Supplemental Digital Content is available in the text.
Abstract
Background.
There is no consensus on rabbit antithymocyte globulin (rATG) dose used for induction immunosuppression in pediatric kidney transplants. We aimed to identify whether a lower rATG dose provides safe and effective immunosuppression compared with a higher dose.
Methods.
We retrospectively analyzed all first-time kidney transplant recipients (aged <21 y) in the North American Pediatric Renal Trials and Collaborative Studies registry since 1998 on mycophenolate mofetil– and tacrolimus-based immunosuppression with rATG induction. An a priori cutoff of 7.5 mg/kg cumulative rATG dose was used to identify low (<7.5 mg/kg) and high (≥7.5 mg/kg) exposure groups. Primary outcome was time to first-acute rejection episode. Secondary outcomes included graft function, patient survival, hospitalizations due to infections, and time to first-posttransplant lymphoproliferative disorder episode.
Results.
Four hundred fifty-five patients met inclusion criteria (59% male, 49% whites, 26% blacks, 38% living donor source). Median cumulative rATG dose was 6.8 mg/kg with a median of 5 doses and a median 1.5 mg/kg/dose introduced at a median of postoperative 0 days. Sixty-four percent received <7.5 mg/kg total rATG. There was no difference in age at transplant, gender, race, end-stage renal disease causes, or HLA mismatch among groups. Time to first-acute rejection was similar (P = 0.07). There was no significant difference in graft or patient survival or time to posttransplant lymphoproliferative disorder. Hospitalization for infection rates was similar.
Conclusions.
These data demonstrate a wide variation in cumulative rATG induction dose. A smaller rATG dose <7.5 mg/kg may provide effective and safe immunosuppression compared with a higher dose.
INTRODUCTION
Short-term pediatric kidney transplant recipient outcomes have significantly improved over the years with enhanced surgical techniques and more potent immunosuppression.1 The use of lymphocyte-depleting induction immunosuppression has led to significant reduction in first-year acute kidney transplant rejection rates2 and allowed many pediatric transplant centers to adopt steroid avoidance or steroid minimization immunosuppression protocols for low-immunologic risk patients.3 Rabbit antithymocyte globulin (rATG) is the most commonly used lymphocyte- depleting induction therapy in both adult and pediatric kidney transplant recipients.4 However, rATG did not receive FDA approval for that induction indication until 2017.5 This may have contributed to wide variation in “standard” dosing practice, as it related to cumulative exposure targets for induction in kidney transplant recipients as each transplant center adopted its own standards and practice.4 Significant cost savings can be realized with lower cumulative rATG exposure,6 and the inherent risk of infection and leukopenia with its use in conjunction with other immunosuppressive drugs favors a more judicious approach in dosing and administration.2 However, a lower dosing strategy may carry with it a higher risk of acute rejection given rATG’s established superiority in preventing acute rejection episodes at a relatively high cumulative dosing threshold of 7.5 mg per kg of body weight.7 In this study, we sought to evaluate prevailing practice patterns in rATG utilization among pediatric kidney transplant centers and to determine whether a lower rATG dose may provide safe and effective immunosuppression compared with a higher rATG dose.
MATERIALS AND METHODS
Study Design
This is a retrospective registry analysis study utilizing the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) transplant registry. The study protocol was submitted to the local institutional review board, which deemed the study to be exempt from further review. NAPRTCS was founded in 1987 as a voluntary registry to capture current practice and trends in immunosuppressive therapy with an ultimate goal of improving the care of pediatric renal allograft recipients in North America.8 At the time of this analysis, registry data were complete through end of calendar year 2016.
Inclusion Criteria
We analyzed all first-time pediatric kidney-only transplant recipients aged younger than 21 years at the time of transplant who were transplanted in 1998 or later. The analysis was limited to those who received rATG induction therapy and maintenance immunosuppression with a tacrolimus and mycophenolate mofetil (MMF)-based regimen at day 30 post index kidney transplant.
Exposure Variable
Based on a single rATG dose of 1.5 mg per kg of body weight, rATG cumulative exposure threshold was set a priori at 5 doses or greater (≥7.5 mg/kg) for the high-dose exposure group and at <5 doses (<7.5 mg/kg) for the low-dose exposure group.
Outcomes
Primary outcome was time to first-acute kidney transplant rejection episode. Secondary outcomes included 6- and 12-month graft function as measured by the modified Schwartz formula in mL/min/1.73m2 as well as comparisons of graft and patient survival,9 hospitalizations due to infections, and time to first-posttransplant lymphoproliferative disorder (PTLD) episode through 5 years post index kidney transplantation.
Statistical Analysis
Categorical demographic and baseline characteristics were summarized by exposure group and compared using Pearson’s chi-squared test. The amount and duration of rATG dosing were summarized numerically by exposure group. Kaplan-Meier survival curves were generated by exposure group for each of the outcome variables of acute rejection, graft failure, death, and PTLD. Differences in the distributions of time to the outcome variable between exposure groups were evaluated using the log-rank test. Time-to-event analyses for outcomes of patient and graft survival, acute rejection, and PTLD were repeated after controlling for steroid use at 30 days. The proportions of patients hospitalized within 12 months of transplant were summarized by exposure group and compared with Pearson’s chi-squared test. Estimated glomerular filtration rate at 6 and 12 months were summarized numerically by exposure group, and the distributions were compared using the Kruskal-Wallis test. Analyses of all outcome measures were repeated in a subset analysis that excluded patients receiving their first dose of rATG beyond 7 days post kidney transplant. Additionally, we performed a subset analysis of “low-risk” recipients limited to white recipients who did not experience delayed graft function.
RESULTS
Cohort Description and Baseline Characteristics of Low-dose and High-dose rATG Exposure Groups
At the time of this analysis, the NAPRTCS registry included data on 12 686 kidney transplant recipients, of whom 9826 were recipients of their first kidney transplant and aged younger than 21 years (Table 1). Of those, 486 met criteria for having received rATG induction and tacrolimus- and MMF-based maintenance regimen at day 30 post index kidney transplant. After exclusion of recipients with missing data on rATG dosing, a total of 440 subjects from 37 participating centers were included in the final analysis (Figure 1). Two hundred fifty-seven were male (58%), 49% were white, 26% black, and 15% Hispanic. Cause of end-stage renal disease was attributed to congenital anomalies of the kidney and urinary tract in 38%, nephrotic syndrome and focal segmental glomerulosclerosis in 20%, other glomerulonephritis in 16%, and other causes in 28%. Thirty-eight percent were living kidney donor recipients. Median age at index kidney transplant was 13 years, and 68% were transplanted in the most recent decade of registry data (2006–2016). Most patients had 1 or 2 antigen mismatches at the HLA-A (1 mismatch [mm] 41.6%; 2 mm 38.4%), HLA-B (1 mm 37%; 2 mm 44.8%), and HLA-DR (1 mm 43.6%; 2 mm 34.3%) loci. Most patients received prophylactic antibiotics (92%) and steroid maintenance immunosuppression at day 30 posttransplant (75.7%). Delayed graft function was observed in 10% of patients during their index kidney transplant hospitalization.
Table 1.
Baseline characteristics of low-dose and high-dose rATG exposure groups
FIGURE 1.
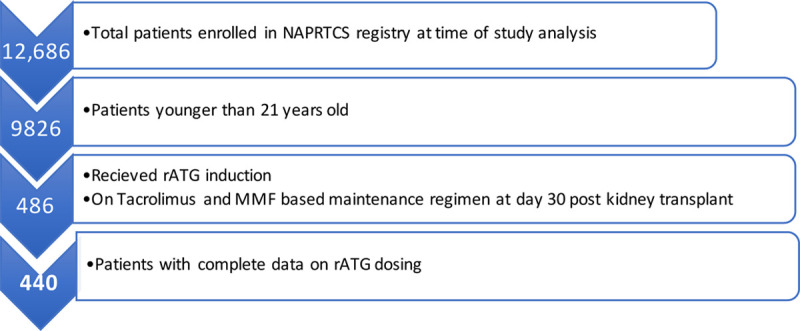
Analysis strategy. MMF, Mycophenolate mofetil; NAPRTCS, North American Pediatric Renal Trials and Collaborative Studies; and rATG, rabbit anti-thymocyte globulin.
Using an a priori cutoff of 7.5 mg/kg cumulative rATG induction dose to stratify the cohort, we identified 64% that received <7.5 mg/kg cumulative rATG (low-dose exposure group) and 36% that received ≥7.5 mg/kg cumulative rATG (high-dose exposure group). The low-dose group received a median of 4 days of rATG therapy with a median cumulative exposure of 5.8 mg/kg versus a median of 6 days of therapy and 9.1 mg/kg cumulative exposure for the high-dose group. There was no significant difference between both groups in terms of age at time of transplant, gender, race, donor source, primary end-stage renal disease cause, degree of HLA-A, B, or DR mismatch, and use of prophylactic antibiotics. However, a higher proportion of the low-dose exposure group was transplanted in the most recent era (P = 0.001) and was steroid free at day 30 post kidney transplant compared with the high-dose exposure group (31% versus 13%; P < 0.001). Delayed graft function was significantly higher in the high-dose exposure group (18% versus 6%; P < 0.001).
rATG Dosing Trends
For the entire cohort, the median cumulative rATG dose was 6.8 mg/kg (range 0.8–23.8 mg/kg) with a median of 5 doses (range 1–20) and a median of 1.5 mg/kg/dose (range 0.4–3.1) introduced at a median of postoperative 0 days (range 0–15) (Figure S1, SDC, http://links.lww.com/TXD/A270). In the registry period analyzed, rATG cumulative exposure dose has declined from an average median of 7.9 mg/kg in the period from 1999 to 2008 to 6.3 mg/kg in the period from 2009 to 2016 (Figure S2, SDC, http://links.lww.com/TXD/A270).
Comparison of Graft and Patient Outcomes Between Low-dose and High-dose rATG Exposure Groups
Time to first episode of acute rejection was similar in both groups as shown in Figure 2, with a Kaplan-Meier estimate with log-rank P value of 0.07. There was a tendency toward an earlier occurrence of the first episode of acute rejection in the high-dose exposure group with 13.8% and 20.7% reaching that outcome at 6 and 12 months, respectively, relative to 6.8% and 12.7% in the low-dose exposure group. Similarly, there was no difference in graft survival between both groups through 5 years of follow-up, P value 0.05, although subjects in the low-dose exposure group tended to have slightly better graft survival at most time points posttransplant (Figure 3). There was no significant difference in patient survival between groups (P = 0.93) (Figure 4). Time to occurrence of PTLD was not significantly different between groups (P = 0.07), although all cases recorded in the registry were in the low-dose exposure group (Figure 5). When the time-to-event analyses described above were repeated controlling for steroid use at 30 days, the findings did not change (time to first episode of acute rejection, P = 0.2; graft survival, P = 0.06; patient survival, P = 0.9; and time to occurrence of PTLD, P = 0.08). All time-to-event outcome measures were then repeated after exclusion of patients receiving their first dose of rATG beyond 7 days post kidney transplant (n = 21) and yielded no statistically significant differences (data not shown). Hospitalization rates due to infections were similar in both exposure groups at 6 and 12 months posttransplant at around 20%, P value 0.98 (Table S1, SDC, http://links.lww.com/TXD/A270). Finally, the low-dose exposure group had better graft function at both 6 and 12 months posttransplant (67.1 and 65.5 mL/min/1.73m2, respectively) relative to the high-dose exposure group (63.5 and 62.5 mL/min/1.73m2, respectively), P value 0.03 (at 6 mo) and 0.04 (at 12 mo; Table S2, SDC, http://links.lww.com/TXD/A270). Subset analysis of a “low-risk” cohort comprising white recipients who did not experience delayed graft function revealed no statistically significant differences in any of the outcome measures above between the low- and high-dose exposure groups while eliminating all differences in baseline characteristics including transplant era (data not shown).
FIGURE 2.
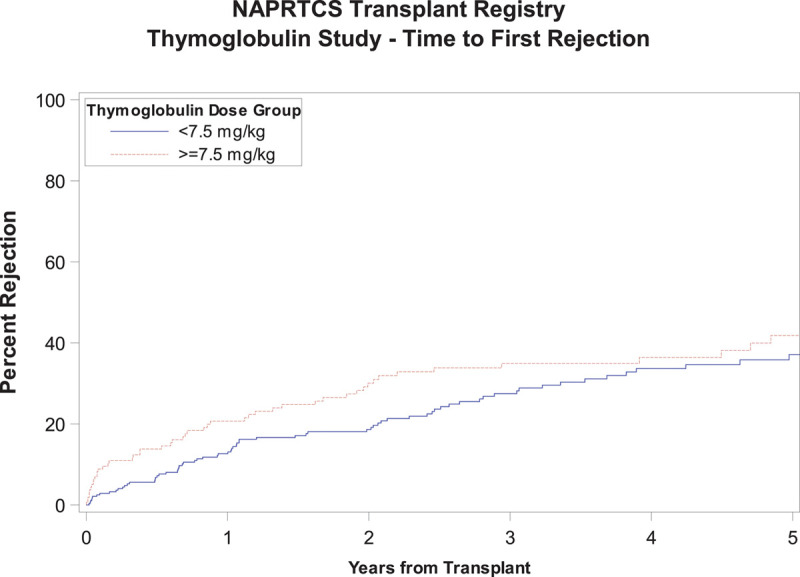
Time to first episode of acute rejection.
FIGURE 3.
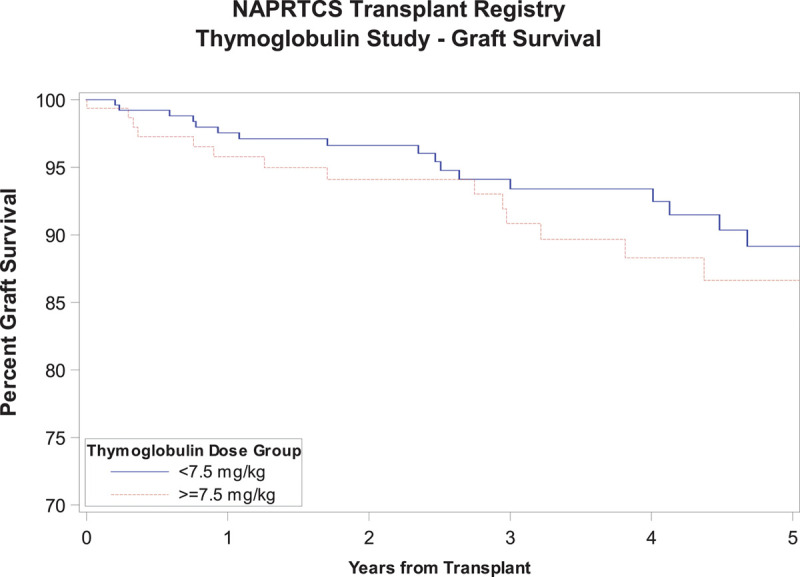
Graft survival.
FIGURE 4.
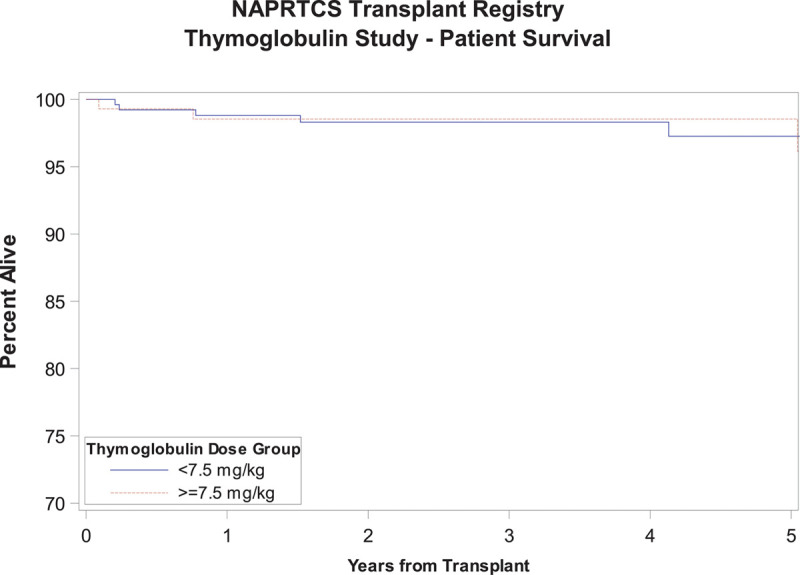
Patient survival.
FIGURE 5.
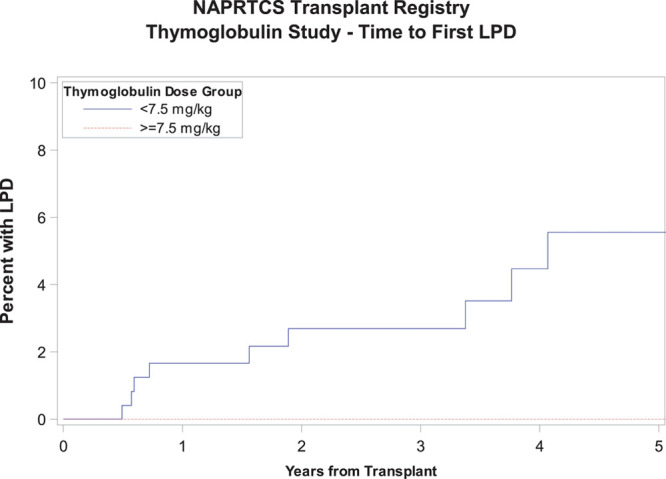
Time to first episode of PTLD. PTLD, posttransplant lymphoproliferative disorder.
DISCUSSION
This is the first study to examine the use of rATG induction for first-time pediatric kidney transplant recipients in a large pediatric-specific registry. rATG use has increased over time, and it is currently the most commonly used induction therapy for kidney transplantation.4 However, the lack of specific dosing recommendations until very recently has led to many immunosuppressive protocols in the adult transplant literature with an overall trend toward a lower cumulative exposure in the most recent era.6,10–12 By utilizing the NAPRTCS registry, a large voluntary database that collects information on pediatric kidney transplants performed across North America,8 we provide a contemporary assessment of the prevailing practice patterns at 37 participating centers with regards to rATG use for induction therapy. We demonstrate a wide variability in rATG cumulative exposure between individual centers, yet an overall trend toward lower exposure in the most recent era. This is consistent with a recent large-scale analysis of the Scientific Registry of Transplant Recipients that encompassed 166 776 US recipients between 2005 and 2014.4 Similar to evolving trends toward lower rATG exposure in adult centers, most recipients in our analysis received <7.5 mg/kg of cumulative rATG induction therapy, suggesting a shift to a total of 4 doses or less at 1.5 mg/kg each.
One concern related to lower rATG dose exposure is the possible increased risk in acute rejection and how that may impact graft survival. Several studies have demonstrated excellent 1-year graft outcomes with low-dose rATG induction strategies. In a comparison to conventional rATG exposure targets of 6–10 mg/kg, Singh et al6 reviewed their outcomes using a tailored rATG cumulative exposure target of 3–6 mg/kg over a 5-year period and found comparable outcomes to those reported in the Scientific Registry of Transplant Recipients. Another study by Grafals et al12 utilizing a much lower rATG cumulative exposure target of either 2.25 or 3.75 mg/kg demonstrated a biopsy-proven acute rejection rate of 10% and 17%, respectively, at 1-year follow-up and similar T-cell subpopulation depletion and repopulation kinetics in both groups. Long-term outcome data are similarly encouraging with a study of 145 adult kidney transplant recipients who received low-dose rATG induction (total cumulative exposure of 3–5 mg/kg), demonstrating superior 8-year graft survival for recipients of both deceased and living donor kidneys compared with those receiving basiliximab induction.11 Although pediatric data comparing rATG induction to basiliximab induction outcomes is limited, Crowson et al13 identified a lower risk of acute rejection in African American pediatric kidney transplant recipients induced with depleting agents that included rATG and alemtuzumab relative to nondepleting agents that included basiliximab and daclizumab. In our study, there was no difference in the time to first episode of acute rejection between the low- and high-rATG exposure groups. In fact, we noted a trend toward earlier onset of acute rejection episodes in the high-dose exposure group. We hypothesized this may have been in part due to differences in baseline characteristics of both groups, with the high-dose exposure group reflecting a relatively older transplant era cohort and with a higher proportion of patients who experienced delayed graft function. Further analysis of a “low-risk” cohort limited to white recipients and excluding patients with delayed graft function eliminated those baseline differences between the low- and high-dose exposure groups and upheld the original analysis finding of no difference in time to first episode of acute rejection between groups. Graft survival in our study was similar in both the low-dose and high-dose exposure groups through 5 years of follow-up.
Another major concern with lymphocyte depleting induction therapy is the higher risk of opportunistic infection and possible increase in malignancy rates particularly PTLD.2,14,15 Further intensification of immunosuppression in recipients who develop acute rejection episodes can further compound that risk. Our study did not identify a difference in rate of hospitalizations due to infections between groups. Although not statistically significant, one potential concern is the aggregation of all PTLD cases in the low-dose exposure arm. Given the lack of granular data regarding Epstein–Barr virus recipient and donor mismatch in the NAPRTCS registry, it is difficult to ascertain the significance of this finding. Additionally, the registry lacked detailed descriptions of oral maintenance immunosuppression burden,such as drug dosage and therapeutic drug levels, to allow further examination of this trend. Nevertheless, the occurrence of PTLD underscores the inherent risks associated with rATG induction and immunosuppression in general, and we need to pursue evidence-based strategies to lower cumulative immunosuppression exposure in low-risk pediatric recipients. One potential strategy may involve tailored rATG dosing based on individual recipient T-cell depletion kinetics as determined by daily measurement of peripheral blood CD3 T-cell subset counts. This has been piloted successfully in adult kidney transplant recipient cohorts in which intermittent rATG doses were administered when the CD3 count exceeded a predefined threshold of either 10 or 20 cells/mm3 depending on the cohort, resulting in adequate T-cell depletion and significant reduction in cumulative exposure and therapy cost compared with standard daily dosing.16,17 Pediatric experience in this regard is limited and would benefit from a prospective study design to assess the safety and efficacy of a similar personalized approach. The NAPRTCS registry did not collect data on CD3 counts, and, as such, a description of T-cell depletion kinetics relative to rATG induction dose is not possible.
Our study has several limitations: Although the NAPRTCS registry collects data from many pediatric kidney transplant centers in North America, it is a voluntary registry and as such, is not inclusive of all practice patterns regarding rATG use, particularly those from smaller institutions that may not be contributing to the registry. The retrospective registry data limits the ability to draw firm conclusions regarding causation as it relates to outcomes and rATG cumulative exposure, although the trends observed are encouraging to pursue those questions in a prospective manner. Also, registry data are limited by lack of granular outcomes not collected by the registry, such as data on leukopenia, viremia, and donor-specific antibody screens. However, the robust registry infrastructure of NAPRTCS and longitudinal data collection dating back to 1998 for our study allows a comprehensive analysis of time trends and long-term outcomes that would be difficult to accomplish with other study designs. Of note, our eligible subject number was significantly lower than the total registry count for subjects receiving rATG induction due to the inclusion criteria requirement of a tacrolimus- and MMF-based maintenance regimen. We believe limiting our analysis to this subset provides the most relevant outcomes data reflective of contemporary immunosuppression practice patterns at most pediatric kidney transplant centers in the current era. Our analysis is also limited in its ability to provide generalizations applicable to recipients on steroid-free immunosuppression protocols given that most patients (75.7%) were still receiving steroids at day 30 posttransplant. However, the comparable acute rejection outcomes between the low- and high-dose rATG exposure groups despite a relatively higher proportion of steroid-free recipients in the low-dose exposure group suggests that steroid-free immunosuppression protocols did not have a negative detrimental effect on graft outcomes in those patients. Similarly, controlling for steroid use at day 30 post kidney transplant did not significantly alter our findings. Finally, we were unable to compare outcomes between rATG induction in either dose group to outcomes in recipients induced with nondepleting agents (such as basiliximab) or no induction at all, as that was beyond the scope of this registry analysis, although we look forward to examining those differences in future studies.
In conclusion, we have demonstrated a wide variability in rATG cumulative exposure for induction immunosuppression in first-time pediatric kidney transplant recipients with an overall trend toward lower exposure in the most recent era that does not appear to be associated with inferior graft and recipient outcomes in this registry. Future studies should utilize more granular outcome measures such as development of viremia, leukopenia, and donor-specific antibodies in relation to various rATG exposure thresholds. This will inform future practice regarding optimal rATG induction therapy dosing in low-risk pediatric kidney transplant patients without compromising graft and recipient outcomes.
ACKNOWLEDGMENTS
We would like to acknowledge the NAPRTCS study group and participants for making this research possible.
Supplementary Material
APPENDIX A:
List of NAPRTCS Investigators at Sites that Contributed the Data in this Report
Footnotes
Published online 21 August, 2020.
Members of NAPRTCS Investigators are listed in Appendix A.
This work was supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. V.R.D. is supported in part by NIH grant R01DK102981. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Statistical analysis for this study was provided by the The Emmes Company, LLC with funding from a NAPRTCS special studies award.
V.R.D. received honoraria from Sanofi Genzyme, Bristol-Myers-Squibb, Atara Bio, and CareDx and grant support from Atara Bio and CareDx. I.F.A., K.M., S.G., and R.A.B. have no relevant financial relationships to disclose.
I.F.A. and V.R.D. participated in research design, performance of the research, data analysis and interpretation, and writing of the article. K.M. participated in research design, performance of the research, data analysis, and interpretation. S.G. participated in data analysis and interpretation and writing of the article. R.A.B. participated in research design and data analysis and interpretation. All authors approved the final article draft for publication.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med. 2014; 371:549–558 [DOI] [PubMed] [Google Scholar]
- 2.Hill P, Cross NB, Barnett ANR, et al. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017; 1:CD004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nehus E, Liu C, Hooper DK, et al. Clinical practice of steroid avoidance in pediatric kidney transplantation. Am J Transplant. 2015; 15:2203–2210 [DOI] [PubMed] [Google Scholar]
- 4.Dharnidharka VR, Naik AS, Axelrod DA, et al. Center practice drives variation in choice of US kidney transplant induction therapy: a retrospective analysis of contemporary practice. Transpl Int. 2018; 31:198–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(FDA) USFDA. April 21, 2017, Approval Letter—Thymoglubin. 2017. Available at https://www.fda.gov/media/104907/download. Accessed November 21, 2019.
- 6.Singh N, Rossi AP, Savic M, et al. Tailored rabbit antithymocyte globulin induction dosing for kidney transplantation. Transplant Direct. 2018; 4:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan DC, Daller JA, Lake KD, et al. Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006; 355:1967–1977 [DOI] [PubMed] [Google Scholar]
- 8.The Emmes Company LLC. NAPRTCS—History & Leadership. 2019 Available at https://naprtcs.org/study-details/history-leadership. Accessed November 21, 2019.
- 9.Alkandari O, Hebert D, Langlois V, et al. Validation of serum creatinine-based formulae in pediatric renal transplant recipients. Pediatr Res. 2017; 82:1000–1006 [DOI] [PubMed] [Google Scholar]
- 10.Mohty M, Bacigalupo A, Saliba F, et al. New directions for rabbit antithymocyte globulin (Thymoglobulin((R> in solid organ transplants, stem cell transplants and autoimmunity. Drugs. 2014; 74:1605–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laftavi MR, Alnimri M, Weber-Shrikant E, et al. Low-dose rabbit antithymocyte globulin versus basiliximab induction therapy in low-risk renal transplant recipients: 8-year follow-up. Transplant Proc. 2011; 43:458–461 [DOI] [PubMed] [Google Scholar]
- 12.Grafals M, Smith B, Murakami N, et al. Immunophenotyping and efficacy of low dose ATG in non-sensitized kidney recipients undergoing early steroid withdrawal: a randomized pilot study. PLoS One. 2014; 9:e104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowson CN, Reed RD, Shelton BA, et al. Lymphocyte-depleting induction therapy lowers the risk of acute rejection in African American pediatric kidney transplant recipients. Pediatr Transplant. 2017; 21 doi: 10.1111/petr.12823. [DOI] [PubMed] [Google Scholar]
- 14.Puliyanda DP, Stablein DM, Dharnidharka VR. Younger age and antibody induction increase the risk for infection in pediatric renal transplantation: a NAPRTCS report. Am J Transplant. 2007; 7:662–666 [DOI] [PubMed] [Google Scholar]
- 15.Marks WH, Ilsley JN, Dharnidharka VR. Posttransplantation lymphoproliferative disorder in kidney and heart transplant recipients receiving thymoglobulin: a systematic review. Transplant Proc. 2011; 43:1395–1404 [DOI] [PubMed] [Google Scholar]
- 16.Peddi VR, Bryant M, Roy-Chaudhury P, et al. Safety, efficacy, and cost analysis of thymoglobulin induction therapy with intermittent dosing based on CD3+ lymphocyte counts in kidney and kidney-pancreas transplant recipients. Transplantation. 2002; 73:1514–1518 [DOI] [PubMed] [Google Scholar]
- 17.Djamali A, Turc-Baron C, Portales P, et al. Low dose antithymocyte globulins in renal transplantation: daily versus intermittent administration based on T-cell monitoring. Transplantation. 2000; 69:799–805 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


