Abstract
INTRODUCTION:
To assess the effects of sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) on acid reflux and esophageal motor function and to evaluate the observation of esophageal adenocarcinoma (EAC) after bariatric surgery.
METHODS:
We searched 5 databases for adults who underwent SG or RYGB and had esophageal pH test and/or esophageal manometry before and after surgery. A separate systemic search of observational studies and a retrospective review at 3 institutions of adults who developed EAC after these surgeries were conducted. Outcomes were changes in manometric and pH parameters and EAC cases after SG and RYGB.
RESULTS:
A total of 27 nonrandomized studies (SG: 612 patients; RYGB: 470 patients) were included. After SG, lower esophageal sphincter pressure and esophageal body amplitude were decreased and the risk of ineffective esophageal motility was increased. Total and recumbent acid exposure times were increased. After RYGB, an increased risk of ineffective esophageal motility was observed. Total, upright, and recumbent acid exposure times were decreased. The total reflux episodes remained unchanged but with increased nonacid reflux and decreased acid reflux events. Including our largest series, 31 EAC cases have been reported to date after SG and RYGB.
DISCUSSION:
This systematic review demonstrates increased acid reflux after SG and decreased acid reflux after RYGB. An observed increased nonacid reflux after RYGB might contribute to failure of gastroesophageal reflux disease improvement. This refluxate might be noxious to the esophagus, warranting further studies. RYGB might not entirely preserve esophageal function as previously believed.
INTRODUCTION
A global pandemic of obesity has become a serious threat to human health in the modern era (1,2). Bariatric surgery is the most effective and sustainable option in the treatment armamentarium against obesity. Sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) are now the 2 most commonly performed weight-loss surgeries worldwide according to the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Global Registry, representing 45.9% and 38.3% of all procedures, respectively (3). SG has recently gained increasing popularity given its relatively simple operation and comparable weight-loss outcomes (4,5). However, its benefits are limited by potential effects on esophageal function, specifically gastroesophageal (GE) reflux disease (GERD) and motility disorders (6,7). The issue of GERD after SG is of utmost importance because it impacts patients' quality of life, potentially heightens the risk of esophageal cancer, and could ultimately lead to surgical revision. A meta-analysis of more than 10,000 patients found an incidence of new-onset GERD and Barrett's esophagus of 23.6% and 6%, respectively, after SG (8). The precise mechanism of GERD after SG has remained unclear, with a leading hypothesis that it results from anatomical changes leading to increased intraluminal pressure, reduced gastric compliance, and impaired antireflux barrier (6).
On the other hand, RYGB has been proposed as the bariatric surgery of choice for patients with medically complicated obesity because most parietal cells are excluded from the gastric pouch, decreasing the amount of acid that can be produced in proximity to the esophagus (9–11). However, a subset of patients has persistent symptoms and GERD-related complications, including esophageal adenocarcinoma (EAC), despite RYGB (12–14).
Most studies assessing postbariatric surgery GERD have relied on clinical symptoms. However, the recent Lyon Consensus objectively defines GERD on the basis of erosive esophagitis on upper endoscopy or esophageal acid exposure time (AET) on ambulatory reflux monitoring (15). Furthermore, data regarding postoperative changes in GE reflux and esophageal motility are often conflicting and limited to small case series (7,11). Further investigation of postbariatric surgery patients with ambulatory reflux monitoring and esophageal manometry might provide insight regarding esophageal motor function and physiology and the mechanisms responsible for GERD (16–18). We, therefore, conducted a systematic review and meta-analysis to summarize the effects of SG and RYGB on acid reflux and esophageal motor function. To evaluate the observation of EAC after bariatric surgery, we also conducted an observational study of all reported cases of EAC after SG and RYGB across 3 tertiary-care centers in 3 states and a systematic review.
MATERIALS AND METHODS
This study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (19).
Search strategy
A comprehensive search of several databases from each database's inception to September 12th, 2019, was conducted with no language restrictions. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by a medical reference librarian (L.J.P.) with input from the study's principal investigator (B.K.A.D.). Controlled vocabulary supplemented with keywords was used to search for esophageal manometry or esophageal pH test in adult patients who underwent SG or RYGB. References of selected retrieved articles were also manually reviewed for additional potentially relevant studies. Two investigators (R.M. and V.J.) independently reviewed the eligibility of the retrieved articles. Any disagreement was resolved by discussion and consensus with a third investigator (P.U.). The detailed search strategies are available in Supplementary Item 1, Supplemental Digital Content 1, http://links.lww.com/CTG/A343. In addition to the systematic search of the databases detailed earlier, the methodology used for our cohort study and a systematic review of EAC after bariatric surgery is available in Appendix 1, Supplemental Digital Content 2, http://links.lww.com/CTG/A348. These were then summarized along with our systematic search of the literature (see Supplementary Item 5, Supplementary Digital Content 3, http://links.lww.com/CTG/A347).
Study selection and quality assessment
Eligible studies were retrospective or prospective cohort studies or clinical trials that must meet the following inclusion criteria: (i) adult participants (aged older than 18 years) with obesity; (ii) underwent either SG or RYGB; (iii) performed esophageal pH test with or without impedance and/or esophageal manometry before and at least 1 month after surgery and reported at least 1 parameter of these tests. Case reports, editorial letters, reviews, and meta-analysis were excluded. If there was more than 1 eligible study that reported duplicate data from the same group of patients, only 1 study with the most comprehensive information was selected for inclusion. Two investigators (K.V. and N.W.) independently evaluated the methodological quality (risk of bias) of individual studies using the Newcastle-Ottawa Scale (20), which assessed each study in 3 areas; (i) the selection of the study groups; (ii) the comparability of the groups; and (iii) the ascertainment of the outcome of interest.
Data extraction
The following data were independently extracted by 2 authors (K.V. and N.W.) using a standardized data extraction form containing the following items: (i) general information: first author name, study location, year of publication, study design, and duration of follow-up; (ii) patient characteristics: age, sex, body mass index, GERD, and number of patients undergoing each test; (iii) outcomes: parameters of esophageal pH test and esophageal manometry. The corresponding authors of the included articles were contacted if additional data were required for the meta-analysis.
Outcomes
The outcomes were changes in esophageal manometric parameters including lower esophageal sphincter (LES) resting pressure, LES length, esophageal body amplitude, intragastric pressure, and the rate of ineffective esophageal motility (IEM) and changes in esophageal pH monitoring parameters including DeMeester score, total esophageal AET (the percentage of time where pH is below 4), AET in the upright position and recumbent position, total number of reflux episodes, number of reflux episodes in the upright position and recumbent position, and number for acid and nonacid reflux episodes after surgery compared with before surgery. The definition of IEM was defined based on the manometric technique and classification used by each individual study.
Statistical analysis
The proportions for categorical variables and the mean or median for continuous variables were extracted from each study. The pooled mean difference (MD) or risk ratio (RR) and 95% confidence intervals (CIs) were estimated using a random-effects model with the approach described by DerSimonian and Laird. Where the mean and SD of the change from baseline to endpoint were not reported in the original studies, the following equations were used to calculate them:
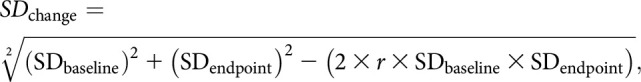 |
where r represents the correlation coefficient. We performed a sensitivity analysis using r of 0.4, 0.6 and 0.8 for our meta-analyses, and the results did not significantly changed indicating that our analyses were robust to this assumption. We used r of 0.4 in our meta-analyses (21). The heterogeneity of effect size estimates across the studies was quantified using the Q statistic and I2 (P < 0.10 was considered significant). I2 values of 0, <25%, 25%–49%, and ≥50% indicated no, low, moderate, and high heterogeneity, respectively (22). If mean and SD were not available, median was converted to mean using the formulas from the Cochrane Handbook for Systematic Reviews of Interventions (21). All analyses were performed using OpenMetaAnalyst software (CEBM, Brown University, Providence, RI).
RESULTS
Study selection
The initial search yielded 353 articles. After the exclusion of 108 duplicate articles, 245 articles underwent title and abstract review. A total of 194 articles were excluded at this stage because they clearly did not fulfill the eligibility criteria, leaving 51 articles for full-text review. Finally, 27 studies (15 studies for SG (23–37), 10 studies for RYGB (38–47), and 2 studies for both surgeries (13,48)) were eligible for inclusion in the systematic review and meta-analysis. The flow diagram of the study selection process is shown in Supplementary Item 2, Supplemental Digital Content 4, http://links.lww.com/CTG/A344.
Characteristics and quality of included studies
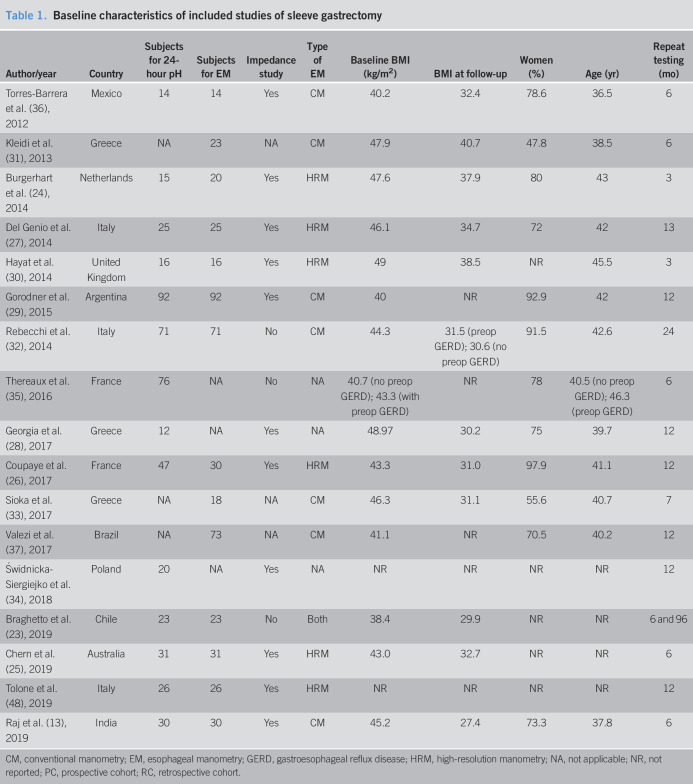
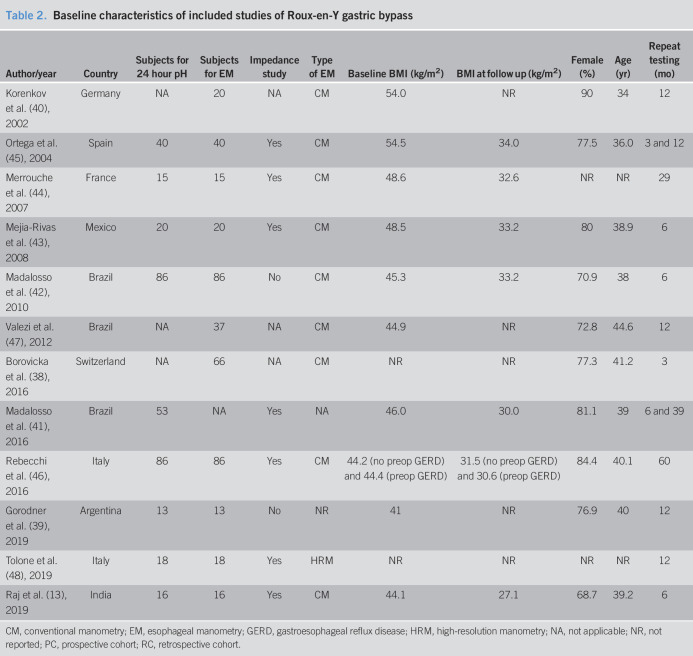
The 27 included studies were conducted in Europe (n = 16), South America (n = 9), Asia (n = 1), and Oceania (n = 1). All studies were either retrospective or prospective cohort studies. The interval between surgery and reassessment of the esophageal function ranged from 3 to 96 months. Sample sizes ranged from 12 to 92 subjects. A 24-hour pH test was used to assess esophageal pH in all studies, in which 16 studies used 24-hour intraluminal impedance-pH catheter. For manometric evaluation, 15 studies used conventional esophageal manometry using a water perfusion catheter with 4–8 pressure sensors, 6 studies used high-resolution manometry using 36 solid-state circumferential sensors spaced 1 cm apart, and 1 study used both methods. The characteristics of included studies are summarized in Tables 1 and 2. The quality assessment of included studies was satisfactory and is available in Supplementary Item 3, Supplemental Digital Content 5, http://links.lww.com/CTG/A345. GERD symptoms after bariatric surgery were variably reported among the included studies using different definitions. We summarized GERD symptoms and endoscopic findings after bariatric surgery compared with their baseline values in Supplementary Item 4, Supplemental Digital Content 6, http://links.lww.com/CTG/A346.
Table 1.
Baseline characteristics of included studies of sleeve gastrectomy
CM, conventional manometry; EM, esophageal manometry; GERD, gastroesophageal reflux disease; HRM, high-resolution manometry; NA, not applicable; NR, not reported; PC, prospective cohort; RC, retrospective cohort.
Table 2.
Baseline characteristics of included studies of Roux-en-Y gastric bypass
CM, conventional manometry; EM, esophageal manometry; GERD, gastroesophageal reflux disease; HRM, high-resolution manometry; NA, not applicable; NR, not reported; PC, prospective cohort; RC, retrospective cohort.
Esophageal manometric changes
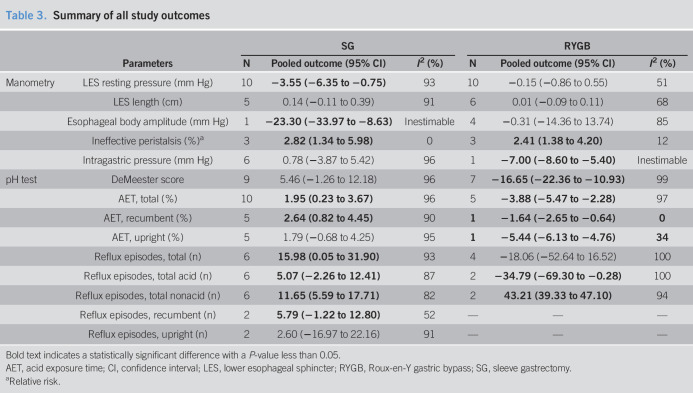
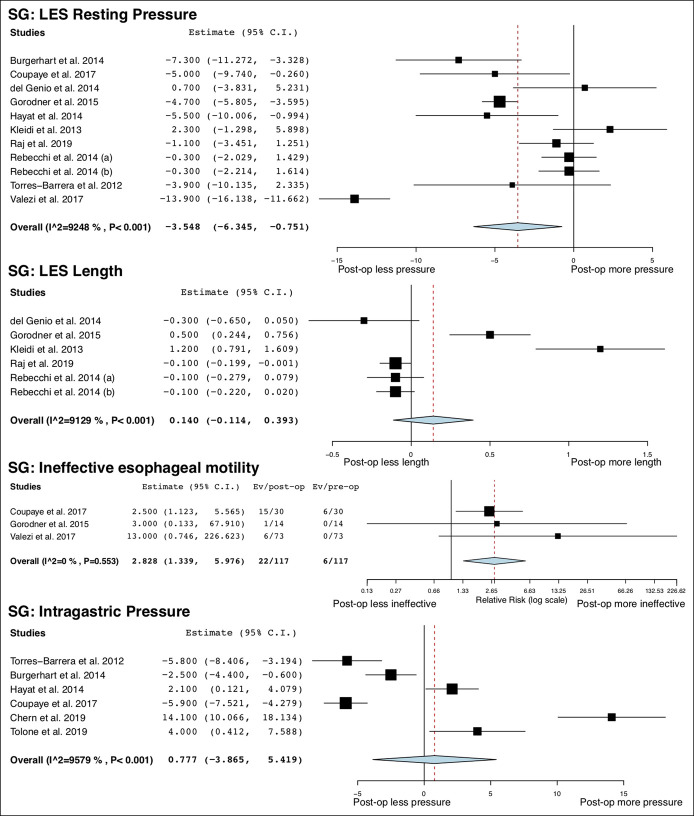
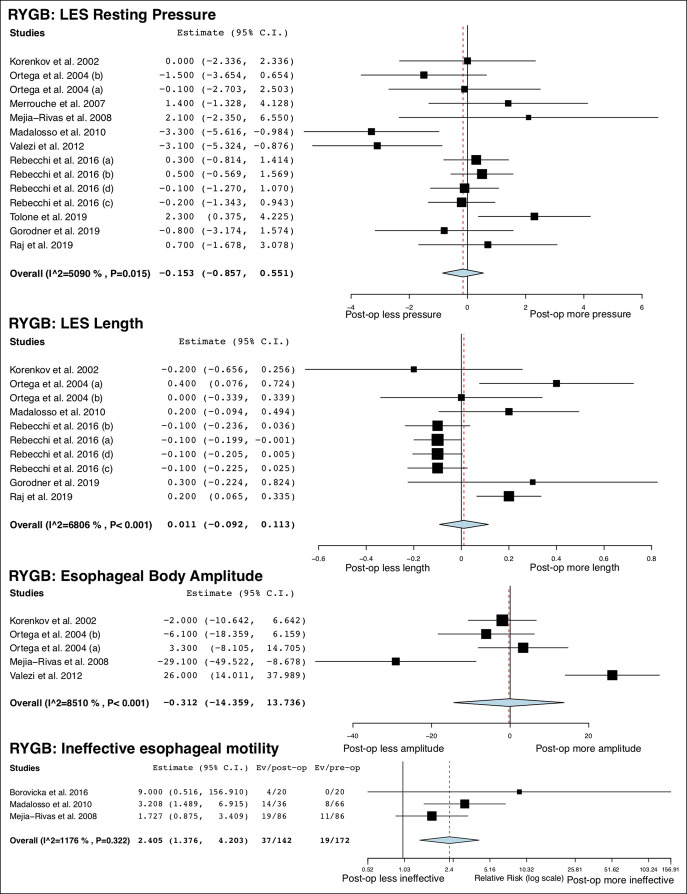
A total of 14 studies (13,23–27,29–33,36,37,48) (492 patients) and 11 studies (13,38–40,42–48) (417 patients) provided information on esophageal manometric changes after SG and RYGB, respectively. LES resting pressure was significantly decreased after SG (MD = −3.55 mm Hg; 95% CI −6.35 to −0.75; I2 = 93%) but did not change after RYGB (MD = −0.15 mm Hg; 95% CI −0.86 to 0.55; I2 = 51%). LES length was not changed after both surgeries. Esophageal body amplitude was significantly decreased after SG (MD = −23.3 mm Hg; 95% CI −33.97 to −8.63, 1 study) but did not change after RYGB (MD = −0.31 mm Hg; 95% CI −14.36 to 13.74; I2 = 85%). Intragastric pressure was not altered after SG but was decreased after RYGB (MD = −7.00 mm Hg; 95% CI −8.60 to −5.40, 1 study). The risk of IEM was significantly increased after both SG (RR = 2.82; 95% CI 1.34 to 5.98; I2 = 0%) and RYGB (RR = 2.41; 95% CI 1.38 to 4.20; I2 = 12%). All studies (29,37,38,42,43) used conventional method (49) to identify IEM except for 1 study (26) that used high-resolution manometry (50). The overall study outcomes of esophageal manometry are summarized in Table 3, and the forest plots are outlined in Figures 1 and 2.
Table 3.
Summary of all study outcomes
Bold text indicates a statistically significant difference with a P-value less than 0.05.
AET, acid exposure time; CI, confidence interval; LES, lower esophageal sphincter; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Relative risk.
Figure 1.
Forest plots of changes in esophageal manometric parameters after SG. SG, sleeve gastrectomy.
Figure 2.
Forest plots of changes in esophageal manometric parameters after RYGB. RYGB, Roux-en-Y gastric bypass.
Changes in esophageal pH monitoring parameters
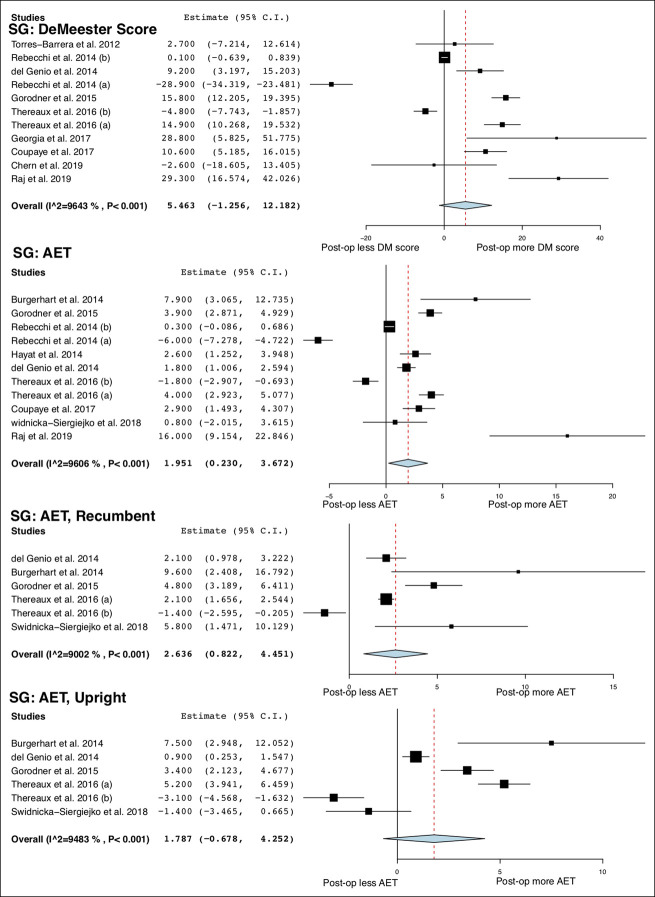
A total of 14 studies (13,23–30,32,34–36,48) (498 patients) and 9 studies (13,39,41–46,48) (347 patients) measured changes in parameters of esophageal pH monitoring after SG and RYGB, respectively. The DeMeester score did not significantly change after SG, whereas it was significantly decreased after RYGB (MD = −16.65; 95% CI −22.36 to −10.93; I2 = 99%). AET was increased after SG (MD = 1.95%; 95% CI 0.23 to 3.67; I2 = 96%) but decreased after RYGB (MD = −3.88%; 95% CI −5.47 to −2.28; I2 = 97%). After SG, AET in the recumbent position was significantly increased (MD = 2.64%; 95% CI 0.82 to 4.45; I2 = 90%) but was not altered in the upright position. After RYGB, AETs in the recumbent (MD = −1.64%; 95% CI −2.65 to −0.64, 1 study) and upright position (MD = −5.44%; 95% CI −6.13 to −4.76, 1 study) were significantly decreased.
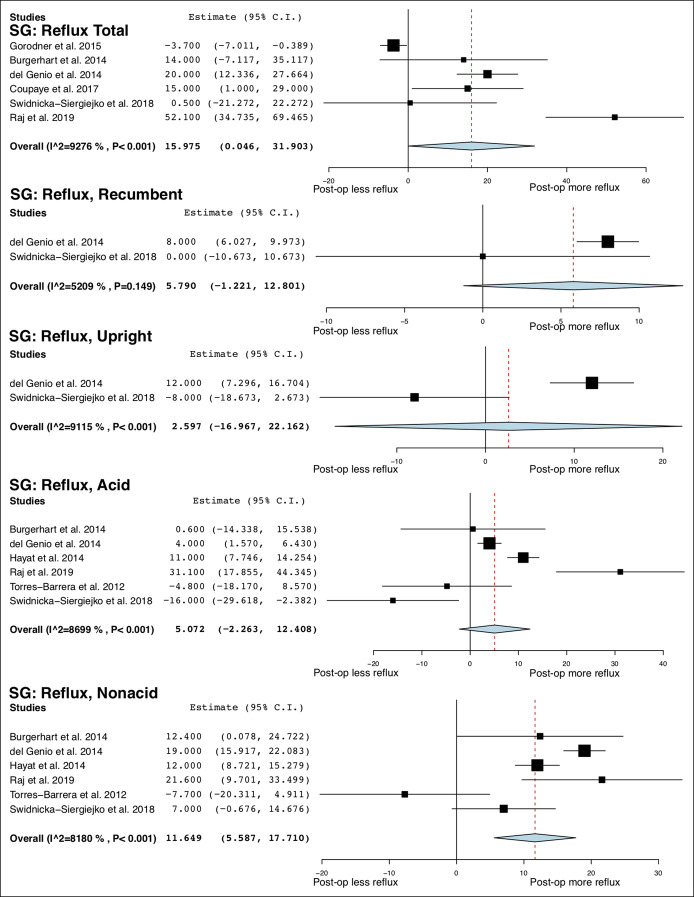
On intraluminal impedance monitoring, the total number of reflux episodes and nonacid reflux episodes were increased after SG, whereas the number of acid reflux episodes was not. After RYGB, the total number of reflux episodes was not altered, but the number of acid reflux episodes was decreased (MD = −34.79 95% CI −69.30 to −0.28; I2 = 100%), and the number of nonacid reflux episodes was increased (MD = 43.21; 95% CI 39.33 to 47.10; I2 = 94%). After SG, total reflux episodes were not significantly altered in the recumbent and upright position. No studies of RYGB reported these outcomes. The overall study outcomes of esophageal pH monitoring are summarized in Table 4, and the forest plots are outlined in Figures 3–5.
Table 4.
Characteristics of patients with esophageal adenocarcinoma after bariatric surgery
AC, adenocarcinoma; BE, Barrett's esophagus; C, chemotherapy; E, endoscopic mucosal resection; F, female; GE, gastroesophageal junction; GERD, gastroesophageal reflux disease; HGD, high-grade dysplasia; M, male; NA, not available; PPI, proton pump inhibitor; R, radiation; RYGB, Roux-en-Y gastric bypass; S, surgery; SG, sleeve gastrectomy.
Figure 3.
Forest plots of changes in esophageal pH monitoring parameters after SG. SG, sleeve gastrectomy.
Figure 5.
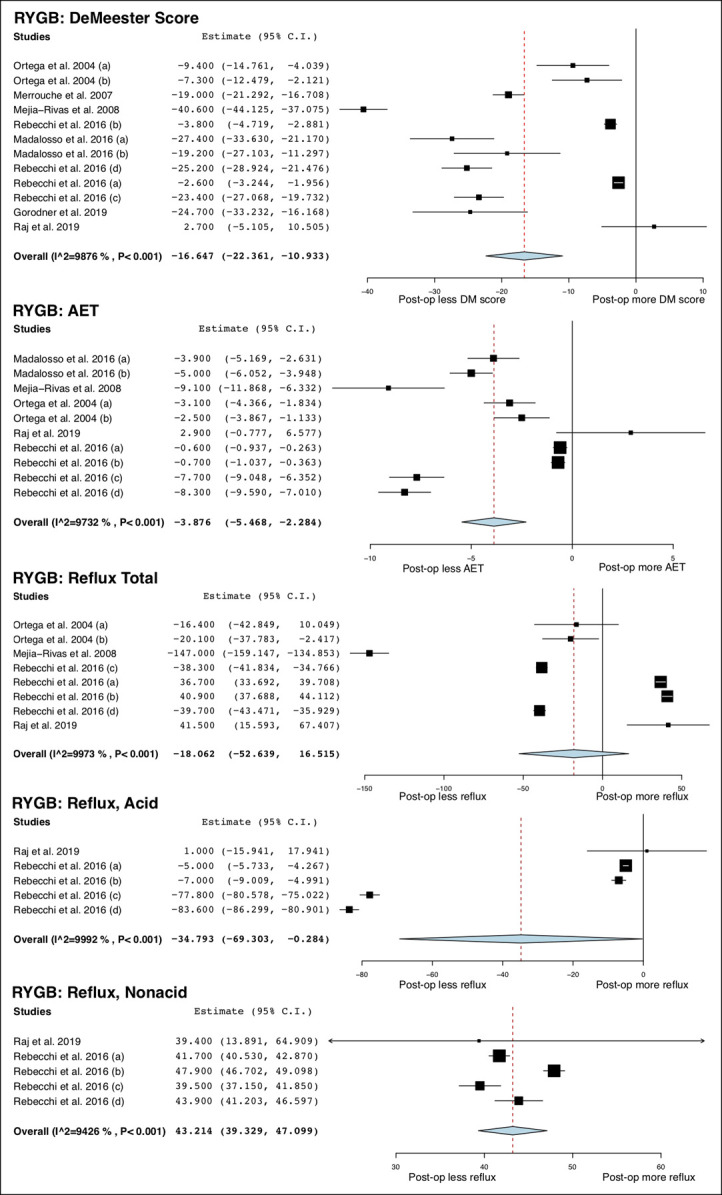
Forest plots of changes in esophageal pH monitoring parameters after RYGB. RYGB, Roux-en-Y gastric bypass.
Figure 4.
Forest plots of changes in esophageal pH monitoring parameters after SG (continued). SG, sleeve gastrectomy.
Esophageal adenocarcinoma after bariatric surgery
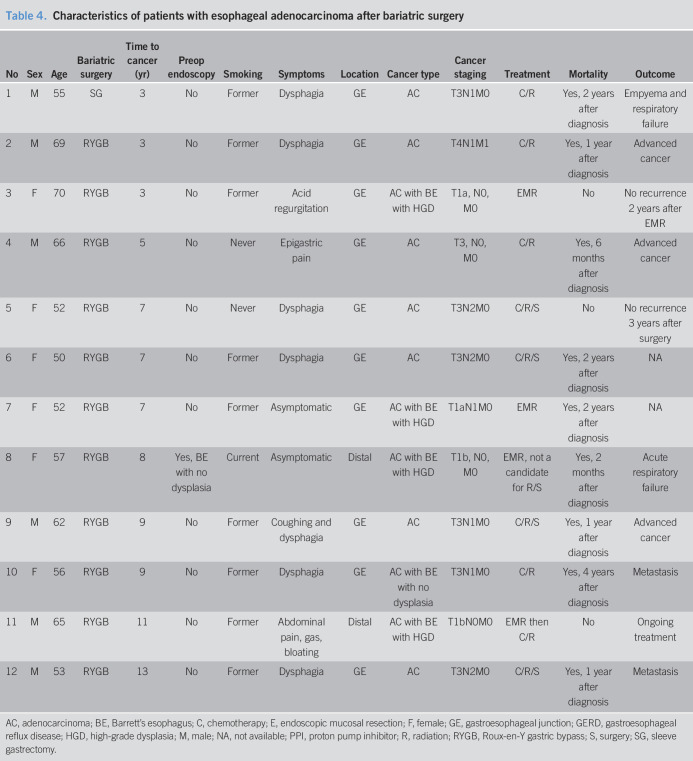
At our institution between January 1, 1995, and September 30, 2019, 12 patients were diagnosed as EAC after bariatric surgery (RYGB: 11, SG: 1) with a mean age of 58.8 ± 7.3 years and 50% women. The median duration after bariatric surgery to cancer diagnosis was 7 (interquartile range 3.5–9) years. A single patient had a preoperative upper endoscopy, which showed Barrett's esophagus with no dysplasia. Most patients (66.7%) had GERD symptoms, and 11 patients (91.7%) were taking proton pump inhibitor before cancer diagnosis. Dysphagia was present at the time of EAC diagnosis in most patients (58.3%). Two patients were asymptomatic, and cancer was diagnosed with a routine or surveillance upper endoscopy. All cases of EAC were localized to the distal esophagus or GE junction. EAC arouse in the background of Barrett's esophagus in 5 patients (41.7%), whereas 2 patients had an early-stage cancer amenable for endoscopic curative treatment. A total of 8 patients (66.7%) died within 2 years after diagnosis (Table 4).
Esophageal adenocarcinoma after bariatric surgery: systematic review
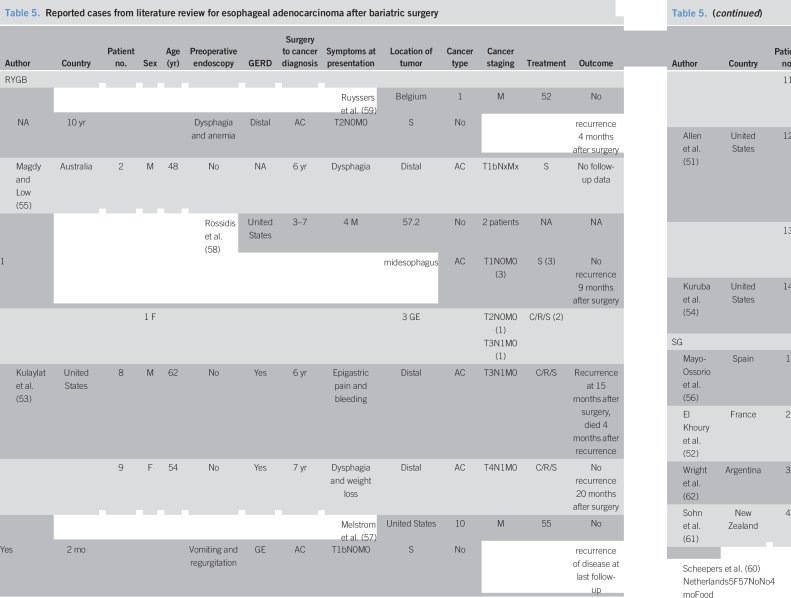
Study selection is detailed in Appendix 1, Supplemental Digital Content 2, http://links.lww.com/CTG/A348. Twelve studies (RYGB: 14 patients; SG: 5 patients) (51–62) were included in the systematic review. Fourteen patients (7 studies (51,53–55,57–59)) were diagnosed as EAC from 2 months to 24 years after RYGB. The patients' age ranged from 36 to 62 years with 16.7% women. None of the patients underwent an upper endoscopy preoperatively. Eight patients had GERD before the cancer diagnosis. Common presenting symptoms were dysphagia (42.9%) and heartburn/regurgitation (21.4%). Most cancers were localized at distal esophagus or GE junction (78.5%). None of the patients presented at early stage (T1a) that was amenable for endoscopic curative resection (Table 5).
Table 5.
Reported cases from literature review for esophageal adenocarcinoma after bariatric surgery
AC, adenocarcinoma; BE, Barrett's esophagus; C, chemotherapy; EMR, endoscopic mucosal resection; F, female; GE, gastroesophageal junction; GERD, gastroesophageal reflux disease; M, male; NA, not available; PPI, proton pump inhibitor; R, radiation; RYGB, Roux-en-Y gastric bypass; S, surgery; SG, sleeve gastrectomy.
Five patients (5 studies (52,56,60–62)) were diagnosed as EAC from 4 months to 5 years after SG. The patients' age ranged from 44 to 57 years with 60% women. Two patients underwent a preoperative upper endoscopy. Of those, 1 patient was found to have Barrett's esophagus with no dysplasia, whereas the other patient had no evidence of esophagitis or Barrett's esophagus. Two patients had GERD before cancer diagnosis. All cancers were localized at distal esophagus or GE junction. Only 1 patient had early-stage cancer (T1a) amenable for endoscopic mucosal resection. The follow-up data were limited up to 1 year (Table 5).
DISCUSSION
GERD is highly prevalent in morbidly obese patients (63). Bariatric surgery has been shown to alter the natural course of GERD and esophageal motor function in many different ways with a possible considerable associated risk of EAC (10,11,13). Our study aimed to provide more insights into this important matter.
SG is largely considered as a refluxogenic procedure although controversies exist because improvement of GERD after SG has been observed in some studies (32,64–66). With the use of a gold standard diagnostic test for GERD, our meta-analysis demonstrated conclusively an increased AET after surgery, which confirmed the above-mentioned statement (8). Regarding the plausible mechanisms, proposed theories have been related to the stomach size reduction that potentially increases intragastric pressure and reduces gastric compliance (6). Previous studies that yielded positive findings measured gastric physiologic changes either with no preoperative data for comparison or at perioperative period (67,68). This meta-analysis found no significant change in intragastric pressure after SG. One explanation could be the counteracting effects of weight loss against stomach remodeling that alters the stomach pressure in the opposite way (69,70).
We found a significant decrease in LES pressure after SG that could be one of the potential mechanisms. The role of LES on GERD pathogenesis is well described because LES is the main antireflux barrier of a complex anatomic high-pressure zone. The compromised LES pressure could be caused by surgical manipulation of the supporting structure surrounding the GE junction, mainly the sling fibers (6). Our study also observed increased AET and reflux episodes in the recumbent position but not in the upright position. These findings further support our hypothesis that impaired LES function or compromised antireflux barrier could be the culprit increasing the tendency of acid reflux in the supine position. Once reflux occurs, mechanisms including chemical clearance with salivary buffering and mechanical clearance through esophageal peristalsis aid in clearance of the gastric refluxate from the esophagus. This study found a higher risk of ineffective peristalsis and decreased amplitude after SG, which potentially suggests impaired esophageal clearance of gastric refluxate that results in prolonged acid exposure to the esophagus and esophagitis (71,72). Given these findings, patients with coexistent GERD might not represent ideal candidates for SG and warrant careful counseling and surveillance if opted for this surgical option.
RYGB has long been known as a reflux-protective bariatric surgery. Our study supported this hypothesis, which demonstrated decreases in DeMeester score and AET after surgery. Previous literature also suggested that this surgery preserved esophageal function (10,73). Although this study found an increased rate of IEM after RYGB, it seemed to have a minimal impact on esophageal motor function overall with no postoperative changes in LES pressure, LES length, and esophageal body amplitude. Intraluminal impedance detected no change in GE reflux events overall; however, the proportion of nonacid reflux events increased after RYGB. The reduction in acid reflux episodes likely reflects reduced gastric parietal cells, given antral resection (74). Given the lack of effect on GE reflux frequency, persistent GERD symptoms might reflect volume regurgitation and mechanical stimulation in a subset of patients (75).
Including our largest series, 31 EAC cases in total have been reported to date after SG and RYGB. In our cohort, most patients presented with GERD and almost half had cancer arising from Barrett's esophagus. SG is shown to increase acid reflux, and this could theoretically result in an increased risk of Barrett's esophagus and EAC. On the other hand, RYGB is shown to decrease acid reflux; however, the effect of RYGB on the development or progression of Barrett's esophagus or EAC remains unclear. The observation of persistent impedance-detected gastric refluxate, whereas less acidic after RYGB, might still prove noxious to the esophagus and warrants further studies (72,76,77). Although a 7.6-year population-based cohort found no increased incidence of EAC after SG or RYGB compared with non-surgery control groups, this phenomenon might require a long-term follow-up, and these effects might not be equally distributed across the population (78).
Our study has several limitations. First, although this meta-analysis combined all available studies, our dataset was still relatively small. A preplanned subgroup analysis of different follow-up intervals could not be performed. Second, there was a high heterogeneity across studies. This could be from differences in the duration of follow-up, methods used for manometric evaluation (conventional vs high resolution), patient selection criteria for surgery (with or without GERD), and surgical technique that was not well standardized. Third, almost all studies (25/27 studies) were single-arm studies. The comparison of esophageal pH and manometric parameter changes between RYGB and SG to determine their relative difference could not be performed. Finally, there is a possibility of reporting bias because there were 6 studies that had inadequate data for meta-analysis. We attempted to contact authors, but no data were available to us. For the analysis of EAC, given the nature of the included studies and our study with no preoperative endoscopy in most studies and no control group for comparison, our study could at best report the observation of EAC after bariatric surgery and no definite association between them could be determined. We tried to minimize bias by including only patients who were newly diagnosed with EAC between 3 and 15 years after bariatric surgery.
In summary, our study found increased acid reflux after SG and decreased acid reflux after RYGB based on pH study. These findings suggest that RYGB might be the bariatric surgery of choice in patients with coexistent GERD. However, the frequency of intraluminal impedance-detected total GE reflux events is unaffected by RYGB, and weakly and nonacid reflux events might still contribute to postoperative GERD in a subset of patients. Given several reported EAC cases after RYGB, the long-term effects of this operation on the rates of Barrett's esophagus and EAC warrant further investigation and careful patient monitoring. RYGB remains a gold standard bariatric surgery for GERD but might not entirely preserve esophageal function as previously believed. Increasing understanding in underlying pathophysiology of GERD after bariatric surgery would lead to a better patient allocation and more insights into postbariatric GERD management.
CONFLICTS OF INTEREST
Guarantor of the article: Barham K. Abu Dayyeh, MD, MPH.
Specific author contributions: V.J., K.R., and B.K.A.D. conceived the study, searched the literature, extracted the data, assessed the quality of the studies, and drafted the manuscript. L.J.P. searched the literature. P.U. and M.H.M. performed the statistical analysis and drafted the manuscript. E.J.V., K.V., N.W., D.B.M, and R.M. extracted the data, assessed the quality of the studies, and drafted the manuscript. All authors read and approved the final manuscript.
Financial support: None to report.
Potential competing interests: B.A.K.D. is a consultant for Metamodix, BFKW, DyaMx, Boston Scientific, USGI medical, and Endo-TAGSS. He received research support from Apollo Endosurgery, USGI, Spatz Medical, Boston Scientific, GI Dynamics, Cairn Diagnostics, Aspire Bariatrics, and Medtronic. He served as a speaker for Johnson and Johnson, Endogastric Solutions, and Olympus. All authors read and approved the final manuscript.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A343, http://links.lww.com/CTG/A344, http://links.lww.com/CTG/A345, http://links.lww.com/CTG/A346, http://links.lww.com/CTG/A347, http://links.lww.com/CTG/A348
REFERENCES
- 1.Bray GA. Obesity: Historical development of scientific and cultural ideas. Int J Obes 1990;14:909–26. [PubMed] [Google Scholar]
- 2.Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil Steril 2017;107:833–9. [DOI] [PubMed] [Google Scholar]
- 3.Himpens J, Ramos A, Welbourn R, et al. Fourth IFSO Global Registry Report 2018. Dendrite Clinical Systems Ltd: Henley-on-Thames, 2018. UKISBN 978–0–9929942-7-3. [Google Scholar]
- 4.Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: The SM-BOSS randomized clinical trial. JAMA 2018;319:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salminen P, Helmio M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA 2018;319:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol 2015;21:10348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sioka E, Tzovaras G, Perivoliotis K, et al. Impact of laparoscopic sleeve gastrectomy on gastrointestinal motility. Gastroenterol Res Pract 2018;2018:4135813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeung KTD, Penney N, Ashrafian L, et al. Does sleeve gastrectomy expose the distal esophagus to severe reflux?: A systematic review and meta-analysis. Ann Surg 2020;271:257–65. [DOI] [PubMed] [Google Scholar]
- 9.Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010;24:2647–69. [DOI] [PubMed] [Google Scholar]
- 10.Naik RD, Choksi YA, Vaezi MF. Consequences of bariatric surgery on oesophageal function in health and disease. Nat Rev Gastroenterol Hepatol 2016;13:111–9. [DOI] [PubMed] [Google Scholar]
- 11.Savarino E, Marabotto E, Savarino V. Effects of bariatric surgery on the esophagus. Curr Opin Gastroenterol 2018;34:243–8. [DOI] [PubMed] [Google Scholar]
- 12.Frezza EE, Ikramuddin S, Gourash W, et al. Symptomatic improvement in gastroesophageal reflux disease (GERD) following laparoscopic Roux-en-Y gastric bypass. Surg Endosc 2002;16:1027–31. [DOI] [PubMed] [Google Scholar]
- 13.Raj PP, Bhattacharya S, Misra S, et al. Gastroesophageal reflux-related physiologic changes after sleeve gastrectomy and Roux-en-Y gastric bypass: A prospective comparative study. Surg Obes Relat Dis 2019;15:1261–9. [DOI] [PubMed] [Google Scholar]
- 14.Scozzari G, Trapani R, Toppino M, et al. Esophagogastric cancer after bariatric surgery: Systematic review of the literature. Surg Obes Relat Dis 2013;9:133–42. [DOI] [PubMed] [Google Scholar]
- 15.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018;67:1351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazzoni M, de Bortoli N, Frazzoni L, et al. Impedance-pH monitoring for diagnosis of reflux disease: New perspectives. Dig Dis Sci 2017;62:1881–9. [DOI] [PubMed] [Google Scholar]
- 17.Gyawali CP, Patel A. Esophageal motor function: Technical aspects of manometry. Gastrointest Endosc Clin N Am 2014;24:527–43. [DOI] [PubMed] [Google Scholar]
- 18.Yadlapati R. High-resolution esophageal manometry: Interpretation in clinical practice. Curr Opin Gastroenterol 2017;33:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braghetto I, Korn O. Late esophagogastric anatomic and functional changes after sleeve gastrectomy and its clinical consequences with regards to gastroesophageal reflux disease. Dis Esophagus 2019;32:doz020. [DOI] [PubMed] [Google Scholar]
- 24.Burgerhart JS, Schotborgh CA, Schoon EJ, et al. Effect of sleeve gastrectomy on gastroesophageal reflux. Obes Surg 2014;24:1436–41. [DOI] [PubMed] [Google Scholar]
- 25.Chern Y, Maani J, Fisher OM, et al. Objective Assessment of Reflux Before and After Laparoscopic Sleeve Gastrectomy: High Resolution Manometry and pH Impedence (Abstract) In: Chinese Society for Metabolic & Bariatric Surgery (CSMBS) Annual Conference. Guangzhou, China; 2019.
- 26.Coupaye M, Gorbatchef C, Calabrese D, et al. Gastroesophageal reflux after sleeve gastrectomy: A prospective mechanistic study. Obes Surg 2018;28:838–45. [DOI] [PubMed] [Google Scholar]
- 27.Del Genio G, Tolone S, Limongelli P, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg 2014;24:71–7. [DOI] [PubMed] [Google Scholar]
- 28.Georgia D, Stamatina T, Maria N, et al. 24-h multichannel intraluminal impedance PH-metry 1 year after laparocopic sleeve gastrectomy: An objective assessment of gastroesophageal reflux disease. Obes Surg 2017;27:749–53. [DOI] [PubMed] [Google Scholar]
- 29.Gorodner V, Buxhoeveden R, Clemente G, et al. Does laparoscopic sleeve gastrectomy have any influence on gastroesophageal reflux disease? Preliminary results. Surg Endosc 2015;29:1760–8. [DOI] [PubMed] [Google Scholar]
- 30.Hayat J, Mansour S, Wan1 A, et al. Acid and non-acid gastro-oesophageal reflux following sleeve gastrectomy (abstract). Surg Endosc 2014;28:S1–S53. [Google Scholar]
- 31.Kleidi E, Theodorou D, Albanopoulos K, et al. The effect of laparoscopic sleeve gastrectomy on the antireflux mechanism: Can it be minimized? Surg Endosc 2013;27:4625–30. [DOI] [PubMed] [Google Scholar]
- 32.Rebecchi F, Allaix ME, Giaccone C, et al. Gastroesophageal reflux disease and laparoscopic sleeve gastrectomy: A physiopathologic evaluation. Ann Surg 2014;260:909–14; discussion 914–5. [DOI] [PubMed] [Google Scholar]
- 33.Sioka E, Tzovaras G, Tsiopoulos F, et al. Esophageal motility after laparoscopic sleeve gastrectomy. Clin Exp Gastroenterol 2017;10:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Świdnicka-Siergiejko AK, Wroblewski E, Hady HR, et al. Esophageal pH and impedance reflux parameters in relation to body mass index, obesity-related hormones, and bariatric procedures. Pol Arch Intern Med 2018;128:594–603. [DOI] [PubMed] [Google Scholar]
- 35.Thereaux J, Barsamian C, Bretault M, et al. pH monitoring of gastro-oesophageal reflux before and after laparoscopic sleeve gastrectomy. Br J Surg 2016;103:399–406. [DOI] [PubMed] [Google Scholar]
- 36.Torres-Barrera GA, Vazquez-Elizondo G, Bosques FJ, et al. Impact of sleeve gastrectomy in the occurrence of gastro esophageal reflux disease (abstact). Gastroenterology 2012:Tu1141. [Google Scholar]
- 37.Valezi AC, Herbella FA, Mali-Junior J, et al. Preoperative manometry for the selection of obese people candidate to sleeve gastrectomy. Arq Bras Cir Dig 2017;30:222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borovicka J, Krieger-Grubel C, van der Weg B, et al. Effect of morbid obesity, gastric banding and gastric bypass on esophageal symptoms, mucosa and function. Surg Endosc 2017;31:552–60. [DOI] [PubMed] [Google Scholar]
- 39.Gorodner MV, Matucci A, Sole L, et al. Does Roux-en-Y gastric bypass really cure gastroesophageal reflux disease? Analysis of objective data (abstract). Surg Endosc 2019:S139. [DOI] [PubMed] [Google Scholar]
- 40.Korenkov M, Kohler L, Yucel N, et al. Esophageal motility and reflux symptoms before and after bariatric surgery. Obes Surg 2002;12:72–6. [DOI] [PubMed] [Google Scholar]
- 41.Madalosso CA, Gurski RR, Callegari-Jacques SM, et al. The impact of gastric bypass on gastroesophageal reflux disease in morbidly obese patients. Ann Surg 2016;263:110–6. [DOI] [PubMed] [Google Scholar]
- 42.Madalosso CA, Gurski RR, Callegari-Jacques SM, et al. The impact of gastric bypass on gastroesophageal reflux disease in patients with morbid obesity: A prospective study based on the montreal consensus. Ann Surg 2010;251:244–8. [DOI] [PubMed] [Google Scholar]
- 43.Mejia-Rivas MA, Herrera-Lopez A, Hernandez-Calleros J, et al. Gastroesophageal reflux disease in morbid obesity: The effect of Roux-en-Y gastric bypass. Obes Surg 2008;18:1217–24. [DOI] [PubMed] [Google Scholar]
- 44.Merrouche M, Sabate JM, Jouet P, et al. Gastro-esophageal reflux and esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg 2007;17:894–900. [DOI] [PubMed] [Google Scholar]
- 45.Ortega J, Escudero MD, Mora F, et al. Outcome of esophageal function and 24-hour esophageal pH monitoring after vertical banded gastroplasty and Roux-en-Y gastric bypass. Obes Surg 2004;14:1086–94. [DOI] [PubMed] [Google Scholar]
- 46.Rebecchi F, Allaix ME, Ugliono E, et al. Increased esophageal exposure to weakly acidic reflux 5 years after laparoscopic Roux-en-Y gastric bypass. Ann Surg 2016;264:871–7. [DOI] [PubMed] [Google Scholar]
- 47.Valezi AC, Herbella FA, Junior JM, et al. Esophageal motility after laparoscopic Roux-en-Y gastric bypass: The manometry should be preoperative examination routine? Obes Surg 2012;22:1050–4. [DOI] [PubMed] [Google Scholar]
- 48.Tolone S, Savarino E, de Bortoli N, et al. Esophageal high resolution manometry can unravel the mechanisms by which different bariatric techniques produce different reflux exposure (abstract). In: The Society for Surgery of the Alimentary Tract. San Diego, CA; 2019. p. S-1393. [DOI] [PubMed]
- 49.Pandolfino JE, Kahrilas PJ; American Gastroenterological A. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005;128:209–24. [DOI] [PubMed] [Google Scholar]
- 50.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen JW, Leeman MF, Richardson JD. Esophageal carcinoma following bariatric procedures. JSLS 2004;8:372–5. [PMC free article] [PubMed] [Google Scholar]
- 52.El Khoury L, Benvenga R, Romero R, et al. Esophageal adenocarcinoma in Barrett's esophagus after sleeve gastrectomy: Case report and literature review. Int J Surg Case Rep 2018;52:132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kulaylat AN, Sahajwani S, Staveley-O'Carroll KF, et al. Reconstructive options for gastroesophageal junction adenocarcinoma after Roux-en-Y gastric bypass. J Thorac Cardiovasc Surg 2013;146:1296–8. [DOI] [PubMed] [Google Scholar]
- 54.Kuruba R, Jawad M, Karl RC, et al. Technique of resection of esophageal adenocarcinoma after Roux-en-Y gastric bypass and literature review of esophagogastric tumors after bariatric procedures. Surg Obes Relat Dis 2009;5:576–81. [DOI] [PubMed] [Google Scholar]
- 55.Magdy M, Low K. Oesophageal cancer post bariatric Roux-en-Y gastric bypass. In: IFSO-APC. Guangzhou, China; 2019.
- 56.Mayo-Ossorio MDLA, Pacheco-Garcia JM, Bengoechea-Trujillo A, et al. Right coloplasty esophagectomy in esophageal adenocarcinoma one year after laparoscopic sleeve gastrectomy. In: 24th IFSO World Congress. Madrid, Spain; 2019.
- 57.Melstrom LG, Bentrem DJ, Salvino MJ, et al. Adenocarcinoma of the gastroesophageal junction after bariatric surgery. Am J Surg 2008;196:135–8. [DOI] [PubMed] [Google Scholar]
- 58.Rossidis G, Browning R, Hochwald SN, et al. Minimally invasive esophagectomy is safe in patients with previous gastric bypass. Surg Obes Relat Dis 2014;10:95–100. [DOI] [PubMed] [Google Scholar]
- 59.Ruyssers M, Jawad R, Gys B, et al. Ivor lewis oesophagectomy for a distal adenocarcinoma of the oesophagus 10 years after Roux-en-Y gastric bypass (RYGB). Obes Surg 2019;29:2713–4. [DOI] [PubMed] [Google Scholar]
- 60.Scheepers AF, Schoon EJ, Nienhuijs SW. Esophageal carcinoma after sleeve gastrectomy. Surg Obes Relat Dis 2011;7:e11–2. [DOI] [PubMed] [Google Scholar]
- 61.Sohn S, Fischer J, Booth M. Adenocarcinoma of the gastro-oesophageal junction after sleeve gastrectomy: A case report. ANZ J Surg 2017;87:E163–E164. [DOI] [PubMed] [Google Scholar]
- 62.Wright FG, Duro A, Medici JR, et al. Esophageal adenocarcinoma five years after laparoscopic sleeve gastrectomy. A case report. Int J Surg Case Rep 2017;32:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Serag H. The association between obesity and GERD: A review of the epidemiological evidence. Dig Dis Sci 2008;53:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berry MA, Urrutia L, Lamoza P, et al. Sleeve gastrectomy outcomes in patients with BMI between 30 and 35-3 years of follow-up. Obes Surg 2018;28:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daes J, Jimenez ME, Said N, et al. Improvement of gastroesophageal reflux symptoms after standardized laparoscopic sleeve gastrectomy. Obes Surg 2014;24:536–40. [DOI] [PubMed] [Google Scholar]
- 66.Pallati PK, Shaligram A, Shostrom VK, et al. Improvement in gastroesophageal reflux disease symptoms after various bariatric procedures: Review of the bariatric outcomes longitudinal database. Surg Obes Relat Dis 2014;10:502–7. [DOI] [PubMed] [Google Scholar]
- 67.Mion F, Tolone S, Garros A, et al. High-resolution impedance manometry after sleeve gastrectomy: Increased intragastric pressure and reflux are frequent events. Obes Surg 2016;26:2449–56. [DOI] [PubMed] [Google Scholar]
- 68.Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy—Volume and pressure assessment. Obes Surg 2008;18:1083–8. [DOI] [PubMed] [Google Scholar]
- 69.El-Serag HB, Tran T, Richardson P, et al. Anthropometric correlates of intragastric pressure. Scand J Gastroenterol 2006;41:887–91. [DOI] [PubMed] [Google Scholar]
- 70.Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: A challenge to esophagogastric junction integrity. Gastroenterology 2006;130:639–49. [DOI] [PubMed] [Google Scholar]
- 71.Fornari F, Callegari-Jacques SM, Scussel PJ, et al. Is ineffective oesophageal motility associated with reflux oesophagitis? Eur J Gastroenterol Hepatol 2007;19:783–7. [DOI] [PubMed] [Google Scholar]
- 72.Tack J, Pandolfino JE. Pathophysiology of gastroesophageal reflux disease. Gastroenterology 2018;154:277–88. [DOI] [PubMed] [Google Scholar]
- 73.Clements RH, Gonzalez QH, Foster A, et al. Gastrointestinal symptoms are more intense in morbidly obese patients and are improved with laparoscopic Roux-en-Y gastric bypass. Obes Surg 2003;13:610–4. [DOI] [PubMed] [Google Scholar]
- 74.Siilin H, Wanders A, Gustavsson S, et al. The proximal gastric pouch invariably contains acid-producing parietal cells in Roux-en-Y gastric bypass. Obes Surg 2005;15:771–7. [DOI] [PubMed] [Google Scholar]
- 75.Boeckxstaens GE, Smout A. Systematic review: Role of acid, weakly acidic and weakly alkaline reflux in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2010;32:334–43. [DOI] [PubMed] [Google Scholar]
- 76.Gutschow CA, Bludau M, Vallbohmer D, et al. NERD, GERD, and Barrett's esophagus: Role of acid and non-acid reflux revisited with combined pH-impedance monitoring. Dig Dis Sci 2008;53:3076–81. [DOI] [PubMed] [Google Scholar]
- 77.Oh DS, Demeester SR. Pathophysiology and treatment of Barrett's esophagus. World J Gastroenterol 2010;16:3762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouchard P, Demyttenaere SV, Court O, et al. Esophageal cancer after sleeve gastrectomy: A population-based cohort study. In: Obesity Week 2019. Las Vegas, NV; 2019. p. A156. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.