Abstract
This study assessed the association of sirtuin type 1 (SIRT1) and survivin expression with the clinicopathological features and survival of esophageal squamous cell carcinoma (ESCC) patients after concurrent chemoradiotherapy.
SIRT1 and survivin proteins were immunohistochemically stained in 93 ESCC tissue specimens.
SIRT1 was expressed in ESCC (80.6% vs 25.8% in normal mucosae) and survivin was expressed in 67.7% of ESCC vs 19.4% normal tissues (P < .01), and SIRT1 expression was associated with survivin expression (r = 0.39, P < .05). Furthermore, expression of both SIRT1 and survivin was associated with tumor size, depth of tumor invasion, tumor differentiation, lymph node metastasis, advanced clinical stage, and chemoradiotherapy (P < .05) as well as poor progression-free survival (PFS; P < .05) of ESCC patients after concurrent chemoradiotherapy (P < .05). Patient age, chemotherapy, tumor size, clinical stage, lymph node metastasis, and SIRT1 and survivin expression were independent PFS predictors (P < .05).
Expression of both SIRT1 and survivin was associated with poor ESCC PFS.
Keywords: esophageal squamous cell cancer, prognosis, sirtuin type 1, survivin
1. Introduction
Esophageal cancer remains a significant global health concern;[1] for example, in China, esophageal cancer is among the most commonly diagnosed cancers and was the third major cause of cancer-related death in 2015.[2] Histologically, esophageal cancer can generally be divided into squamous cell carcinoma (ESCC) and adenocarcinoma, and the incidence of ESCC shows significant variation geographically compared with other malignancies, with very high incidence rates in China, Iran, South Africa, France, and Italy.[3] ESCC is typically diagnosed at an advanced pathologic stage of disease and associated with very poor outcomes because esophageal cancer cells are highly invasive and rapidly metastatic,[4,5] leading to a 5-year survival rate of only 10% to 15%.[6–8] To date, the exact etiology of esophageal cancer remains to be defined. Tobacco smoking and alcohol consumption are known risk factors for ESCC, and nutritional factors, like pickled vegetables, have also been linked to ESCC development.[3] Esophageal cancer development may involve multiple genetic alterations, as characterized by Hanahan and Weinberg[9] as 6 essential hallmarks of cancer cells, that is, self-sufficiency in growth, insensitivity to growth-inhibition, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and invasion and metastasis. Thus, further studies of these molecular alterations could help us to better understand the pathogenesis of ESCC and support the development of novel strategies and/or biomarkers for early detection, molecular targeting therapy or prevention, and prediction of prognosis in this now deadly disease.
Towards this end, our group focuses on the identification and evaluation of biomarkers for early detection of ESCC and prediction of prognosis. Silent mating type information regulation 2 homolog 1, also known as sirtuin type 1 (SIRT1), is a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase that deacetylates proteins that contribute to cellular reactions to stressors or longevity.[10,11] Research has shown that SIRT1 is involved in DNA damage repair, cell cycle, apoptosis, and oxidative stress in cells.[12] Moreover, SIRT1 possesses an anti-apoptotic effect and promotes carcinogenesis,[13] and SIRT1 overexpression occurs in a variety of solid tumors.[13] In ESCC, a previous study revealed that SIRT1 overexpression is an independent prognostic factor for patients.[14] However, another previous study showed that although SIRT1 expression is frequently upregulated in gastrointestinal cancers, its prognostic value in these cancer patients remains inconclusive.[15]
Survivin is a member of the inhibitor-of-apoptosis protein family and functions to suppress caspase activation, thereby leading to negative apoptosis regulation.[16,17] Indeed, overexpression of survivin is associated with tumor progression and induces anticancer drug resistance; thus, survivin represents a potential therapeutic target for control of different human cancers.[16,17] In ESCC, survivin overexpression is associated with poor clinical outcomes,[18,19] while inhibition of survivin induces the sensitivity of tumor cells to radiation.[20] SIRT1 is able to deactivate the p53 protein but stimulate autophagy.[21,22] In contrast, p53 can inhibit survivin expression at the transcriptional level.[23] p53 mutations and dysfunctions in ESCC are well established and contribute to ESCC development and progression.[3] Thus, in the present study, we collected tissue specimens from 93 cases of ESCC for immunostaining of SIRT1 and survivin proteins and then analyzed the association of their expression with clinicopathological features and prognosis in ESCC patients who had received concurrent chemoradiotherapy.
2. Materials and methods
2.1. Patients and specimens
Tissue samples were obtained from 93 ESCC patients in the Department of Pathology, The People Hospital of Linyi (Shandong, China). These patients received medical care between August 2010 and October 2012. The inclusion criteria were:
-
(i)
provision of informed consent for participation in this study by the patient or representing family member before surgery;
-
(ii)
histologically confirmed diagnosis of ESCC without distant metastasis or other malignancies; and
-
(iii)
no treatment with chemoradiotherapy or other anti-cancer therapy before surgery. ESCC patients were diagnosed surgically according to the World Health Organization classifications[24] and staged following the American Joint Committee on Cancer TNM classifications.[25] All patients underwent total esophagectomy and radical lymph node dissection, and 50 patients also received concurrent chemoradiotherapy with standard cisplatin and 5-fluorouracil doses and radiotherapy with 50 Gy doses. Normal esophageal tissues were collected from >5 cm away from tumor lesions for use as non-tumor control samples, and the margin included no cancer cells. This study was approved by the Ethics Committee of The People Hospital of Linyi (Shandong, China), and all participants or their next of kin provided written informed consent before their enrollment in this study.
2.2. Follow-up of patients
Each patient was routinely followed up every 3 months in the first 2 years and then every 6 months thereafter. Overall survival (OS) was defined as the time from diagnosis to the date of death or the last follow-up, while the progression-free survival (PFS) was calculated between the time of tumor diagnosis and the date of disease progression. All 93 patients were followed up until February 2015 for a median follow-up period of 30 months (range, 5–54 months).
2.3. Immunohistochemistry
ESCC tissue specimens and paired esophageal non-cancerous squamous mucosae were routinely embedded in paraffin and sectioned into 4 to 5 μm thick tissue sections. In brief, the sections were first deparaffinized twice in xylene and rehydrated in a series of ethanol (100%–50%) and in deionized water. Antigen retrieval was performed in a pressure cooker in 10 mmol/L sodium citrate solution (pH 6.0) for 2 to 2.5 minute, while the activity of endogenous peroxidase was prevented by immersion in 3% H2O2 in deionized water at room temperature for 10 minute. After being washed 3 times in phosphate-buffered saline (PBS), the sections were blocked in a normal serum (Polink-1 PV6000 secondary antibody detection kit, Zhongshan Goldenbridge Co., Ltd., Beijing, China) at room temperature for 30 minute and then with 50 μL of a monoclonal anti-SIRT1 antibody (Abcam, Rockville, MD) at a dilution of 1:200 or with an anti-survivin antibody (Epi Biotechnology Co. Ltd., San Francisco, CA) at a dilution of 1:200 at 37°C for 60 minute. The sections were then washed 3 times in PBS and incubated with a secondary antibody from the detection kit. Next, the sections were further washed with PBS 3 times and then subjected to color reaction with 3, 3-diaminobenzidin (DAB) solution from the detection kit in the dark at room temperature for approximately 10 minute. After being counterstained with Harris hematoxylin (Zhongshan Goldenbridge Biotechnology Co., Ltd.), dehydrated in ethanol, and cleared in xylene, the sections were mounted with a neutral gum. The negative control sections were incubated with PBS instead of the primary antibody.
The immunostained sections were viewed and scored under a light microscope by a pathologist who had no knowledge of the identity of the patients. The nuclear staining (brown color) was regarded as positive for survivin expression, while the nuclear and cytoplasmic staining (brown color) was considered as positive staining for SIRT1 expression. For each section, we selected 5 high power (400x) fields to assess the proportion of immunostained tumor cells and the nuclear staining intensity of the entire sections. The staining intensity scores ranged from 0 (negative), 1 (weak staining), 2 (moderate), and 3 (strong), while the percent cell staining scores were 0 (no staining), 1 (1%–25%), 2 (26%–50%), 3 (51%–75%), and 4 (76%–100%). The staining index was obtained by addition of these 2 scores together, and if the staining index was more than 4, the case was considered as positive for survivin and SIRT1 expression according to a previous study.[14,24]
2.4. Statistical analysis
The SPSS 21.0 software package (SPSS Inc., Chicago, IL) was utilized for all statistical analyses of our current data. The association of SIRT1 and survivin expression with clinicopathological variables was assessed using the Chi-squared test or Fisher exact probability test, if appropriate, while the association between SIRT1 and survivin expression was analyzed by using the Spearman test and the association of SIRT1 and survivin expression with OS and PFS was plotted by using Kaplan–Meier curves and statistically analyzed using the log rank test. The Cox regression test was used for multivariate analysis of independent prognostic predictors. A P value ≤.05 was considered statistically significant.
3. Results
3.1. Characteristics of patients
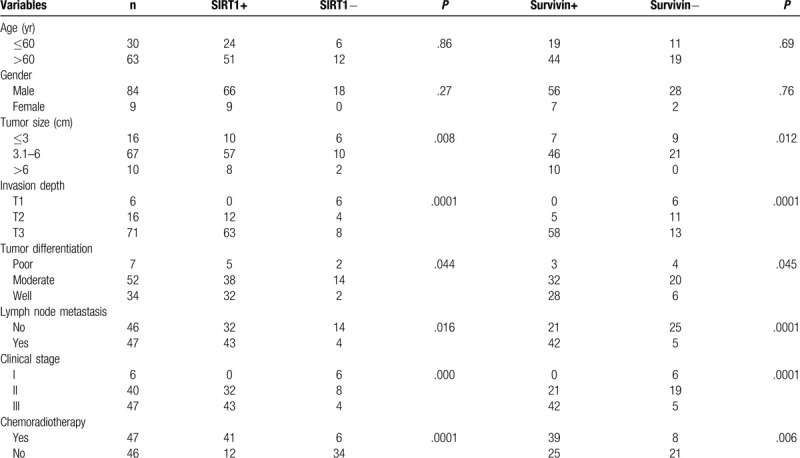
For this study, ESCC specimens were collected from 93 patients with a median age at diagnosis of 63 years (range, 41–78 years). Tumor infiltration was observed into the mucosal layer in 6 patients, into the muscular layer in 16 patients, and into the fiber membrane layer in 82 patients. Moreover, tumors were well differentiated in 34 cases, moderately differentiated in 52 cases, and poorly differentiated in 7 cases. ESCC was classified as stage I in 6 patients, stage II in 40 patients, and stage III in 47 patients (Table 2). All of the 93 patients were followed-up until February 2015 for a median follow-up period of 30 months (range, 5–54 months).
Table 2.
Association of sirtuin type 1 and survivin expression with clinicopathological features of esophageal squamous cell carcinoma patients.
3.2. Overexpression of SIRT1 and survivin proteins in ESCC tissues
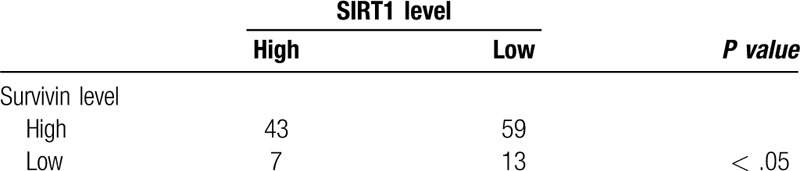
Our immunohistochemical staining analysis showed that survivin expression was localized in the nuclei of tumor cells, whereas positive staining for SIRT1 expression was seen in the cytoplasm of tumor cells (Fig. 1). Overall, SIRT1 protein was expressed in 80.6% (75/93) of ESCC tissues compared to only 25.8% (24/93) of the normal squamous epithelia specimens (χ2 = 56.16, P < .01). Furthermore, survivin was expressed in 67.7% (63/93) of ESCC tissues vs 19.4% (18/93) of the normal squamous epithelium samples (χ2 = 44.28, P < .01). Survivin expression was associated with SIRT1 expression in ESCC tissues (r = 0.39, P < .05; Table 1).
Figure 1.
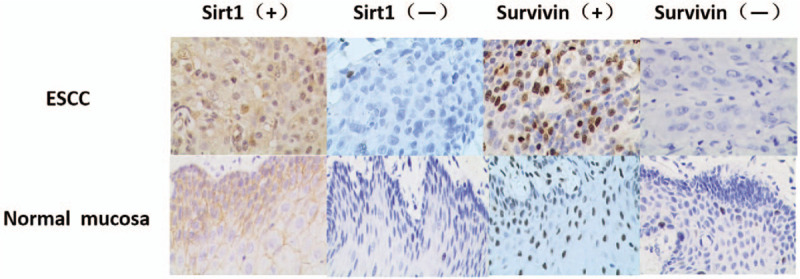
Differential expression of sirtuin type 1 and survivin in esophageal squamous cell carcinoma tissues. Paraffin-embedded tissue sections of esophageal squamous cell carcinoma tissues and normal squamous mucosae were prepared and immunostained with anti- sirtuin type 1 or anti-survivin antibodies. All magnification, 200×.
Table 1.
Association of sirtuin type 1 expression with Survivin expression in esophageal squamous cell carcinoma tissue samples.
3.3. Associations of SIRT1 or survivin expression with clinicopathological features of patients
SIRT1 expression was associated with tumor size, depth of tumor invasion, tumor differentiation, lymph node metastasis, advanced clinical stage, and chemoradiotherapy (P < .05). Furthermore, survivin expression was linked to tumor size, depth of tumor invasion, tumor differentiation, lymph node metastasis, advanced tumor clinical stage, and chemoradiotherapy (P < .05). However, our analysis showed no association of SIRT1 and survivin expression with patient age or gender (P > .05; Table 2).
3.4. Association of SIRT1 and survivin expression with OS and PFS of ESCC patients
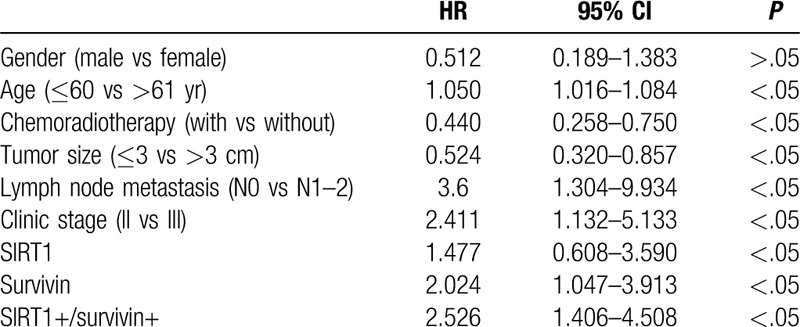
Expression of SIRT1 and survivin individually and in combination was associated with OS (χ2 = 9.97, P < .01; χ2 = 13.97, P < .01, respectively) and PFS (t = 2.45, P < .05; t = 4.532, P < .01, respectively) (Fig. 2, Table 3). Our multivariate analysis showed that age, chemotherapy, tumor size, lymph node metastasis, clinical stage, and SIRT1 and survivin expression were all independent predictors for PFS in ESCC patients after concurrent chemoradiotherapy (P < .05; Table 4).
Figure 2.
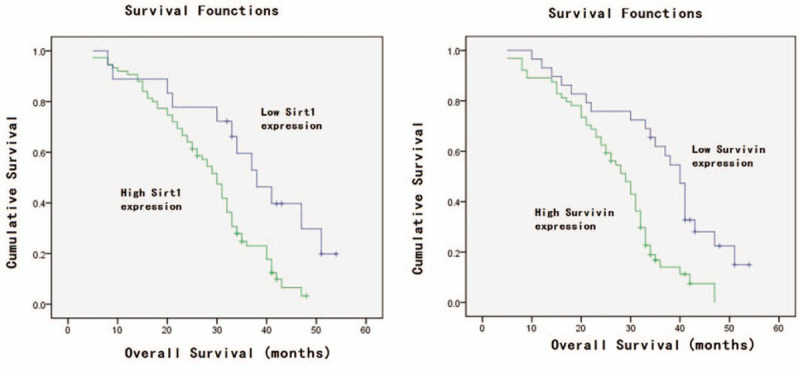
Kaplan–Meier curves for progression-free survival stratified by sirtuin type 1 (SIRT1) and survivin expression. A, SIRT1; B, survivin; and C, SIRT1 and survivin.
Table 3.
Association of different sirtuin type 1 and survivin expression statuses with overall survival and progression-free survival in esophageal squamous cell carcinoma patients.
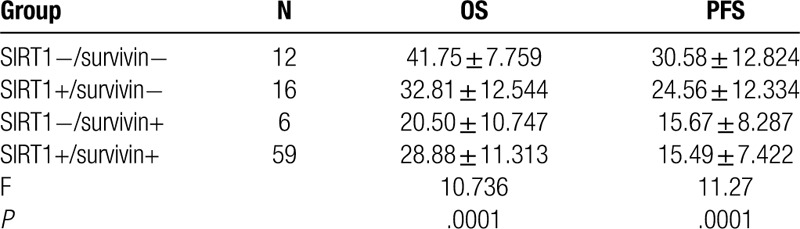
Table 4.
Multivariate analysis of overall survival of esophageal squamous cell carcinoma patients.
4. Discussion
ESCC is frequently detected at advanced clinical stages, which contributes to its poor prognosis. Thus, the identification and evaluation of biomarkers for the prediction of survival could help medical oncologists to effectively manage ESCC patients. In our current study, we re-assessed the association of SIRT1 and survivin protein expression with OS and PFS in ESCC patients. We found that both SIRT1 and survivin were expressed in ESCC tissues more often than in normal tissue specimens. SIRT1 expression was associated with tumor invasion, tumor differentiation, lymph node metastasis, and advanced clinical stage (P < .05), while survivin expression was associated with tumor size, depth of tumor invasion, tumor differentiation, lymph node metastasis, and advanced tumor clinical stage (P < .05). Moreover, expression of SIRT1 and survivin, individually and in combination, was associated with poor OS and PFS among ESCC patients after concurrent chemoradiotherapy. In addition, age, chemotherapy, tumor size, lymph node metastasis, clinical stage, and SIRT1 and survivin expression were all independent predictors for OS and PFS among ESCC patients.
SIRT1 has been shown to be involved in the tumorigenesis and lymph node metastasis of various types of human cancers.[26–29] In ESCC, a previous study reported that SIRT1 also possesses a tumor-promoting role and linked SIRT1 expression to ESCC T stage [26], while another study showed that basal SIRT1 protein levels are higher in ESCC tissues than in control samples and SIRT1 overexpression is significantly associated with tumor TNM stage and lymph node metastasis.[30] SIRT1 overexpression was also shown to be associated with poor ESCC prognosis,[31] and high SIRT1 expression in ESCC was suggested as an independent diagnostic and prognostic marker for ESCC.[31] Our current study confirmed these data and indicated that SIRT1 expression is associated with ESCC lymph node metastasis, advanced tumor clinical stage, and poor OS and PFS.
Survivin functions to inhibit caspase activation, thereby promoting cell survival.[16] Survivin is overexpressed in a variety of human cancers and associated with poor prognosis in ESCC.[18,19] Our current study also confirmed these previous data. Our hypothesis that the combination of these 2 proteins could serve as a prognostic biomarker for survival prediction was based on the relationship of these two proteins with p53 protein. It is well established that p53 is highly mutated in ESCC, and SIRT1 was reported to deactivate the p53 protein.[22] In addition, p53 can inhibit survivin expression.[23] In this regard, overexpression of SIRT1 could lead to inactivation of wild-type p53 and subsequently induce survivin expression in ESCC, promoting ESCC development and progression, and this mechanism is supported by a previous review article showing that SIRT1 can negatively regulates expression of beta-catenin and survivin, although the review article classified SIRT1 as a tumor suppressor.[29] In contrast, a previous study showed that SIRT1 expression is not associated with survivin expression in ESCC, but with epidermal growth factor receptor (EGFR) expression,[32] which is inconsistent with our current findings. Thus, further study is needed to specifically define the relationship and role of SIRT1 and survivin in ESCC. Our current study demonstrated that the combination of these 2 proteins was a significant predictor for poor survival among ESCC patients.
Chemoradiotherapy can reduce loco-regional recurrence of ESCC and improve outcomes after the surgery. Thus, detection of these 2 proteins could help medical oncologists to predict treatment responses and PFS in ESCC patients. However, our current study does have limitations; for example, the current study was retrospective, descriptive, and not well controlled for a particular chemoradiotherapy among the ESCC patients. The sample size was also relatively small.
5. Conclusions
Both SIRT1 and survivin were expressed more frequently in ESCC tissues than in normal tissues, and their expression was associated with lymph node metastasis and poor OS and PFS. Further studies are needed to confirm our findings and determine the clinical value of these proteins as a prognostic biomarker in ESCC as well as to investigate the underlying molecular mechanisms leading to their expression and action in ESCC.
Acknowledgments
The authors would like to thank the Department of Thoracic Surgery, Linyi People's Hospital.
Author contributions
LY and XZ conceived and designed the experiments; QZ and SW prepared the manuscript; LL and NJ performed the experiments; LY analyzed the data. All authors read and approved the final manuscript.
Footnotes
Abbreviations: ESCC = esophageal squamous cell carcinoma, OS = overall survival, PBS = phosphate-buffered saline, PFS = progression-free survival, SIRT1 = sirtuin type 1.
How to cite this article: Yan L, Zhao Q, Liu L, Jin N, Wang S, Zhan X. Expression of SIRT1 and survivin correlates with poor prognosis in esophageal squamous cell carcinoma. Medicine. 2020;99:34(e21645).
This study was supported in part by a grant from Shandong Medical and Health Development Plan 2014WS0288.
The authors have no conflicts of interest to disclose.
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Xu XC. Risk factors and gene expression in esophageal cancer. Methods Mol Biol 2009;471:335–60. [DOI] [PubMed] [Google Scholar]
- [4].Akutsu Y, Matsubara H, Shuto K, et al. Clinical and pathologic evaluation of the effectiveness of neoadjuvant chemoradiation therapy in advanced esophageal cancer patients. World J Surg 2009;33:1002–9. [DOI] [PubMed] [Google Scholar]
- [5].Talsma K, van Hagen P, Grotenhuis BA, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM classification for esophageal cancer. Ann Surg Oncol 2012;19:2142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen H, Wang Z, Yang Z, et al. Prospective study of adjuvant radiotherapy on preventing lymph node metastasis after Ivor-lewis esophagectomy in esophageal cancer. Ann Surg Oncol 2013;20:2721–6. [DOI] [PubMed] [Google Scholar]
- [7].Cao B, Shi Q, Wang W. Higher expression of SIRT1 induced resistance of esophageal squamous cell carcinoma cells to cisplatin. J Thorac Dis 2015;7:711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lv L, Shen Z, Zhang J, et al. Clinicopathological significance of SIRT1 expression in colorectal adenocarcinoma. Med Oncol 2014;31:965. [DOI] [PubMed] [Google Scholar]
- [9].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- [10].Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun 1999;260:273–9. [DOI] [PubMed] [Google Scholar]
- [11].Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci Am 2006;294:48–51, 54–47. [DOI] [PubMed] [Google Scholar]
- [12].Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res 2009;69:1702–5. [DOI] [PubMed] [Google Scholar]
- [13].Cheung CH, Huang CC, Tsai FY, et al. Survivin–biology and potential as a therapeutic target in oncology. Onco Targets Ther 2013;6:1453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ma MC, Chiu TJ, Lu HI, et al. SIRT1 overexpression is an independent prognosticator for patients with esophageal squamous cell carcinoma. J Cardiothorac Surg 2018;13:25.doi:10.1186/s13019-018-0718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu S, Jiang J, Liu J, et al. Meta-analysis of SIRT1 expression as a prognostic marker for overall survival in gastrointestinal cancer. Oncotarget 2017;8:62589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sah NK, Khan Z, Khan GJ, et al. Structural, functional and therapeutic biology of survivin. Cancer Lett 2006;244:164–71. [DOI] [PubMed] [Google Scholar]
- [17].Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer 2003;3:46–54. [DOI] [PubMed] [Google Scholar]
- [18].Li C, Li Z, Zhu M, et al. Clinicopathological and prognostic significance of survivin over-expression in patients with esophageal squamous cell carcinoma: a meta-analysis. PLoS One 2012;7:e44764.doi: 10.1371/journal.pone.0044764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xia H, Chen S, Huang H, et al. Survivin over-expression is correlated with a poor prognosis in esophageal cancer patients. Clin Chim Acta 2015;446:82–5. [DOI] [PubMed] [Google Scholar]
- [20].Liu X, Zhao Y, Zhang W, et al. Inhibition of survivin enhances radiosensitivity of esophageal cancer cells by switching radiation-induced senescence to apoptosis. Onco Targets Ther 2018;11:3087–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 2008;105:3374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu T, Ma X, Ouyang T, et al. SIRT1 reverses senescence via enhancing autophagy and attenuates oxidative stress-induced apoptosis through promoting p53 degradation. Int J Biol Macromol 2018;117:225–34. [DOI] [PubMed] [Google Scholar]
- [23].Mirza A, McGuirk M, Hockenberry TN, et al. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene 2002;21:2613–22. [DOI] [PubMed] [Google Scholar]
- [24].Shen Z, Ren Y, Ye D, et al. Significance and relationship between DJ-1 gene and surviving gene expression in laryngeal carcinoma. Eur J Histochem 2011;55:e9.doi: 10.4081/ejh.2011.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li C, Wang L, Zheng L, et al. SIRT1 expression is associated with poor prognosis of lung adenocarcinoma. Onco Targets Ther 2015;8:977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen GQ, Tian H, Yue WM, et al. SIRT1 expression is associated with lymphangiogenesis, lymphovascular invasion and prognosis in pN0 esophageal squamous cell carcinoma. Cell Biosci 2014;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001;107:137–48. [DOI] [PubMed] [Google Scholar]
- [28].Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001;107:149–59. [DOI] [PubMed] [Google Scholar]
- [29].Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta 2010;1804:1684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].TNM Classification of the Esophagus. International Union Against Cancer. 6th edNew York: J Willy Sons; 2002. [Google Scholar]
- [31].He Z, Yi J, Jin L, et al. Overexpression of Sirtuin-1 is associated with poor clinical outcome in esophageal squamous cell carcinoma. Tumour Biol 2016;37:7139–48. [DOI] [PubMed] [Google Scholar]
- [32].Han F, Zhang S, Liang J, et al. Clinicopathological and predictive significance of SIRT1 and peroxisome proliferator-activated receptor gamma in esophageal squamous cell carcinoma: the correlation with EGFR and survivin. Pathol Res Pract 2018;214:686–90. [DOI] [PubMed] [Google Scholar]


