Abstract
Background.
The impact of functional status on survival among simultaneous pancreas-kidney transplant (SPKT) candidates and recipients is not well described.
Methods.
We examined national Scientific Registry of Transplant Recipients (SRTR) data for patients listed for SPKT in the United States (2006–2019). Functional status was categorized by center-reported Karnofsky Performance Score (KPS). We used Cox regression to quantify associations of KPS at listing and transplant with subsequent patient survival, adjusted for baseline patient and transplant factors (adjusted hazard ratio, 95% LCLaHR95%UCL). We also explored time-dependent associations of SPKT with survival risk after listing compared with continued waiting in each functional status group.
Results.
KPS distributions among candidates (N = 16 822) and recipients (N = 10 316), respectively, were normal (KPS 80–100), 62.0% and 57.8%; capable of self-care (KPS 70), 23.5% and 24.7%; requires assistance (KPS 50–60), 12.4% and 14.2%; and disabled (KPS 10–40), 2.1% and 3.3%. There was a graded increase in mortality after listing and after transplant with lower functional levels. Compared with normal functioning, mortality after SPKT rose progressively for patients capable of self-care (aHR, 1.001.181.41), requiring assistance (aHR, 1.061.311.60), and disabled (aHR, 1.101.552.19). In time-dependent regression, compared with waiting, SPKT was associated with 2-fold mortality risk within 30 days of transplant. However, beyond 30 days, SPKT was associated with reduced mortality, from 52% for disabled patients (aHR, 0.260.480.88) to 70% for patients with normal functioning (aHR, 0.260.300.34).
Conclusions.
While lower functional status is associated with increased mortality risk among SPKT candidates and recipients, SPKT can provide long-term survival benefit across functional status levels in those selected for transplant.
INTRODUCTION
Simultaneous pancreas-kidney transplant (SPKT) offers the best long-term outcomes for type 1 diabetes mellitus associated with advanced chronic kidney disease (CKD) or end-stage kidney disease (ESKD).1,2 Successful pancreas transplant achieves insulin independence and good metabolic control, improving and stabilizing many of the complications of insulin-dependent diabetes, and restoring kidney function prevents dialysis-related morbidity.3 Overall, SPKT recipients gain increased life expectancy and quality of life compared with type 1 diabetic ESKD patients who remain on dialysis,4,5 albeit with moderately increased perioperative risks compared with kidney transplant alone.5,6 Further, the patient population seeking SPKT is evolving, as improvements in diabetes care, advanced insulin delivery technologies, and delayed CKD progression have resulted in an older population living with prevalent disease and an expansion of SPKT to selected patients with type 2 diabetes.7-9 The average age of SPKT recipients in 2017 was 43 years, compared with 38 years in 1995.10 In 2018, 26.7% of US SPKT candidates and 23.1% of recipients were aged older than 50 years.7 As diabetic patients present for transplant evaluation at progressively older ages with concomitant higher comorbidity burdens, frailty and impaired functional status have become important considerations in clinical assessment and management.11,12
Frailty is a global measure of physical function characterized by decreased strength, decreased endurance, muscle wasting, and lack of physiological reserve.13,14 Frailty has been studied extensively in patients with CKD, and, more recently, in kidney transplant populations, using a number of different frailty scales and approaches.15,16 Significant associations between frailty and poor outcomes after kidney transplant have been reported, including a high risk of mortality.14,17,18 While data on the impacts of frailty on kidney transplant outcomes are emerging,17-20 there is a paucity of information on the impact of measures of frailty on pancreas transplant outcomes, including SPKT-related outcomes. Given that SPKT is a high-risk procedure than kidney transplant alone,21 some transplant programs may avoid the additional risk of pancreas transplant surgery in frail diabetic patients.
The Karnofsky Performance Score (KPS), a categorical assessment tool for functional impairment, has been commonly used for many chronic disease assessments, including among transplant candidates and recipients.22,23 US transplant centers report KPS at the times of listing and transplant to the Organ Procurement and Transplantation Network (OPTN) national registry.22 KPS is a simple scale that ranks patients from normal functioning (100%) to dead (0%) in 10% increments. KPS is easily determined and nationally reported.22 Though not initially intended as a measure of frailty, it is the only measure among data collected by the Scientific Registry of Transplant Recipients (SRTR) that can be used as a surrogate measure. In a recent study of adult deceased-donor kidney transplant recipients, KPS at transplant was found to be an independent predictor of posttransplant outcomes.24 The impact of functional status assessed by KPS on outcomes related to SPKT, however, is not well described. To help address this knowledge gap, we sought to characterize the associations of KPS with patient survival in a large national cohort of US SPKT candidates and recipients. Specifically, we examined survival according to baseline KPS, as well as the impact of SKPT on survival (perioperatively and longer term) in relation to candidate functional status.
MATERIALS AND METHODS
Data Source and Sampling
This study used data from the SRTR. The SRTR system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the OPTN. The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors. The primary samples included adult (age ≥18 y) SPKT candidates and recipients from 2006 to 2019. The latest follow-up status date posttransplant was December 9, 2019. Patients with unknown functional status (n = 561) were excluded.
Functional Status, Covariate, and Outcome Definitions
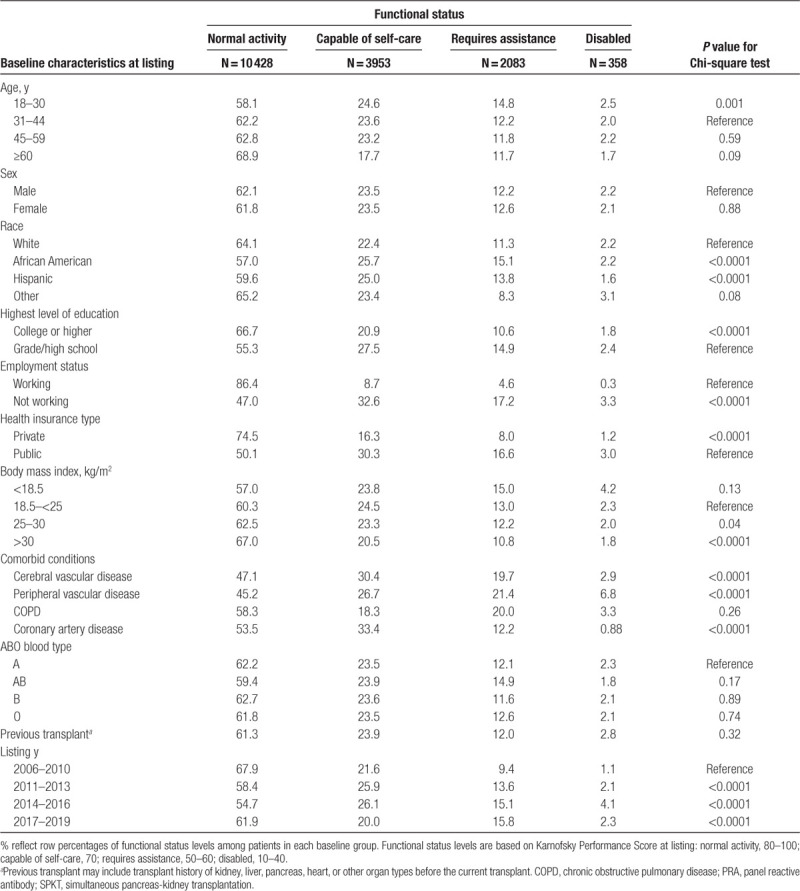
Functional status at the time of listing for SPKT candidates and at the time of transplant for SPKT recipients was defined by center-reported KPS (Table S1, SDC, http://links.lww.com/TXD/A273). KPS is a categorical classification system with progressive but arbitrary increases in assigned performance status at 10% intervals without use of the intervening numbers and so was categorized for analysis.22,23 Patients were categorized into 4 groups: normal (80–100), capable of self-care (70), requires assistance (50–60), and disabled (10–40).22,23,25 Transplant recipient clinical and demographic characteristics, and characteristics of the donated organ and other transplant factors, were defined by the OPTN Transplant Candidate Registration and Transplant Recipient Registration forms (Table 1). The primary outcome was mortality, as reported by transplant centers to OPTN and supplemented with the Social Security Death Master File. Kidney graft failure was defined as return to maintenance dialysis or retransplant. Pancreas graft failure was defined by center reporting. Graft loss was considered as all-cause and included graft loss due to death, per the methods of the SRTR.26
Table 1.
Functional status distribution according to baseline traits among US candidates listed for SPKT, 2006–2019
Outcome and Statistical Analyses
Clinical characteristics of the study sample are described as counts with proportions. Continuous variables were categorized into clinically relevant strata. Missing categorical covariate data were grouped with the absence of a characteristic when such categories were relevant or into a category distinct from the reference group. Missing covariate status was included as a regression parameter, as per our standard analytic approach.27-31 The clinical characteristics of patients with lower than normal functional status (caring for self, requiring assistance, or disability) were compared with patients with normal functional status using the Chi-squared test.
At-risk time for death began at the time of listing for SPKT candidates and at the time of transplant for SPKT recipients, with censoring at the last follow-up or the study end. Patient survival was estimated using the Kaplan-Meier method. At-risk time for the Kaplan-Meier estimates while on the waiting list included censoring at transplant to constrain estimates to the period between listing and transplant. Waitlisting was considered the principle of intention to treat, and, therefore, at-risk time was not censored at removal from the waiting list for reasons other than transplant or death.
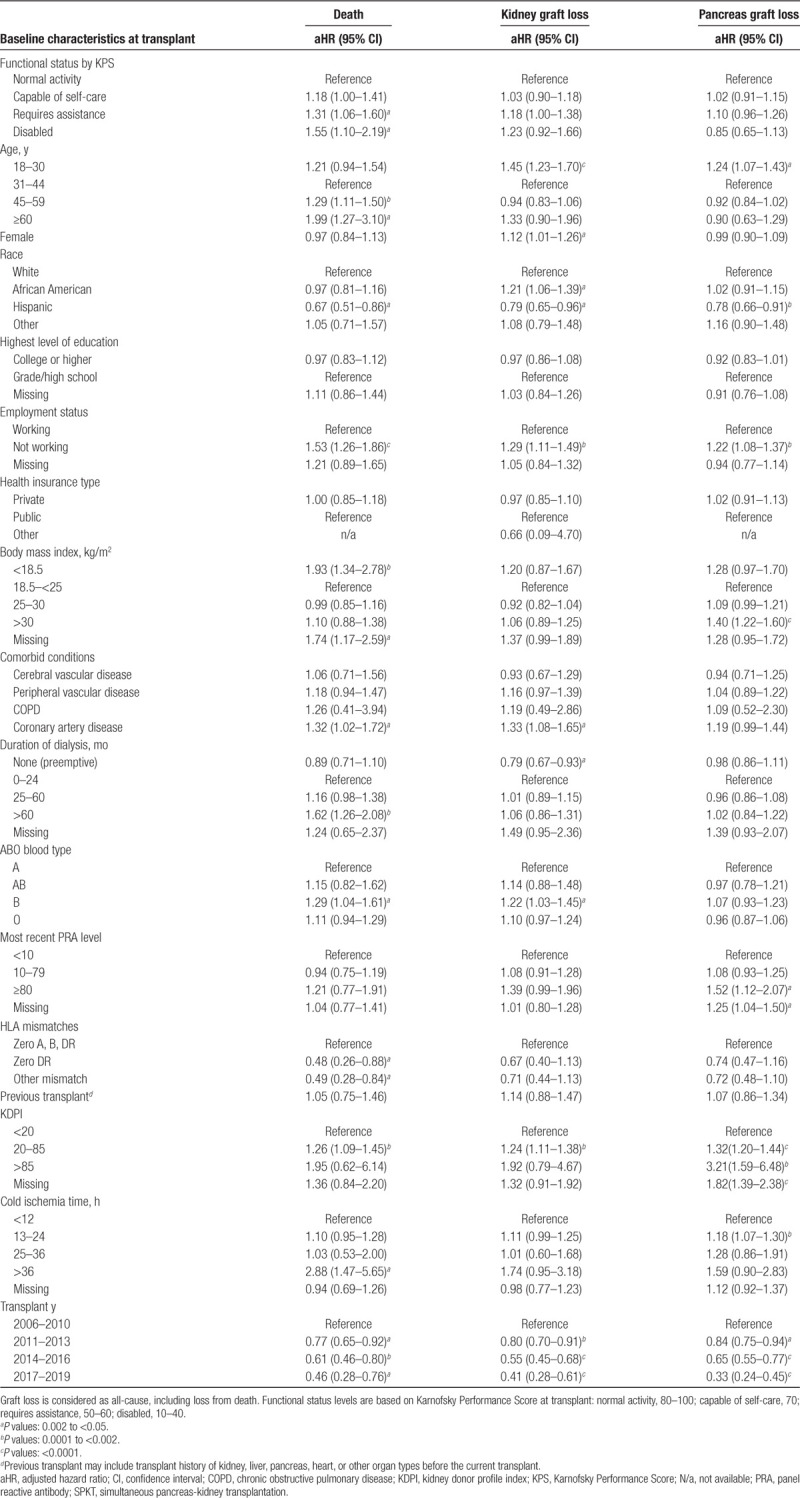
The adjusted association of functional status at the time of SPKT with mortality (adjusted hazard ratio, 95% LCLaHR95%UCL) and with graft failure after transplant was assessed using multivariable Cox proportional hazards analysis, adjusted for baseline recipient, donor and transplant factors (Table 2). Patients with normal functional status were selected as the reference group for comparison of the impact of KPS level. The association of SPKT, as a time-dependent exposure, with mortality compared with continued waiting without transplant was assessed for each functional status category using multivariable stratified Cox regression, including adjustment for other baseline clinical factors (Table S2, SDC, http://links.lww.com/TXD/A273). Based on a priori hypothesis that mortality risk differs between the early and late periods after transplant, mortality risk associated with SPKT versus waiting was partitioned by time into within ≤30 and >30 days of SPKT. A 2-sided P value of <0.05 was considered statistical significance. All analyses were performed using SAS for Windows, version 14.
Table 2.
Adjusted associations of functional status at transplant and other baseline clinical factors with death and graft loss after SPKT
RESULTS
Sample and Baseline Characteristics
Between 2006 and 2019, 16 822 eligible patients were listed for SPKT in the United States; 10 316 patients underwent SPKT in the study period and 20 received a kidney alone after SPKT listing. No patients received a pancreas alone after SPKT listing. The sample comprised 58.6% men and 41.4% women; racial distribution includes 62.3% white, 21.7% African American, 12.8% Hispanic, and 3.2% other races (Table S2, SDC, http://links.lww.com/TXD/A273). Among the SPKT candidates, 62% were at normal functional status, 24% were capable of self-care, 12% required assistance, and 2% were disabled. In contrast, among the SPKT recipients, 58% were at normal functional status, 25% were capable for self-care, 14% required assistance, and 3% were disabled. Compared with normal functional status, decreased functional status (requiring assistance or disabled) was more common among candidates who were African American, had lower education level, were not working, or were publicly insured (Table 1). Low KPS was more common among SPKT candidates with, versus without, cerebrovascular disease and peripheral vascular disease. Overall, patterns of reduced functional status among SPKT candidates according to baseline traits showed patterns similar to those observed among SPKT recipients (Table S3, SDC, http://links.lww.com/TXD/A273).
Association of Functional Status Levels With Mortality After Listing and After Transplant
A graded decline in patient survival with lower functional status at the time of listing was observed in SPKT candidates. Estimated 5-year patient survival after listing was 85.7% for patients with normal functional status, 82.4% for patients capable of self-care, 83.8% for patients requiring assistance, and 76.8% for patients with disability (Figure 1A). When censoring for SPKT or kidney transplant alone, estimated 5-year patient survival on the waiting list was 77.5% for patients with normal functional status, 74.7% for patients capable of self-care, 76% for patients requiring assistance, and 65.9% for patients with disability (Figure 1B).
FIGURE 1.
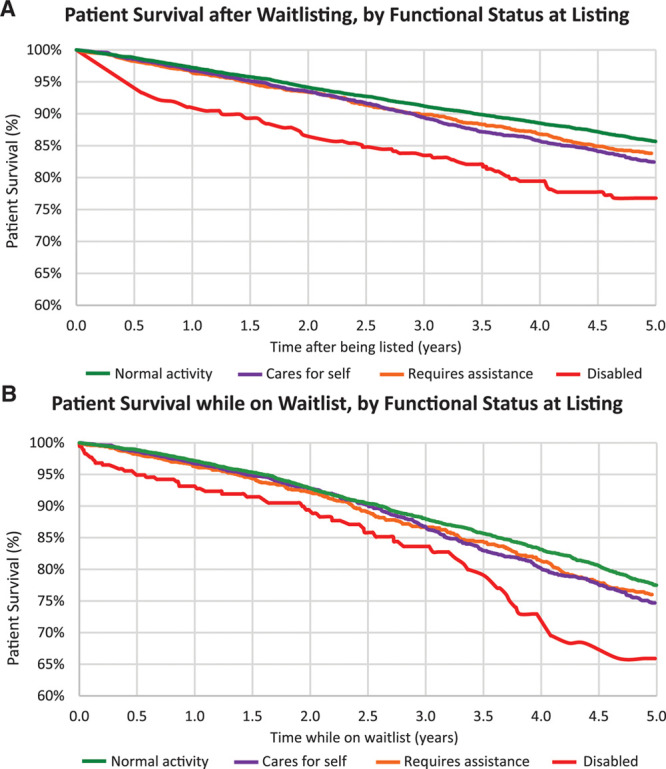
Patient survival after listing for SPKT without censoring for transplant (A), and while on the waiting list censored at transplant (B) according to functional status at listing. SPKT, simultaneous pancreas-kidney transplant.
Lower functional status levels at the time of transplant among SPKT recipients were also associated with a graded decline in posttransplant survival (Figure 2). The estimated 5-year patient survival after transplant was 91.4% for patients with normal functional status, 89.5% for patients capable of self-care, 88.4% for patients requiring assistance, and 85.7% for patients with disability. Compared with mortality risk after SPKT for patients with normal functioning, after adjustment for patient, donor, and transplant factors, risk rose progressively for patients capable of self-care (aHR, 1.001.181.41), requiring assistance (aHR, 1.061.311.60), and disabled (aHR, 1.101.552.19) (Figure 3; Table 2). KPS at transplant was not significantly associated with kidney or pancreas loss after transplant, except that requiring assistance was associated with kidney allograft loss (aHR, 1.001.181.38).
FIGURE 2.
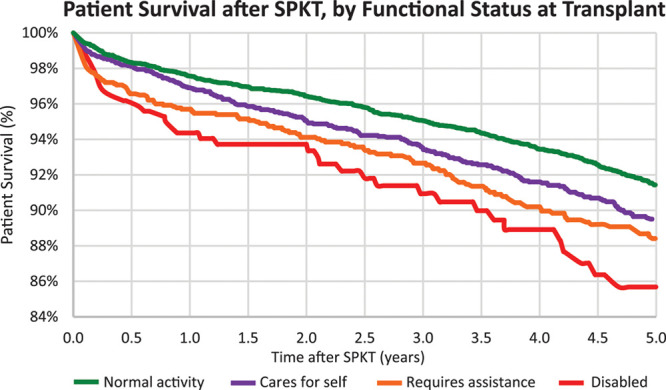
Patient survival after SPKT according to functional status at transplant. SPKT, simultaneous pancreas-kidney transplant.
FIGURE 3.
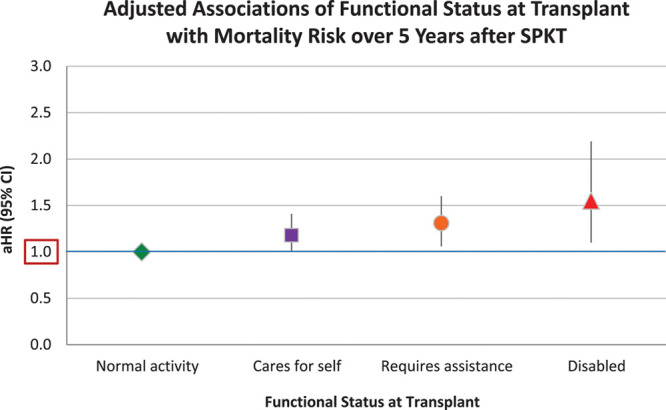
Adjusted associations of functional status at transplant with SPKT recipient mortality risk. *Adjusted for baseline factors in Table 2. aHR, adjusted hazard ratio; CI, confidence interval; SPKT, simultaneous pancreas-kidney transplant.
Impact of Transplant on Patient Mortality, According to Functional Status at Listing
Considered from the perspective of continued waiting without transplant, SPKT was associated with approximately twice the mortality within 30 days of transplant. Associated risks ranged from a 75% increase for patients with normal functional status (aHR, 1.251.752.45) to a trend toward 2-fold mortality for disabled patients (aHR, 0.682.207.08), although statistical significance was not reached for lower functional status, likely due to smaller sample sizes (Figure 4A, Table S4, SDC, http://links.lww.com/TXD/A273). In contrast, beyond 30 days, SPKT was associated with reduced mortality compared with continued waiting, ranging from 52% risk reduction for disabled patients (aHR, 0.260.480.88) to 70% reduction for those with normal functioning (aHR, 0.260.300.34) (Figure 4B).
FIGURE 4.
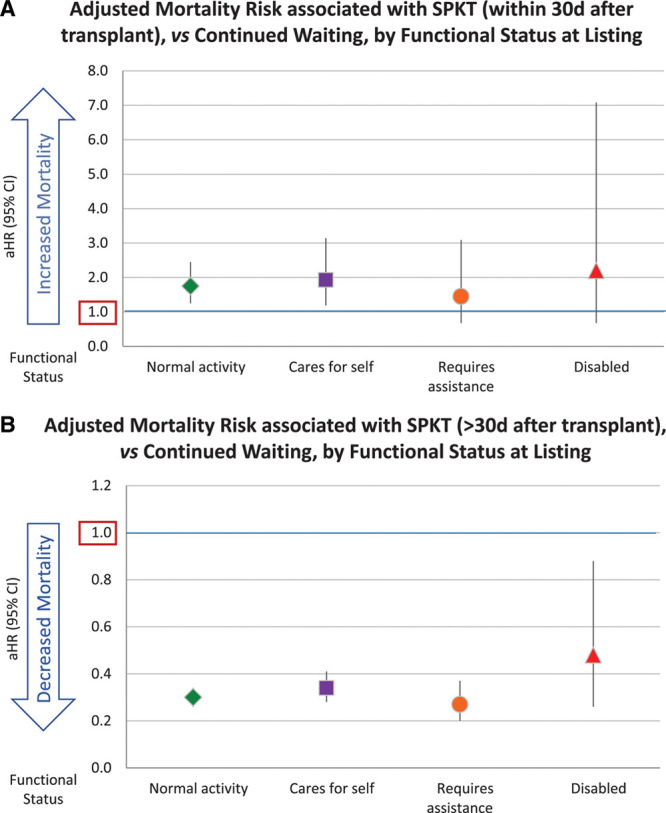
Adjusted mortality risk associated with SPKT (A) within 30 d and (B) after 30 d posttransplant, by functional status at listing. aHR, adjusted hazard ratio; CI, confidence interval; SPKT, simultaneous pancreas-kidney transplant.
DISCUSSION
As progressively older patients with higher comorbidity burdens seek and undergo transplant, measures of frailty including functional status are being recognized as important considerations in transplant practice.32 We examined the survival implications of functional status assessed by KPS, the only existing surrogate measure for frailty/functional status collected in the OPTN registry,22,24,33 in a national US cohort of SPKT candidates and recipients, and observed several key findings: (1) need for assistance or disability was reported for 14.5% of candidates and 17.5% of recipients; (2) lower functional status levels were associated with graded increases in mortality after listing and after transplant; (3) compared with continued waiting, SPKT was associated with 2-fold mortality risk within 30 days of the transplant procedure. However, beyond 30 days, SPKT was associated with reduced mortality, from 52% risk reduction for disabled patients to 70% risk reduction for those with normal functioning.
Recent improvements in diabetes management have extended life expectancy and increased the number of older type 1 diabetes patients living with advanced disease complications.34,35 Previous studies have demonstrated inferior outcomes among SPKT recipients aged older than 50 years,36,37 although more recent reports demonstrate that acceptable outcomes can be obtained with careful medical assessment and patient selection. Therefore, advanced age, in isolation, should not be considered an absolute exclusion criterion for SPKT.38-41 Frailty is more common among older dialysis patients and kidney transplant recipients.32,42 In our study of SPKT candidates and recipients, functional status distributions were fairly similar across age groups, such that reduced functional status was not solely a condition of the elderly. Prior work has reported independent associations of diabetes with frailty among dialysis patients.43,44 Diabetes is thought to contribute directly to the pathophysiology of frailty by increasing the risk of sarcopenia.45 Patients with type 1 diabetes in particular are at risk for sarcopenia due to the catabolic effect of insulin deficiency and excess accumulation of intramyocellular lipids.46 In addition to diabetes, CKD is also associated with frailty through protein energy-wasting, oxidative stress, and chronic inflammation.47 These mechanisms may explain why decreased levels of functional status were observed across age groups in our study of diabetic patients with ESKD. Importantly, our results suggest that functional status is an independent predictor of patient survival in SPKT candidates and recipients, independent of age.
Associations between reduced functional levels assessed by KPS and posttransplant mortality have been reported after deceased-donor kidney transplant,24 liver transplant,48 and lung transplant.49,50 We found that in the context of SPKT, impaired functional status (defined as KPS <70) was common among candidates and recipients (~15%–18%) and was associated with increased mortality after both listing and transplant. Importantly, evaluation and decision-making regarding suitability for SPKT is comprehensive and especially affected by markers of cardiovascular risk, as cardiac comorbidity is common in this population.51 In our study, the association of lower KPS with increased mortality remained significant in the dose-response relationship after adjusting for potential confounders available in the registry, including reported comorbid conditions, supporting that functional assessment is a relevant prognostic tool in this population. The application of functional assessment by KPS and possibly other measures of frailty in SPKT populations, in addition to comprehensive assessment, may improve risk stratification and target waitlist management attention to high-risk groups.
Beyond characterization of higher- versus lower-risk transplant recipients, transplant outcomes compared with continued waiting offer an important patient-centered perspective. Morbidity and mortality risks after SPKT are recognized to be highest within the first 3 months due to the risks of the surgery (such as infection, reperfusion pancreatitis, and enteric anastomotic leak), as well as other perioperative risks such as major adverse cardiac events.7,52 We found that risk of increased perioperative mortality was similar across functional status levels. However, after the perioperative risk period, SPKT was significantly associated with reduced long-term mortality compared with waiting. Importantly, a survival benefit was found across all functional status levels, although benefit trended higher for patients with normal functioning (52% risk reduction among disabled patients versus 70% reduction in those with normal functioning).
Demonstration of long-term survival benefits of SPKT compared with waiting across all functional status groups, including the small number of disabled patients selected for transplant, is an important observation of this study. Frail diabetic candidates with ESKD may be deemed too ill for SPKT at many transplant centers, in part due to concerns for perioperative risks.39 Notably, the number of additions to the SPKT waiting list in the United States steadily declined from 1935 in 2000 to 1228 in 2017.37,53,54 Fewer candidates are added to the waiting list and the number of active candidates decreased by more than half, from 2776 in 2002 to 1039 in 2018.7,37,53,54 Furthermore, the number of active pancreas transplant centers in the United States has been declining; only 10 US centers performed at least 20 pancreas transplants in 2017, and 50% of all centers performed <6 pancreas transplants in 2017.37,53,54 When SPKT is successful, the potential benefits of an insulin-free, dialysis-free state can be substantial. In a study of 1000 SPKT recipients with 22-year follow-up, long-term patient survival after SPKT was better than for all other transplant options for type 1 diabetes patients with ESKD, including those who underwent living donor kidney transplant alone.55 However, expertise and judicious selection are critical to select appropriate candidates for the procedure. Although we lacked information on evaluation and selection protocols in this retrospective registry study, our findings support that SPKT may be an appropriate option to prolong long-term survival for some carefully selected diabetic patients with impaired functional status. These data may encourage reconsideration of some patients currently deemed ineligible for SPKT, although selection by experienced clinicians remains paramount.39
This study has limitations. We conducted a retrospective observational study of national registry data, with the limitations inherent in any such study. The benefit of SPKT may dominantly derive from restoration of kidney function.56 As patients with decreased functional status who were listed and underwent transplant may be selected based on clinical information not included in the registry, these results may not be generalizable to all patients with lower KPS values. Notably, the distribution of functional status levels among SPKT candidates was similar to a recent study of kidney transplant candidates, although the proportion of disability was slightly lower among the SPKT candidates (2% versus 4% among listed kidney candidates).25 In addition, this is an analysis of the US SPKT population, and results may not generalize to SPKT populations in other countries. Although several frailty assessment tools are being used increasingly to measure functional status, there is no standardized selection process for candidates based on these measures.42 KPS may be impacted by observer bias and scoring can vary within and across transplant centers. Nonetheless, benefits of KPS include that it is easily measured and identified in the national registry at listing and at the time of transplant. While we identified significant associations of KPS with survival among SPKT candidates and recipients, we lacked information on interventions and management strategies used by centers for patients with low functional status.
In conclusion, lower center-reported functional status was associated with increased mortality risk among SPKT candidates and recipients. However, SPKT can provide substantial long-term survival benefits across all functional status levels, compared with waiting without SPKT, for patients selected to undergo the procedure. For frail candidates who are otherwise medically acceptable, these data raise intriguing possibilities for intervention, such as targeted physical therapy and nutrition therapy to improve functional status, followed by reassessment for SPKT candidacy for those initially declined. Multidisciplinary involvement of physical therapy and nutritional experts within the management paradigm is vital to strive for reductions in frailty and improved functional status but requires coordinated participation of referring care providers (eg, nephrologists, endocrinologists, dialysis teams) and reimbursement mechanisms through insurance providers.42 Continued efforts are needed to improve and standardize the measurement of functional status and frailty in this population, and to determine whether interventions such as prehabilitation can improve functional status and outcomes, deliver these services to patients in need, and help support SPKT opportunities for all patients who can benefit.
ACKNOWLEDGMENTS
This work was conducted under the auspices of the Hennepin Healthcare Research Institute, contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. KLL receives support from the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation. The opinions, results, and conclusions reported in this article are those of the authors and are independent of the funding sources. The authors thank Huiling Xiao, MS, biostatistician at Saint Louis University for assistance with article preparation and SRTR colleague Nan Booth, MSW, MPH, ELS, for article editing. An abstract describing portions of this work presented as an oral abstract at the American Transplant Congress virtual program, June 2020.
DATA AVAILABILITY STATEMENT
This study was approved by the Saint Louis University Institutional Review Board. Individual participant deidentified data will not be shared by the authors due to restrictions of Data Use Agreements. SRTR registry data can be obtained from the SRTR.
Footnotes
Published online 21 August, 2020.
K.J.W. and R.F.P. are cosenior authors and have contributed equally to this work.
This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. KLL receives support from the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation. The opinions, results, and conclusions reported in this article are those of the authors and are independent of the funding sources.
The authors declare no conflicts of interest.
K.L.L. and M.A.S. participated in study design, acquisition of data and regulatory approvals, analysis, interpretation, and writing of the article. T.A., W.C., J.C.T., and K.J.W. participated in study design, interpretation, and writing of the article. S.-H.C., M.C., D.M.D., D.A., R.O., F.H.C.-R., B.L.K., and R.F.P. participated in interpretation of data, critical review for important intellectual content, and final approval.
REFERENCES
- 1.Weiss AS, Smits G, Wiseman AC. Twelve-month pancreas graft function significantly influences survival following simultaneous pancreas-kidney transplantation. Clin J Am Soc Nephrol. 2009; 4:988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smets YF, Westendorp RG, van der Pijl JW, et al. Effect of simultaneous pancreas-kidney transplantation on mortality of patients with type-1 diabetes mellitus and end-stage renal failure. Lancet. 1999; 353:1915–1919 [DOI] [PubMed] [Google Scholar]
- 3.Dunn TB. Life after pancreas transplantation: reversal of diabetic lesions. Curr Opin Organ Transplant. 2014; 19:73–79 [DOI] [PubMed] [Google Scholar]
- 4.Rana A, Gruessner A, Gruessner RW. Estimation of life-years saved by solid-organ transplant–reply. JAMA Surg. 2015; 150:1015–1016 [DOI] [PubMed] [Google Scholar]
- 5.Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation. 2001; 71:82–90 [DOI] [PubMed] [Google Scholar]
- 6.van Dellen D, Worthington J, Mitu-Pretorian OM, et al. Mortality in diabetes: pancreas transplantation is associated with significant survival benefit. Nephrol Dial Transplant. 2013; 28:1315–1322 [DOI] [PubMed] [Google Scholar]
- 7.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2016 annual data report: pancreas. Am J Transplant. 2018; 18Suppl 1:114–171 [DOI] [PubMed] [Google Scholar]
- 8.Al-Qaoud TM, Odorico JS, Redfield RR., III Pancreas transplantation in type 2 diabetes: expanding the criteria. Curr Opin Organ Transplant. 2018; 23:454–460 [DOI] [PubMed] [Google Scholar]
- 9.Hau HM, Jahn N, Brunotte M, et al. Short and long-term metabolic outcomes in patients with type 1 and type 2 diabetes receiving a simultaneous pancreas kidney allograft. BMC Endocr Disord. 2020; 20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schold JD. Trends in pancreas transplantation in the United States. Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas. 2020, Amsterdam, The Netherlands: Elsevier; 359–364 [Google Scholar]
- 11.Yanase T, Oki Y, Katabami T, et al. New diagnostic criteria of adrenal subclinical Cushing’s syndrome: opinion from the Japan Endocrine Society. Endocr J. 2018; 65:383–393 [DOI] [PubMed] [Google Scholar]
- 12.Perkisas S, Vandewoude M. Where frailty meets diabetes. Diabetes Metab Res Rev. 2016; 32Suppl 1261–267 [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 14.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019; 19:984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harhay MN, Rao MK, Woodside KJ, et al. An overview of frailty in kidney transplantation: measurement, management and future considerations. Nephrol Dial Transplant. 2020 10.1093/ndt/gfaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz EC, Cosio FG, Bernard SL, et al. The relationship between frailty and decreased physical performance with death on the kidney transplant waiting list. Prog Transplant. 2019; 29:108–114 [DOI] [PubMed] [Google Scholar]
- 17.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012; 147:190–193 [DOI] [PubMed] [Google Scholar]
- 18.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015; 15:149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013; 13:2091–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015; 99:805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant. 2015; 20:94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAdams-DeMarco MA, Van Pilsum Rasmussen SE, Chu NM, et al. ; AST Kidney Pancreas Community of Practice Workgroup. Perceptions and practices regarding frailty in kidney transplantation: results of a national survey. Transplantation. 2020; 104:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mor V, Laliberte L, Morris JN, et al. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984; 53:2002–2007 [DOI] [PubMed] [Google Scholar]
- 24.Bui K, Kilambi V, Rodrigue JR, et al. Patient functional status at transplant and its impact on posttransplant survival of adult deceased-donor kidney recipients. Transplantation. 2019; 103:1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheshadri A, Cullaro G, Johansen KL, et al. Association of Karnofsky Performance Status with waitlist mortality among older and younger adults awaiting kidney transplantation. Clin Transplant. 2020; 34:e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scientific Registry of Transplant Recipients (SRTR). Posttransplant outcomes. Available at https://www.srtr.org/reports-tools/posttransplant-outcomes/. Accessed June 7, 2020.
- 27.Alhamad T, Kunjal R, Wellen J, et al. Three-month pancreas graft function significantly influences survival following simultaneous pancreas-kidney transplantation in type 2 diabetes patients. Am J Transplant. 2020; 20:788–796 [DOI] [PubMed] [Google Scholar]
- 28.Alhamad T, Venkatachalam K, Daloul R, et al. Targeting high calcineurin inhibitor levels after acute rejection with less tremor: a new strategy. Transplantation. 2017; 101:e287–e288 [DOI] [PubMed] [Google Scholar]
- 29.Alhamad T, Koraishy FM, Lam NN, et al. Cannabis dependence or abuse in kidney transplantation: implications for posttransplant outcomes. Transplantation. 2019; 103:2373–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lentine KL, Lam NN, Naik AS, et al. Prescription opioid use before and after kidney transplant: implications for posttransplant outcomes. Am J Transplant. 2018; 18:2987–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alhamad T, Malone AF, Brennan DC, et al. Transplant center volume and the risk of pancreas allograft failure. Transplantation. 2017; 101:2757–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007; 18:2960–2967 [DOI] [PubMed] [Google Scholar]
- 33.Bui K, Kilambi V, Mehrotra S. Functional status-based risk-benefit analyses of high-KDPI kidney transplant versus dialysis. Transpl Int. 2019; 32:1297–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abi Khalil C, Roussel R, Mohammedi K, et al. Cause-specific mortality in diabetes: recent changes in trend mortality. Eur J Prev Cardiol. 2012; 19:374–381 [DOI] [PubMed] [Google Scholar]
- 35.Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015; 313:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siskind E, Maloney C, Akerman M, et al. An analysis of pancreas transplantation outcomes based on age groupings–an update of the UNOS database. Clin Transplant. 2014; 28:990–994 [DOI] [PubMed] [Google Scholar]
- 37.Gruessner AC, Gruessner RWG. Pancreas transplantation of US and non-US cases from 2005 to 2014 as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2016; 13:35–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittal S, Smilevska R, Franklin R, et al. An analysis of the association between older recipient age and outcomes after whole-organ pancreas transplantation—a single-centre, retrospective study. Transpl Int. 2020; 33:529–535 [DOI] [PubMed] [Google Scholar]
- 39.Scalea JR, Redfield RR, III, Arpali E, et al. Pancreas transplantation in older patients is safe, but patient selection is paramount. Transpl Int. 2016; 29:810–818 [DOI] [PubMed] [Google Scholar]
- 40.Ablorsu E, Ghazanfar A, Mehra S, et al. Outcome of pancreas transplantation in recipients older than 50 years: a single-centre experience. Transplantation. 2008; 86:1511–1514 [DOI] [PubMed] [Google Scholar]
- 41.Schenker P, Vonend O, Krüger B, et al. Long-term results of pancreas transplantation in patients older than 50 years. Transpl Int. 2011; 24:136–142 [DOI] [PubMed] [Google Scholar]
- 42.Basu A. Role of physical performance assessments and need for a standardized protocol for selection of older kidney transplant candidates. Kidney Int Rep. 2019; 4:1666–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansen KL, Dalrymple LS, Delgado C, et al. Factors associated with frailty and its trajectory among patients on hemodialysis. Clin J Am Soc Nephrol. 2017; 12:1100–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansen KL, Dalrymple LS, Delgado C, et al. Association between body composition and frailty among prevalent hemodialysis patients: a US Renal Data System special study. J Am Soc Nephrol. 2014; 25:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuda Y, Asaoka T, Eguchi H, et al. Clinical impact of preoperative sarcopenia on the postoperative outcomes after pancreas transplantation. World J Surg. 2018; 42:3364–3371 [DOI] [PubMed] [Google Scholar]
- 46.Landi F, Onder G, Bernabei R. Sarcopenia and diabetes: two sides of the same coin. J Am Med Dir Assoc. 2013; 14:540–541 [DOI] [PubMed] [Google Scholar]
- 47.Cachofeiro V, Goicochea M, de Vinuesa SG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008; 111:S4–S9 [DOI] [PubMed] [Google Scholar]
- 48.Dolgin NH, Martins PN, Movahedi B, et al. Functional status predicts postoperative mortality after liver transplantation. Clin Transplant. 2016; 30:1403–1410 [DOI] [PubMed] [Google Scholar]
- 49.Grimm JC, Valero V, 3rd, Kilic A, et al. Preoperative performance status impacts perioperative morbidity and mortality after lung transplantation. Ann Thorac Surg. 2015; 99:482–489 [DOI] [PubMed] [Google Scholar]
- 50.Kilic A, Beaty CA, Merlo CA, et al. Functional status is highly predictive of outcomes after redo lung transplantation: an analysis of 390 cases in the modern era. Ann Thorac Surg. 2013; 96:1804–1811 [DOI] [PubMed] [Google Scholar]
- 51.Tuomilehto J, Borch-Johnsen K, Molarius A, et al. Incidence of cardiovascular disease in type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia. 1998; 41:784–790 [DOI] [PubMed] [Google Scholar]
- 52.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2015 Annual Data Report: pancreas. Am J Transplant. 2017; 17Suppl 1117–173 [DOI] [PubMed] [Google Scholar]
- 53.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2016 Annual Data Report: pancreas. Am J Transplant. 2018; 18Suppl 1114–171 [DOI] [PubMed] [Google Scholar]
- 54.Gruessner AC, Gruessner RWG. Pancreas transplantation for patients with type 1 and type 2 diabetes mellitus in the United States: a registry report. Gastroenterol Clin North Am. 2018; 47:417–441 [DOI] [PubMed] [Google Scholar]
- 55.Sollinger HW, Odorico JS, Becker YT, et al. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg. 2009; 250:618–630 [DOI] [PubMed] [Google Scholar]
- 56.Young BY, Gill J, Huang E, et al. Living donor kidney versus simultaneous pancreas-kidney transplant in type I diabetics: an analysis of the OPTN/UNOS database. Clin J Am Soc Nephrol. 2009; 4:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study was approved by the Saint Louis University Institutional Review Board. Individual participant deidentified data will not be shared by the authors due to restrictions of Data Use Agreements. SRTR registry data can be obtained from the SRTR.


