Abstract
Noninvasive tests for the assessment of liver fibrosis are highly needed for the management of patients with autoimmune hepatitis (AIH). We aimed to investigate the accuracy of red cell distribution width to platelet ratio (RPR) in predicting liver fibrosis in AIH patients. One hundred nineteen AIH patients who underwent liver biopsy were enrolled. Liver fibrosis stage was diagnosed using the Scheuer scoring system. The diagnostic accuracy was evaluated by the area under the receiver operating characteristic curve (AUROC). RPR values in AIH patients with S2-S4 (0.10, interquartile range [IQR] 0.08–0.15), S3-S4 (0.10, IQR 0.09–0.14), and S4 (0.14, IQR 0.09–0.19) were significantly higher than patients with S0-S1 (0.07, IQR 0.06–0.08, P < .001), S0-S2 (0.08, IQR 0.06–0.12, P = .025) and S0-S3 (0.09, IQR 0.07–0.13, P = .014), respectively. The RPR was positively correlated with fibrosis stages (r = 0.412, P < .001), while aspartate transaminase to platelet ratio index (APRI) and fibrosis-4 score (FIB-4) were not significantly associated with fibrosis stages in AIH patients. The AUROCs of RPR in identifying significant fibrosis (S2-S4), advanced fibrosis (S3-S4), and cirrhosis (S4) were 0.780 (95% confidence interval [CI] 0.696–0.865), 0.639 (95% CI 0.530–0.748), and 0.724 (95% CI 0.570–0.878), respectively. The AUROCs of RPR were significantly higher than APRI and FIB-4 in diagnosing significant fibrosis, advanced fibrosis, and cirrhosis. Our study demonstrates that the RPR is a simple predictor of liver fibrosis and is superior to APRI and FIB-4 in identifying liver fibrosis in AIH patients.
Keywords: autoimmune hepatitis, liver fibrosis, noninvasive tests, red cell distribution width
1. Introduction
Autoimmune hepatitis (AIH) is an immune-mediated chronic inflammatory liver disease, which is classically characterized by elevated serum transaminase and immunoglobulin G levels, hypergammaglobulinemia, specific autoantibodies production, and liver interface hepatitis on pathological examination.[1,2] Chronic liver inflammation can result in liver fibrosis, cirrhosis, and hepatic carcinoma in AIH.[3] Given the lack of specific diagnostic markers for AIH patients, majority of patients already have significant fibrosis or even cirrhosis when they are first diagnosed.[3] Evaluating the stages of liver fibrosis is essential for choosing treatment strategies and estimating long-term prognosis for AIH patients.[4]
Liver biopsy (LB) is the gold standard to assess disease activity and liver fibrosis in AIH patients.[4,5] However, LB is not an optimal method for evaluating liver fibrosis due to its invasiveness, high cost, sampling errors, and observer discrepancy.[6,7] In addition, it is difficult to observe the dynamical changes of liver fibrosis by LB. Therefore, noninvasive tests (NITs) for assessing liver fibrosis were developed in the past years. Transient elastography (TE) is a promising method with high accuracy for evaluating liver fibrosis in chronic viral hepatitis.[8–10] However, the predicting accuracy of TE in AIH patients is controversial, since elevated alanine aminotransferase (ALT) levels may influence the accuracy of TE in detecting early stages of fibrosis.[11] In addition, high cost of equipment limits the clinical use of TE in resource-limited settings.[12] Several NITs based on clinical parameters for assessing liver fibrosis have been established, including aspartate transaminase (AST) to platelet (PLT) ratio index (APRI) and the fibrosis-4 score (FIB-4). APRI and FIB-4 were initially proposed to assess liver fibrosis with relatively high accuracy in patients with chronic hepatitis C (CHC).[13,14] APRI and FIB-4 are also recommended to assess significant fibrosis and cirrhosis in both chronic hepatitis B (CHB) and CHC patients by the World Health Organization.[12,15] However, several studies reported that the predicting performances of these 2 NITs in AIH patients were not satisfied.[16,17]
A simpler and easy-to-calculate NIT, red cell distribution width (RDW) to PLT ratio (RPR), was developed to assess liver fibrosis and cirrhosis for CHB patients.[18] The area under the receiver operating characteristic curves (AUROCs) of RPR were 0.825 and 0.884 for diagnosing significant fibrosis and cirrhosis in CHB patients, which were superior to the FIB-4 and APRI.[18] In other liver diseases, RPR also has an excellent performance for predicting liver fibrosis.[19] Wang et al reported that RPR had a higher accuracy than APRI and FIB-4 in identifying significant fibrosis in patients with primary biliary cirrhosis (PBC).[19] However, whether RPR can be used to predict fibrosis stages in AIH patients remains unclear. In the present study, we analyzed the diagnostic accuracy of RPR for significant liver fibrosis, advanced liver fibrosis, and liver cirrhosis in AIH patients. Furthermore, we compared the predicting values of RPR with APRI and FIB-4 for liver fibrosis stages.
2. Methods
2.1. Patients
Between July 2016 and June 2019, a total of 127 consecutive AIH patients from Nanjing Drum Tower Hospital, The Second Hospital of Nanjing and Huai’an No. 4 People's Hospital, who underwent LB were enrolled in the present study. One hundred four (81.9%) of the patients were female and the median age of patients were 53.0 (interquartile range [IQR] 46.0, 60.0) years. AIH patients were diagnosed according to the practice guidelines of the American Association for the Study of Liver Diseases.[4] None of patients received immunosuppressive therapy before LB. Patients with the following conditions were excluded from the study:
-
(1)
combined with other liver diseases, such as viral hepatitis, nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease, PBC, and metabolic liver disease;
-
(2)
co-existence of hepatic carcinoma or other malignant tumor;
-
(3)
severe cardiac, respiratory, renal, hematological, and psychiatric diseases.
Among the 127 patients, 5 patients who were combined with CHB, 2 patients with CHC, and 1 patient with insufficient data were excluded. Finally, 119 AIH patients were included for analysis in this study.
All patients provided written informed consent for the LB, and this study was performed according to the ethics principles of the Declaration of Helsinki and approved by the Ethics Committees of Nanjing Drum Tower Hospital, The Second Hospital of Nanjing and Huai’an No. 4 People's Hospital.
2.2. LB and laboratory test
Ultrasound-guided LB was performed using a 16-gauge disposable needle. All liver specimens were scored by pathologists blinded to patient clinical characteristics. Liver fibrosis stages were evaluated according to the Scheuer scoring system.[20] Liver fibrosis was classified into the following 5 stages: S0, no fibrosis; S1, portal fibrosis without septa; S2, portal fibrosis with rare septa; S3, numerous septa without cirrhosis; and S4, cirrhosis.[20] S2-S4, S3-S4, and S4 are defined as significant liver fibrosis, advanced liver fibrosis, and liver cirrhosis, respectively. We retrospectively reviewed the medical records of the enrolled patients. Demographic and clinical characteristics were recorded within 1 week before LB, including age, sex, blood routine, biochemistry, and immunology tests.
2.3. Computational formula of NITs
The NITs used in the present study were as follows: APRI: (AST (U/L)/ULN of AST)/PLT count (109/L) ×100[13]; FIB-4: (age (years) × AST (U/L))/ ((PLT count (109/L) × (ALT (U/L))1/2)[14]; RPR: RDW (%)/PLT count (109/L).[18]
2.4. Statistical analyses
Continuous variables were presented as the median (IQR) and were compared using the independent t test or Mann–Whitney U test. Categorical variables were expressed as percentages and were analyzed by Chi-square test. The correlation between NITs and liver fibrosis stage was determined using Spearman rank correlation test. Receiver operating characteristic (ROC) curves was performed to evaluate the predictive accuracy of different NITs. The AUROCs and 95% confidential interval (CI) of AUROC were calculated. Differences between the AUROCs were tested using the z-test. The cut-off values were determined by the Youden index which was the optimal combination of sensitivity and specificity. Differences were considered to be significant at a 2-tailed P < .05. All statistical analyses were carried out using the SPSS statistical software version 22.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Study population
A total of 119 AIH patients were included for the analysis. The characteristics of patients were shown in Table 1. The majority of patients were female (83.2%) and median age was 52.5 (IQR 44.5, 59.8) years old. The median levels of PLT, RDW, ALT, and immunoglobulin G were 156.5 (IQR 118.3, 196.5) × 109/L, 13.7 (IQR 12.9, 15.6) %, 81.6 (IQR 40.0, 204.7) U/L, and 15.9 (IQR 12.8, 20.8) g/L, respectively. Eighty-two patients (68.9%) were positive for antinuclear antibody and 17 (14.3%) were positive for anti-smooth muscle antibody. The distributions of each liver fibrosis stage were as follows: S0, 4 (3.4%) patients; S1, 33 (27.7%) patients; S2, 48 (40.3%) patients; S3 22 (18.5%) patients; S4 12 (10.1%) patients. The median values of APRI, FIB-4, and RPR were 1.32 (IQR 0.56, 2.64), 2.69 (IQR 1.35, 5.10), and 0.09 (IQR 0.07, 0.13), respectively.
Table 1.
Baseline characteristics of study patients.
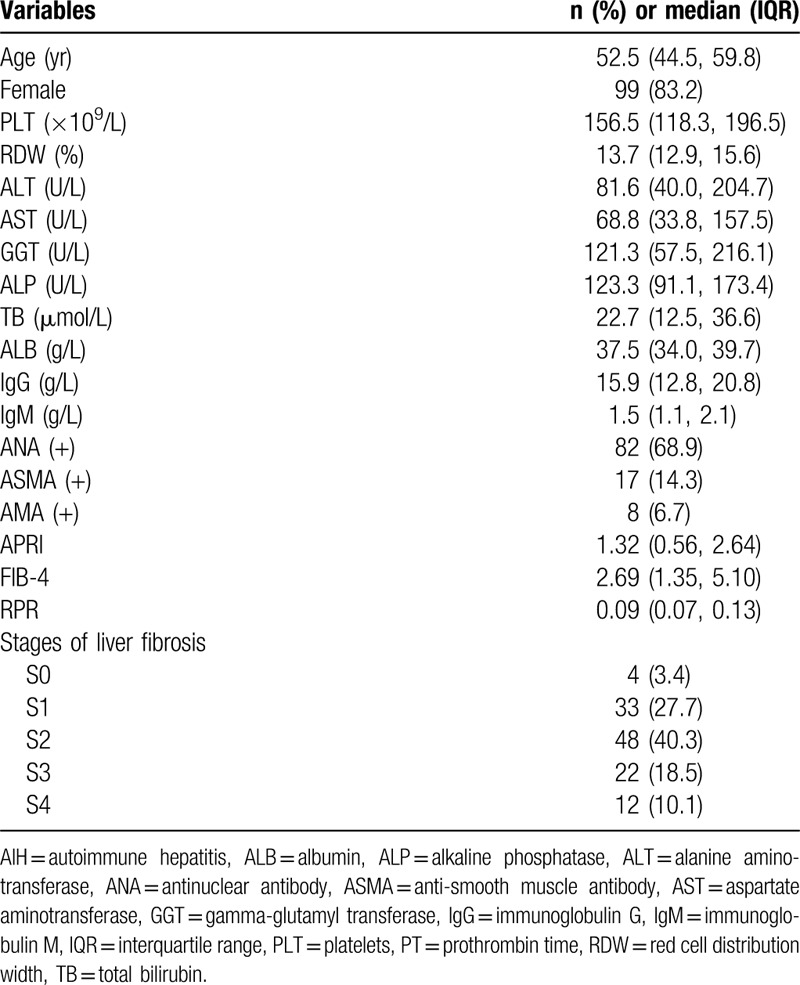
3.2. Comparisons of different NITs according to liver fibrosis stages
The levels of APRI, FIB-4, and FIB-4 in different fibrosis stages were showed in Figure 1. The results showed that RPR values in patients with S2-S4 (0.10, IQR 0.08–0.15), S3-S4 (0.10, IQR 0.09–0.14), and S4 (0.14, IQR 0.09–0.19) were significantly higher than that of patients with S0-S1 (0.07, IQR 0.06–0.08, P < .001), S0-S2 (0.08, IQR 0.06–0.12, P = .018), and S0-S3 (0.09, IQR 0.07–0.13, P = .011), respectively. However, the values of APRI were not significant different between patients with S0-S1 (1.31, IQR 0.48–3.24) and S2-S4 (1.37, IQR 0.57–2.52, P = .991), S0-S2 (1.44, IQR 0.49–3.24) and S3-S4 (1.03, IQR 0.57–1.90, P = .261), S0-S3 (1.41, IQR 0.55–2.82) and S4 (0.89, IQR 0.58–1.40, P = .332). FIB-4 values in patients with S2-S4 (3.22, IQR 1.58–6.41) were significantly higher than that of patients with S0-S1 (1.83, IQR 1.22–3.76, P = .017), while the FIB-4 values were not significant different between patients with S0-S2 (2.62, IQR 1.26–5.18) and S3-S4 (2.99, IQR 1.53–5.06, P = .713), S0-S3 (2.69, IQR 1.29–5.10) and S4 (3.28, IQR 1.72–5.97, P = .689). The RPR was positively correlated with fibrosis stages (r = 0.412, P < .001), while the APRI (r = −0.061, P = .511) and FIB-4 (r = 160, P = .083) were not correlated with fibrosis stages (Fig. 2).
Figure 1.
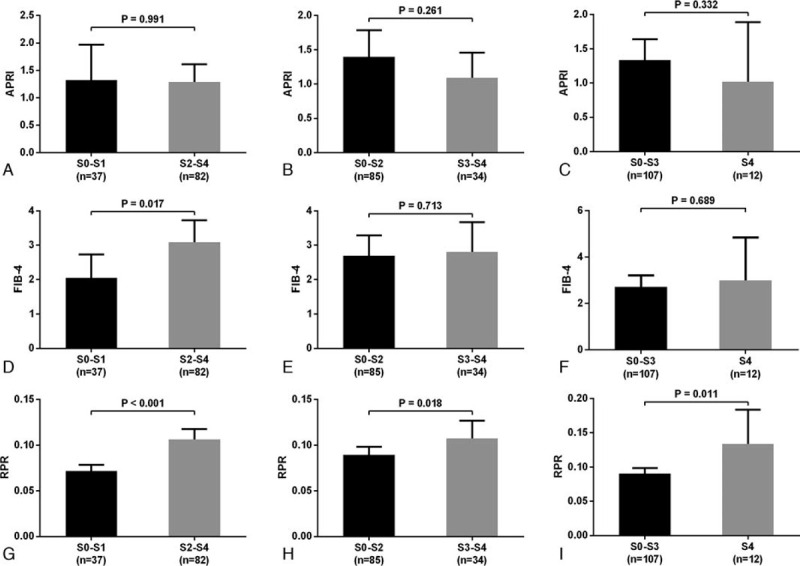
Comparisons of the APRI (A), FIB-4 (B), and RPR (C) levels according to different liver fibrosis stages in AIH patients. AIH = autoimmune hepatitis, APRI = aspartate transaminase to platelet ratio index, FIB-4 = fibrosis-4 score, RPR = red cell distribution width to platelet ratio.
Figure 2.
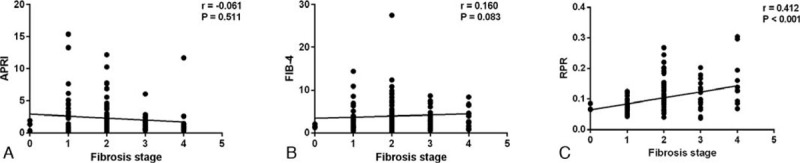
Correlations between different noninvasive tests and liver fibrosis stages.
3.3. Comparisons of diagnostic accuracy between RPR and other NITs
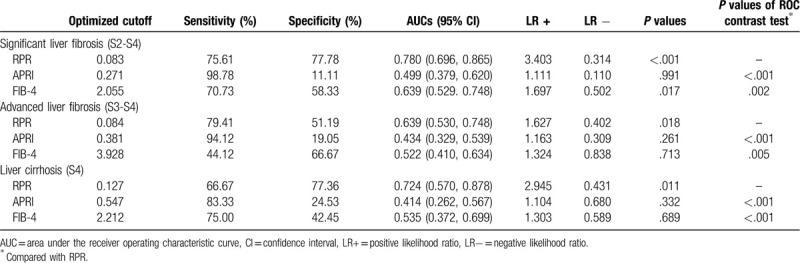
The ROC curves were performed to evaluate the accuracy of RPR, APRI, and FIB-4 in identifying significant fibrosis, advanced fibrosis, and cirrhosis (Fig. 3). The AUROCs of RPR in predicting significant fibrosis, advanced fibrosis, and cirrhosis were 0.780 (95% CI 0.696–0.865), 0.639 (95% CI 0.530–0.748), and 0.724 (95% CI 0.570–0.878), respectively. The optimal cut-off values of RPR were 0.083, 0.084, and 0.127, respectively. The AUROCs of APRI in predicting significant fibrosis, advanced fibrosis, and cirrhosis were 0.499 (95% CI 0.379–0.620, P = .991), 0.434 (95% CI 0.329–0.539, P = .261) and 0.414 (95% CI 0.262–0.567, P = .332), and the AUROCs of FIB-4 in predicting significant fibrosis, advanced fibrosis, and liver cirrhosis were 0.639 (95% CI 0.529–0.748, P = .017), 0.522 (95% CI 0.410–0.634, P = .713) and 0.535 (95% CI 0.372–0.699, P = .689), respectively. In comparison, The AUROCs of RPR were significantly higher than APRI and FIB-4 in diagnosing significant fibrosis, advanced fibrosis, and liver cirrhosis (Table 2).
Figure 3.
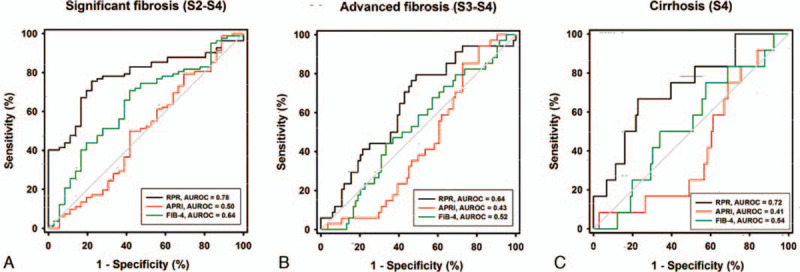
Receiver operating characteristic curve of different non-invasive tests for predicting significant liver fibrosis (A) advanced liver fibrosis (B), and liver cirrhosis (C) in AIH patients. AIH = autoimmune hepatitis.
Table 2.
Diagnostic accuracy of different non-invasive tests for predicting liver fibrosis in patients with autoimmune hepatitis.
4. Discussion
NITs for accurately identifying liver fibrosis stages are highly needed for the clinical management of AIH. Although several NITs have been proposed to predict liver fibrosis with high accuracy in patients with viral hepatitis,[13,18,21] the diagnosis performances of these NITs in AIH patients are still to be explored.
APRI and FIB-4 are 2 most widely used NITs of diagnosing liver fibrosis and are recommended by the World Health Organization guidelines to predict liver fibrosis in CHB and CHC patients in resource-limited settings.[12,15,22–24] However, few studies have reported the performances of APRI and FIB-4 for predicting liver fibrosis in AIH patients. Yuan et al reported that the AUROCs of APRI and FIB-4 were 0.798 and 0.881 for predicting liver cirrhosis in AIH patients.[25] Similar study was reported by Zeng et al which showed that APRI and FIB-4 could diagnose liver cirrhosis with moderate accuracy in AIH patients.[16] However, these 2 studies only investigated the accuracy of APRI and FIB-4 in predicting liver cirrhosis in AIH patients. As compared to the diagnose liver cirrhosis, accurately evaluating early stages of liver fibrosis is more important for AIH patients.[3] Moreover, the sample sizes are very small in these 2 studies.[16,25] In the present study, we assessed the diagnostic performances of these 2 NITs for significant liver fibrosis, advanced liver fibrosis, and liver cirrhosis in AIH patients. However, our results suggested that APRI could not predict significant liver fibrosis, advanced liver fibrosis, and liver cirrhosis. FIB-4 could only identify significant liver fibrosis with a low AUROC of 0.639. Our study demonstrates that APRI and FIB-4 are not good NITs for staging liver fibrosis in AIH patients as in viral hepatitis.
In the present study, we further investigated the novel NIT, RPR, for staging liver fibrosis in AIH patients. The results revealed that the diagnostic performances of RPR for different liver fibrosis stages were significantly higher than that of APRI and FIB-4. RPR was initially established to estimate liver fibrosis in patients with CHB.[18] RPR was demonstrated to predict significant fibrosis and cirrhosis in CHB patients with relatively high accuracy, which was superior to APRI and FIB-4.[18] Since then, several studies have validated the performances of RPR for predicting liver fibrosis in chronic liver diseases.[19,26–28] A retrospective study from Korean indicated that the diagnostic performance of RPR for predicting advanced liver fibrosis and cirrhosis was comparable to FIB-4 and superior to APRI in CHB patients.[26] A systematic meta-analysis also reported that RPR had almost the same diagnostic performance as APRI and FIB-4 in identifying significant liver fibrosis, while was comparable with APRI and inferior to FIB-4 in staging advanced liver fibrosis and cirrhosis in chronic liver diseases.[27] In non-viral liver diseases, Cengiz et al found that the diagnostic accuracy of RPR was comparable with APRI and FIB-4 for predicting significant liver fibrosis, advanced liver fibrosis, and cirrhosis in NAFLD patients.[28] Wang et al, reported that RPR showed a higher accuracy than APRI and FIB-4 for predicting advanced fibrosis in treatment-naïve PBC patients.[19]
Recently, Liu et al assessed the RPR for predicting advanced liver fibrosis in patients with AIH.[17] The study indicated that RPR had the highest accuracy compared to other NITs for predicting advanced liver fibrosis.[17] However, the sample size is relatively simple with only 45 AIH patients included. In addition, this study only investigated the accuracy of RPR in predicting advanced liver fibrosis in AIH patients. Consisted with the study by Liu et al, our study also indicated that RPR could predict advanced liver fibrosis with high accuracy. Furthermore, we evaluated the diagnostic accuracy of RAR for significant liver fibrosis and cirrhosis in AIH patients. Our results showed that PRR could predict significant fibrosis and liver cirrhosis with relative high accuracy.
RPR only contained 2 routine blood routine parameters and the computational formula is relatively simple. Numerous studies reported that RDW was associated with severity of chronic liver diseases.[29–31] A retrospective study by Karagoz et al reported that RDW was significantly increased in CHB patients and can be defined as an independent predictor in liver fibrosis.[29] Our previous study found significantly elevated RDW in patients with CHB related cirrhosis.[30] Kim et al reported that elevated RDW was associated with advanced liver fibrosis in a large cohort of NAFLD.[31] RDW was also demonstrated to be an independent predictor of cirrhosis in AIH.[16,17] Several reasons may interpret the elevation of RDW in AIH patients. Portal hypertension leads to hypersplenism which may increases the destruction of red blood cells.[17] In addition, proinflammatory factor inhibits maturation of red blood cell in AIH patients, which may cause the immature red blood cells into peripheral blood.[32] Moreover, chronic inflammation may impair the iron metabolism, restrain the production of erythropoietin and decrease red blood cell survival which together resulting in the increasing RDW.[33,34] Our previous study also found that the RDW level was positively associated with the severity of liver inflammation in AIH patients.[35] This study revealed that the RDW level was higher in patients with significant liver inflammation than mild inflammation patients, which suggested that RDW may be a promising indicator for reflecting the severity of liver inflammation in AIH patients.[35] PLT is also a well-known independent predictor for liver fibrosis and cirrhosis in chronic liver diseases. The decreased PLT may be caused by hypersplenism and the decreased thrombopoietin production associated with damaged liver cells in liver fibrosis and cirrhosis patients.[36,37]
This study has several limitations. First, our study was retrospective and simple size was relatively small. Thus, the diagnosing value of RPR for liver fibrosis in AIH patients remains to be validated in the future studies. Second, we did not compare the predicting performance between RPR and TE since TE was not a routine measure in our clinics. Third, future studies are also required to investigate whether RPR could predict treatment response and long-term outcomes of AIH patients.
In conclusion, the RPR is a more accurate NIT than APRI and FIB-4 to stage liver fibrosis in patients with AIH. The RPR represents a simple and inexpensive alternative NIT to LB for the management of AIH patients in clinic setting.
Author contributions
Study concept and design: Rui Huang, Chao Wu and Yongfeng Yang; analysis and interpretation of the data: Xiaomin Yan, Yanhong Feng, Jun Chen, Duxian Liu; collection of data: Juan Xia, Lin Li, Weimao Ding; drafting the manuscript: Huali Wang, Jian Wang, and Rui Huang.
Footnotes
Abbreviations: AIH = autoimmune hepatitis, ALT = alanine aminotransferase, APRI = aspartate transaminase to platelet ratio index, AST = aspartate transaminase, AUROC = receiver operating characteristic curve, CHB = chronic hepatitis B, CHC = chronic hepatitis C, FIB-4 = fibrosis-4 score, IQR = interquartile range, LB = liver biopsy, NAFLD = nonalcoholic fatty liver disease, NITs = noninvasive tests, PBC = primary biliary cirrhosis, PLT = platelet, RDW = red cell distribution width, RPR = red cell distribution width to platelet ratio, TE = transient elastography.
How to cite this article: Wang H, Wang J, Xia J, Yan X, Feng Y, Li L, Chen J, Liu D, Ding W, Yang Y, Huang R, Wu C. Red cell distribution width to platelet ratio predicts liver fibrosis in patients with autoimmune hepatitis. Medicine. 2020;99:34(e21408).
HW and JW contributed equally to this work.
All authors have contributed significantly to the work.
The study was supported from the National Science and Technology Major Project of China (2018ZX10302205), National Natural Science Foundation of China (81672025 and 81702011), Medical Science and Technology Development Foundation of Nanjing (ZDX16004 and YKK16118) and Natural Science Foundation of Jiangsu Province for Young Scholar (BK20160121), Jiangsu Science and Technology Development Plan (BE2017605), Jiangsu Provincial Medical Innovation Team (CXTDA2017005) and Nanjing Medical Science and Technique Development Foundation (QRX17121).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Corrigan M, Hirschfield GM, Oo YH, et al. Autoimmune hepatitis: an approach to disease understanding and management. Br Med Bull 2015;114:181–91. [DOI] [PubMed] [Google Scholar]
- [2].Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology 2010;139:58–72.e4. [DOI] [PubMed] [Google Scholar]
- [3].Lohse AW, Mieli-Vergani G. Autoimmune hepatitis. J Hepatol 2011;55:171–82. [DOI] [PubMed] [Google Scholar]
- [4].Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatol Baltim Md 2010;51:2193–213. [DOI] [PubMed] [Google Scholar]
- [5].European Association for the Study of the Liver. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol 2015;63:971–1004. [DOI] [PubMed] [Google Scholar]
- [6].Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatol Baltim Md 2003;38:1449–57. [DOI] [PubMed] [Google Scholar]
- [7].Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614–8. [DOI] [PubMed] [Google Scholar]
- [8].Seo YS, Kim MY, Kim SU, et al. Accuracy of transient elastography in assessing liver fibrosis in chronic viral hepatitis: a multicentre, retrospective study. Liver Int 2015;35:2246–55. [DOI] [PubMed] [Google Scholar]
- [9].Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010;53:1013–21. [DOI] [PubMed] [Google Scholar]
- [10].Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 2009;29:242–7. [DOI] [PubMed] [Google Scholar]
- [11].Boursier J, Zarski J-P, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013;57:1182–91. [DOI] [PubMed] [Google Scholar]
- [12].Abraham P, Aghokeng AF, Andrieux-Meyer I, et al. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- [13].Wai C-T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- [14].Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- [15].Andrieux-Meyer I, Araújo ES, El-Sayed MH, et al. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection: Updated Version. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- [16].Zeng T, Yu J, Tan L, et al. Noninvasive indices for monitoring disease course in Chinese patients with autoimmune hepatitis. Clin Chim Acta 2018;486:135–41. [DOI] [PubMed] [Google Scholar]
- [17].Liu L, Cao J, Zhong Z, et al. Noninvasive indicators predict advanced liver fibrosis in autoimmune hepatitis patients. J Clin Lab Anal 2019;33:e22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen B, Ye B, Zhang J, et al. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PloS One 2013;8:e68780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang H, Xu H, Wang X, et al. Red blood cell distribution width to platelet ratio is related to histologic severity of primary biliary cirrhosis. Medicine 2016;95:e3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 1991;13:372–4. [DOI] [PubMed] [Google Scholar]
- [21].Vallet-Pichard A, Mallet V, Pol S. FIB-4: a simple, inexpensive and accurate marker of fibrosis in HCV-infected patients. Hepatology 2006;44:769. [DOI] [PubMed] [Google Scholar]
- [22].Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015;61:292–302. [DOI] [PubMed] [Google Scholar]
- [23].Seto W-K, Lee C-F, Lai C-L, et al. A new model using routinely available clinical parameters to predict significant liver fibrosis in chronic hepatitis B. PloS One 2011;6:e23077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin C-L, Liu C-H, Wang C-C, et al. Serum biomarkers predictive of significant fibrosis and cirrhosis in chronic hepatitis B. J Clin Gastroenterol 2015;49:705–13. [DOI] [PubMed] [Google Scholar]
- [25].Yuan X, Duan S-Z, Cao J, et al. Noninvasive inflammatory markers for assessing liver fibrosis stage in autoimmune hepatitis patients. Eur J Gastroenterol Hepatol 2019;31:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee HW, Kang W, Kim BK, et al. Red cell volume distribution width-to-platelet ratio in assessment of liver fibrosis in patients with chronic hepatitis B. Liver Int 2016;36:24–30. [DOI] [PubMed] [Google Scholar]
- [27].Cai Y, Liu D, Cui J, et al. Diagnostic accuracy of red blood cell distribution width to platelet ratio for predicting staging liver fibrosis in chronic liver disease patients: a systematic review and meta-analysis. Medicine 2019;98:e15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cengiz M, Ozenirler S. Comparative diagnostic accuracy of red cell distribution width-to-platelet ratio versus noninvasive fibrosis scores for the diagnosis of liver fibrosis in biopsy-proven nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2015;27:1293–9. [DOI] [PubMed] [Google Scholar]
- [29].Karagoz E, Ulcay A, Tanoglu A, et al. Clinical usefulness of mean platelet volume and red blood cell distribution width to platelet ratio for predicting the severity of hepatic fibrosis in chronic hepatitis B virus patients. Eur J Gastroenterol Hepatol 2014;26:1320–4. [DOI] [PubMed] [Google Scholar]
- [30].Huang R, Yang C, Wu K, et al. Red cell distribution width as a potential index to assess the severity of hepatitis B virus-related liver diseases. Hepatol Res 2014;44:E464–70. [DOI] [PubMed] [Google Scholar]
- [31].Kim HM, Kim BS, Cho YK, et al. Elevated red cell distribution width is associated with advanced fibrosis in NAFLD. Clin Mol Hepatol 2013;19:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Allen LA, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail 2010;16:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mao W, Ye B, Lin S, et al. Prediction value of model for end-stage liver disease scoring system on prognosis in the acute on chronic liver failure patients with plasma exchange treatment. ASAIO J 2010;56:475–8. [DOI] [PubMed] [Google Scholar]
- [34].Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628–32. [DOI] [PubMed] [Google Scholar]
- [35].Wang H, Wang J, Huang R, et al. Red blood cell distribution width for predicting significant liver inflammation in patients with autoimmune hepatitis. Eur J Gastroenterol Hepatol 2019;31:1527–32. [DOI] [PubMed] [Google Scholar]
- [36].Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest 1966;45:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kawasaki T, Takeshita A, Souda K, et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol 1999;94:1918–22. [DOI] [PubMed] [Google Scholar]


