:
-
1.
In the resting state, the oxygen saturation of patients ≤93%;
-
2.
PaO2/FiO2 ≤300 mm Hg (1 mm Hg = 0.133 kPa).
-
3.
Respiratory failure occurs and mechanical ventilation is required (non-invasive or invasive ventilator)
-
4.
Shock occurs;
-
5.
Patients with organ failures requiring ICU monitoring and treatment.
According to previous study,[14] the basic reproductive number (R0) was 2.43 (95% CI = 2.42–2.44) and that 92.9% (95% CI = 92.5–93.1%) of total cases were not reported, so many patients might not be reported in Changsha, China. The First Hospital of Changsha city is located in Changsha, Hunan Province, a neighboring province of Hubei province. The ethics commissions approved this study of the First Hospital of Changsha City, and written consents were obtained from the enrolled patients. All of the patients involved in the present study were diagnosed according to World Health Organization interim guidance.[15] The clinical outcomes, including discharge and death, were recorded up to April 28, 2020.
2.2. Data collection
The research team from the Second Xiangya Hospital of Central South University and The First Hospital of Changsha city conducted a comprehensive analysis of the medical information of COVID-19 patients. In this report, we obtained the clinical and epidemiological characteristics, data of laboratory, radiological picture, treatment, and outcomes records using electronic medical records. The medical information, including demographic data (age, gender, and occupation), exposure history, medical history, comorbidities, signs, symptoms, chest computed tomographic (CT) scans, laboratory results, and treatment, such as antibacterial therapy, glucocorticoid therapy, and antiviral therapy, was collected. The durations from exposure to Wuhan to the onset of disease and the course of disease were recorded.
2.3. Real-time reverse transcription polymerase chain reaction (RT-PCR) assay for SARS-CoV-2
The laboratory test assays of 2019-nCoV were conducted according to the WHO recommendation.[16] Laboratory identification of 2019-nCoV was performed in three different institutions: The First Hospital of Changsha City, Hunan Center for Disease Control, and Prevention (Hunan CDC) and Chinese CDC (CCDC). Upper and lower respiratory tract specimens were collected for extracting SARS-CoV-2 RNA. RNA was obtained and further tested by RT-PCR through the same method previously described.[17] Other respiratory viruses (influenza A virus, influenza B virus, respiratory syncytial virus) and parainfluenza virus were also tested in this study.
2.4. Statistical analysis
Continuous variables were described as median and IQR. Categorical variables were expressed using the number and percentages. Mann–Whitney U test was performed for continuous variables, and the X2 test or Fisher's exact test was conducted for categorical variables. A two-sided α < .05 was considered to be statistically significant. Additionally, normality test was also conducted to test whether the data conforms to the normal distribution. All of the statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 17.0 software.
2.5. Role of the funding source
The study funders/sponsors had no role in the design and conduction of the study, including the collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and the decision to submit the manuscript for publication. The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
3. Results
This study population consisted of 201 hospitalized patients aged 1 to 84 with laboratory-confirmed COVID-19 in Changsha by April 28, 2020. Because the data does not conform to the normal distribution, we used the Mann–Whitney U test for continuous variables. Forty-five (22.4%) of the Severe Acute Respiratory Syndrome Coronavirus 2 CoV (SARS-COV-2) infected patients were admitted to the ICU due to a rapid deterioration of clinic condition, and 156 (77.6%) were in isolated wards. The median age of the ICU and non-ICU groups were 57.0 (IQR 46.0–66.0) and 40.0 (IQR 31.0–53.0), respectively. Nine (4.5%) adolescent patients under the age of 18 were all in the non-ICU group. About half of the patients (102 (50.7%)) were male, most of the infected patients work as staff (96 [47.8%]), and other occupations included farmer (5 [2.5%]), self-employed worker (13 [6.5%]), student (13 [6.5%]), retiree (48 [23.9%]), and unemployed (26 [12.9%]). Concerning the epidemiologic history, the number of patients with links to Wuhan was 92 (45.8%). The number of patients exposed to the Wuhan city in the ICU group (26 [57.8%]) was significantly higher than that of the non-ICU group (66 [42.3%]) (P = .048). The median time between onset and admission was five days (IQR 3–8) in both the ICU and non-ICU groups. Additionally, the incubation period of the disease was six days (IQR 4–9) in the ICU group, 6 days (IQR 3.0–7.3) in the non-ICU group, and 6 days (IQR 3.8–8.0) in total. As of April 28, 2020, a total of 199 patients (99%) had been discharged, and 2 patients (1%) had died. Of the 45 patients admitted to the ICU, 43 (95.6%) had been discharged, and 2 (4.4%) had died (P = .049 < .05) (Table 1).
Table 1.
Epidemiologica characteristics of COVID-19 patients.

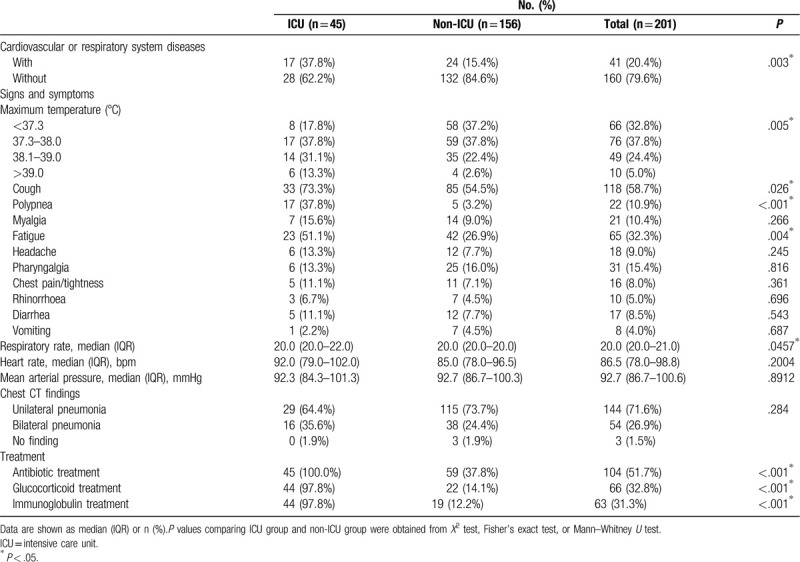
Seventeen (37.8%) patients of the ICU group and 24 patients (5.4%) of the non-ICU group had cardiovascular or respiratory system diseases. The most common symptoms were fever (135 [67.2%]), dry cough (118 [58.7%]), fatigue (65 [32.3%]), and pharyngalgia (31 [15.4%]). Less common symptom were polypnea (22 [10.9%]), myalgia (21 [10.4%]), pharyngalgia (31 [15.4%]), headache (18 [9.0%]), diarrhea (17 [8.5%]), chest tightness (16 [8.0%]), rhinorrhoea (10 [5.0%]), and vomiting (8 [4.0%]). Compared with the non-ICU patients, patients admitted to ICU were more likely to report high-grade fever, dry cough, polypnea, and fatigue. Of the 201 patients, most patients had low (76 [37.8%]) to moderate (49 [22.4%]) grade fever or normal temperature (66 [32.8%]). One hundred and forty-four (71.6%) enrolled patients showed unilateral pneumonia by chest CT scan (Fig. 1). Fifty-four (26.9%) patients showed bilateral pneumonia, and three (1.5%) patients showed no abnormal signs or symptoms. Resting heart rate and mean arterial blood pressure did not differ between the two groups (P > .05). Compared with patients who did not receive ICU care, patients who received ICU care had a higher respiratory rate (P = .0457). Forty-five (100%) patients, 44 (97.8%) patients, and 44 (97.8%) patients in the ICU group received treatments of antibiotic, glucocorticoid, and immunoglobulin, respectively; the frequency of patients who received no ICU care receiving these treatments was lower (Table 2).
Figure 1.
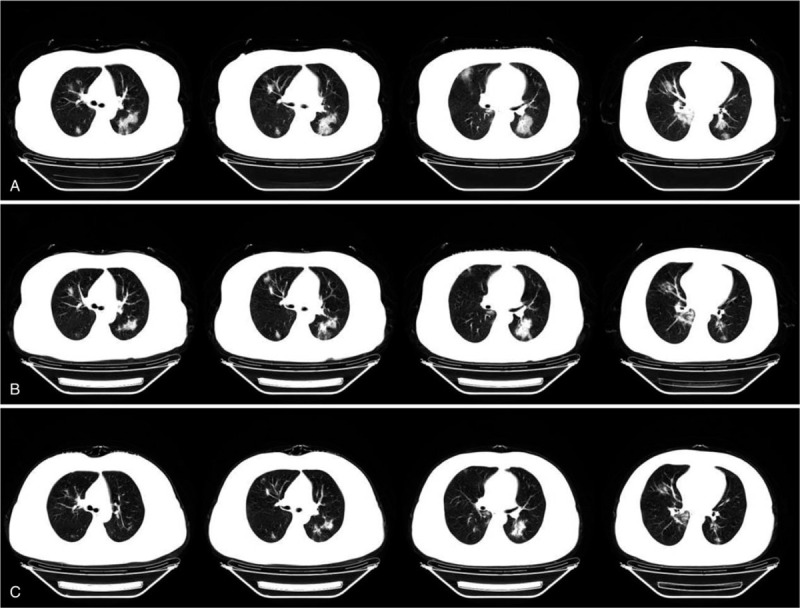
(A) Chest CT was obtained on hospital day 2, multiple high-density exudation were observed in bilateral lung. (B) Chest CT was obtained on hospital day 5. The exudation in both lungs were absorbed and the GGO than before. (C) Chest CT was obtained on hospital day 8. The lesions of bilateral lung were further absorbed and a few cord shadows were formed.
Table 2.
Clinical characteristics and treatment of COVID-19 patients.
The laboratory findings differed significantly between the ICU and non-ICU groups. Concerning the blood counts of patients on admission, the white blood cell count of 75 patients (37.3%) was <4 × 109/L, and the lymphocyte count of 46 patients (22.9%) was <0.8 × 109/L. Compared with the non-ICU group, high levels of ESR (median 52.0 [IQR 36.0–72.0]) CRP (median 42.4 [IQR 22.0–73.9]), and PCT (median 0.05 [0.05–0.08]) were observed in the ICU group. Prolonged prothrombin time (median 12.0 [IQR 11.3–12.5]) and a higher level of D-dimer (median 0.25 [IQR 0.14–0.52]) on admission were found in the ICU patients. Levels of ALA, AST, CK, and LDH were significantly increased in the ICU group. These laboratory results were recorded on the first day after admission for all of the patients, and then on those who later received ICU care or not (Table 3).
Table 3.
Laboratory results of COVID-19 patients.

4. Discussion
The number of COVID-19 patients is increasing, and so is the death toll.[11,18] Several studies[9–13] reported the epidemiological and clinical characteristics of COVID-19 patients, providing information for SARS-CoV-2 evolution, infectivity, transmissibility, and pathogenicity. In this study, we reported a total of 201 patients with SARS-CoV-2 infection outside of Wuhan, China. Among them, 45 (22.4%) patients required ICU care, and 156 (77.6%) were admitted to the isolation ward of the First Hospital of Changsha City. Patients in the ICU group were older, while no significant difference in sex ratio was found between the two groups, which suggests that age may be a risk factor for a poor outcome. The occupational composition of the ICU group differed from the non-ICU group, and the number of patients exposed to Wuhan city in the ICU group was significantly higher than that of the non-ICU group, which indicates that the exposure history to Wuhan city may affect the outcome of COVID-19 patients. No significant differences in the duration and incubation were found between the ICU group and the non-ICU group. As of April 28, 2020, 199 (99%) of 201 patients had been discharged. Two (1%) patient of the ICU group in this study died. More patients had a poor prognosis in ICU group compared to non-ICU group (P < .05) and this overall mortality was lower than that reported for Wuhan (4.3%).[11]
In terms of clinical features, patients with cardiovascular or respiratory diseases were more likely to require ICU care. In addition, the maximum temperature of patients in the ICU group was significantly higher than that in the non-ICU group, and more patients in the ICU group presented fatigue and anhelation. Moreover, the respiratory rate of cases in the ICU group was higher than those of the non-ICU group, which may be attributed to inflammation of the lungs. Patients in the ICU group must breathe more frequently to provide the oxygen required compared to patients in the non-ICU group.
The most common laboratory abnormalities observed in the present report included depressed WBC, neutrocytes, lymphocytes, prolonged prothrombin time, and elevated ESR, CRP, PCT, and LDH. Previous studies indicated the role of neutrophil biology and related signaling in COVID-19. Yu et al reported that elevated levels of neutrophil activation in COVID-19 patients were related to higher risk of morbid thrombotic complications.[19] Liu et al indicated that neutrophil-to-lymphocyte ratio was an independent risk factor of the in-hospital mortality for COVID-19 patients.[20] The rising trend in neutrophil-to-lymphocyte ratio may indicate a risk of death for participants with COVID-19.[21] The interplay between STAT3 and STAT5 signaling pathways from normal and diseasespecific G-CSFR may lead to abnormal neutrophil productions.[22]
Compared with the non-ICU group, numerous laboratory abnormalities were detected in the ICU group. A previous study[10] indicated that SARS-CoV-2 might mainly act on lymphocytes, including T lymphocytes. SARS-CoV-2 could induce a cytokine storm in the body, thereby generating a series of immune responses and causing changes in peripheral white blood cells and lymphocytes. Additionally, several reports confirmed that the decrease of lymphocytes indicates that coronavirus consumed many immune cells and inhibited cellular immune function, which might lead to exacerbations of COVID-19 patients.[23] In this report, lower levels of neutrocytes and lymphocytes were detected in the ICU group, which may be caused by the cellular immune deficiency of the ICU group. In addition, higher levels of ESR, CRP, and PCT were detected in the ICU group, which indicated higher levels of inflammation in the ICU group. A longer prothrombin time was found in the ICU group, which might represent coagulation activation in the ICU group. Compared with non-ICU patients, higher levels of ALA, AST, CK, CK-MB CREA, and LDH were detected in patients of the ICU group, which was similar to previous reports.[11] These abnormalities suggest that the SARS-CoV-2 infection may be associated with myocardial injury, hepatic injury, and kidney injury. It may also be that people with these organ dysfunctions are more likely to be infected by SARS-CoV-2.
According to the suggestion of The Diagnosis and Treatment of Pneumonitis with 2019-nCoV Infection (DTPI) published by the National Health Commission of the PRC, all of the patients in this study were given lopinavir, and ritonavir tablets (2 pills BID peros), which were used for HIV infection in the past, combined with interferon alfa-2b injection (5 million IU add into 2 mL of sterile water, inhalation BID), and 51.7% received antibacterial agents. During the Severe Acute Respiratory Syndrome (SARS) period in 2003, usage of high doses of glucocorticoids caused a series of sequelae in survivors such as osteonecrosis of the femoral head and glucose metabolism disorders. Because of this lesson, we gave a small dose (40–80 mg/day) of glucocorticoid therapy in a short period (5 days) and adjusted the dose and time of medication according to the dynamic changes of the patient's chest CT imaging to control the immune response in the lungs. The patients avoided the occurrence of cytokine storms, thereby reducing the risk of complications, such as acute ARDS, in patients. Glucocorticoid therapy was given to 32.8% of patients.
There are several limitations to this study. First, COVID-19 was diagnosed by RT-PCR using throat swab samples, while no serum was obtained to assess the viremia. Second, few patients were included in this study. A multi-center study with a more significant number of patients should be performed. Third, since most patients involved in the present study were still hospitalized at the time of submission, it is difficult to evaluate the risk factors for adverse outcomes.
5. Conclusions
In this single-center study of 201 COVID-19 patients in Changsha, China, 22.4% of patients were admitted to ICU. We found that the risk of cellular immune deficiency, hepatic injury, and kidney injury was higher in ICU group, which should be monitored. Because SARS-nCoV-2 has pandemic potential, careful monitoring is essential, and more information about this disease is still needed for clinical management.
Acknowledgments
The authors would like to thank all of the co-investigators and colleagues who made this study possible. The authors would like to thank Changsha CDC, Hunan CDC, and CCDC for their assistance with laboratory testing. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this revised manuscript.
Author contributions
Conceptualization: Jian Zhou and Zhiguo Zhou.
Data curation: Jian Zhou, Jingjing Sun, Dixuan Jiang and Zhiguo Zhou.
Funding acquisition: Jian Zhou and Zhiguo Zhou.
Investigation: Jian Zhou and Zhiguo Zhou.
Methodology: Jian Zhou, Jingjing Sun, Dixuan Jiang and Zhiguo Zhou.
Validation: Jian Zhou, Ziqin Cao and Wanchun Wang.
Writing – original draft: Jian Zhou, Kang Huang, Fang Zheng and Yuanlin Xie.
Writing – review & editing: Jian Zhou and Zhiguo Zhou.
Footnotes
Abbreviations: ALA = alanine aminotransferase, ARDS = Acute respiratory distress syndrome, AST = aspartate aminotransferase, CCDC = Chinese Center for Disease Control and Prevention, CK = creatine kinase, CK-MB = creatine kinase-MB, COVID-19 = Coronavirus Disease 2019, CREA = creatinine, CRP = C-reactive protein, CT = computed tomographic, DTPI = The Diagnosis and Treatment of Pneumonitis with COVID-19 Infection, ESR = erythrocyte sedimentation rate, ICU = Intensive Care Unit, LDH = lactate dehydrogenase, PT = procalcitonin, PT = prolonged prothrombin time, RT-PCR = Real-time reverse transcriptase-polymerase chain reaction, RT-PCR = Real-Time Reverse Transcription Polymerase Chain Reaction, SARS-CoV-2 = Severe Acute Respiratory Syndrome Coronavirus 2 CoV, SPSS = Statistical Package for the Social Sciences, WBC = white blood cell.
How to cite this article: Zhou J, Sun J, Cao Z, Wang W, Huang K, Zheng F, Xie Y, Jiang D, Zhou Z. Epidemiological and clinical features of 201 COVID-19 patients in Changsha city, Hunan, China. Medicine. 2020;99:34(e21824).
JZ and JS contributed equally to this work.
This study was funded by the Innovative Major Emergency Project Funding against the New Coronavirus Pneumonia in Hunan Province (Grant No. 2020SK3014), Health and Family Planning Commission Fund Project in Hunan Province (Grant No. B2017209), Natural Science Foundation of Hunan Province (Grant No. 2018JJ2452), New Coronavirus Pneumonia Emergency Project of Changsha Science and Technology Bureau (Grant No. kq2001010 and kq2001008), the Mittal Innovation Project of Central South University (Grant No. GCX20190879Y) and the Fundamental Research Funds for the Central Universities of Central South University (Grant No. 2018zzts930). The study funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Ethics approval: This study was approved by the First Hospital of Changsha City Committee for Clinical Research and written consents were obtained from the enrolled patients. All of the methods were in accordance with the Declaration of Helsinki.
Consent to participate: All of the participants provided their written informed consent to participate in this study. All of the methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication: Written informed consent to publish has been received from each enrolled patient.
Availability of data and materials: The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Hui DS, I.A.E., Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020;91:264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol 2020;92:401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wuhan Municipal Health Commission. Report of novel coronavirus-infected pneumonia in China. Published January 20, 2020.Available at http://wjw.wuhan.gov.cn/front/web/showDetail/2020012009077 [accessed January 31, 2020]. [Google Scholar]
- [5].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].World Health Organization. Novel Coronavirus (2019-nCoV) situation reports. Situation report - 21. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports). February 10, 2020 [Google Scholar]
- [7].Johns Hopkins University CSSE. Wuhan coronavirus (2019-nCoV) global cases (https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6). January 24, 2020. [Google Scholar]
- [8].Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xiong Y, Sun D, Liu Y, et al. Clinical and high-resolution CT features of the COVID-19 Infection: comparison of the initial and follow-up changes. Invest Radiol 2020;55:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol 2020;55:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andrea M, Martina B, Sebastiano B, et al. Estimation of unreported novel coronavirus (SARS-CoV-2) infections from reported deaths: a susceptible–exposed–infectious–recovered–dead model. J Clin Med 2020;9:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. January 28, 2020.Available at: https://www.who.int/publications-detail/clinical-managementof-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected [accessed January 31, 2020]. [Google Scholar]
- [16].Laboratory diagnostics for novel coronavirus. WHO 2020 (https://www.who.int/health-topics/coronavirus/laboratory-diagnostics-for-novel-coronavirus). January 17, 2020. [Google Scholar]
- [17].Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chang, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA 2020;323:1092–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zuo Y, Zuo M, Yalavarthi S, et al. Neutrophil extracellular traps and thrombosis in COVID-19. medRxiv 2020;04.30.20086736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].2020;Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. 81:e6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].2020;Ye W, Chen G, Li X, et al. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. 1:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pankaj D, Kenneth DG. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol 2017;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu WJ, Zhao M, Liu K, et al. T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral Res 2017;137:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


