Supplemental Digital Content is available in the text
Keywords: stereotactic body radiotherapy, radiotherapy, stage I, non-small cell lung cancer, meta-analysis
Abstract
Background:
Stereotactic body radiotherapy (SBRT) superseded conventional radiotherapy (CRT) for the treatment of patients with inoperable early stage non-small cell lung cancer (NSCLC) over a decade ago. However, the direct comparisons of the outcomes of SBRT and CRT remain controversial. This meta-analysis was performed to compare the survival and safety of SBRT and CRT in patients with inoperable stage I NSCLC.
Methods:
We systematically searched the Cochrane Library, Embase, PubMed, Web of Science, Ovid MEDLINE, ScienceDirect, Scopus and Google Scholar for relevant articles. Overall survival (OS), progression-free survival (PFS), lung cancer-specific survival (LCSS), local control rate (LCR) and adverse effects (AEs) were the primary outcomes.
Results:
We identified 11,110 articles, 17 of which were eventually included in this study; these 17 articles had 17,973 patients (SBRT: 7395; CRT: 10,578). Compared to CRT for the treatment of inoperable stage I NSCLC, SBRT had superior survival in terms of OS (hazard ratio [HR]: 0.66, 95% confidence interval [CI]: 0.62-0.70, P < .00001), LCSS (HR: 0.42 [0.35-0.50], P < .00001), and PFS (HR: 0.34 [0.25-0.48], P < .00001). The 4-year OS rate (OSR); 4-year LCSS rate (LCSSR); 3-year local control rate (LCR); 5-year PFS rate (PFSR) with SBRT were all higher than those with CRT. With regard to all-grade AEs, the SBRT group had a significantly lower rate of dyspnea, esophagitis and radiation pneumonitis; no significant difference was found in grade 3-5 AEs (risk ratio [RR]: 0.68 [0.30-1.53], P = .35).
Conclusions:
With better survival and a lower rate of dyspnea, esophagitis and radiation pneumonitis than CRT, SBRT appears to be more suitable for patients with inoperable stage I NSCLC.
1. Introduction
Lung cancer remains a major contributor to cancer-related mortality and cancer incidence worldwide[1]; its annual incidence is predicted to increase continually for at least the next few decades.[2] Recent advances in screening have made substantial progress with regard to detecting early-stage lung cancer.[3] As far as stage I non-small cell lung cancer (NSCLC) patients are concerned, the current standard therapy is surgery.[4] However, multiple comorbidities and poor physical performance status result in nearly 20% of stage I NSCLC patients being unable to tolerate surgery.[5] Conventional radiotherapy (CRT) has been used as the standard noninvasive strategy for more than a decade.[6] However, given the low 5-year survival of 10% to 22%,[7,8] CRT has long puzzled clinicians.
The past decade has seen great advances in stereotactic body radiotherapy (SBRT), and SBRT has gradually superseded CRT in clinical practice for the treatment of patients with inoperable early-stage NSCLC. The CHISEL trial reported that the rate of 2-year local control for inoperable Stage I NSCLC patients was 89% with SBRT compared with 65% with CRT.[9] However, Borst et al[10] demonstrated that SBRT had a significant dose-response relationship to radiation pneumonitis. In addition, the SPACE trial revealed that no apparent difference was observed in overall survival or local control between patients treated with SBRT and CRT.[11] It is controversial whether the SBRT can replace CRT.
This meta-analysis aimed to directly compare the efficacy and safety of CRT and SBRT for inoperable stage I NSCLC.
2. Materials and methods
The PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines guided the performance of this meta-analysis (Supplementary Digital Content, Table 1).[12]
2.1. Search strategy
The following internet sources were used to retrieve the relevant literature:
-
(1)
PubMed;
-
(2)
Web of Science;
-
(3)
Embase;
-
(4)
Cochrane Library;
-
(5)
Ovid MEDLINE;
-
(6)
ScienceDirect;
-
(7)
Scopus;
-
(8)
Google Scholar.
We updated the last search on September 3, 2019. “Stereotactic body radiotherapy” and “lung neoplasms” were the key terms used in the search. The complete search is outlined in Appendix 1. For the further identification of eligible articles, we identified relevant references of the retrieved literature as well. We set no limitations on language.
2.2. Selection criteria
We performed this search pursuant to the PICOS guidelines:
-
(1)
participants: stage I NSCLC patients who were inoperable, high-risk operable (the factors were:
-
(2)
intervention and comparison: SBRT versus CRT (including conventional radiotherapy,[15,16,19,21,26] conventional fractionated radiotherapy,[17,20,23,25,27] 3D conformal radiotherapy,[9,11,13,14,18] and accelerated hypofractionated radiotherapy[13]);
-
(3)
outcomes: OS (overall survival), OS rate (OSR), local control rate (LCR), lung cancer-specific survival (LCSS), LCSS rate (LCSSR), progression-free survival (PFS), PFS rate (PFSR) and adverse effects (AEs); and
-
(4)
study design: RCTs and cohort studies.
Protocols, abstracts, meta-analyses, animal experiments, articles with duplicated data and studies without original data were excluded.
2.3. Data extraction
The following data were extracted independently by 2 investigators: first author, country, publication year, number of participants, participant characteristics (age, sex, tumor size, TNM stage, histology, performance status (PS), medically inoperable rate), treatment characteristics (type, dose, time), median follow-up, study design, antitumor efficacy indices (OS, OSR, LCSS, LCSSR, LCR, PFS, and PFSR) and the types and quantity of AEs (all-grade AEs and grade 3-5 AEs). Disagreements on any terms were resolved by a third investigator.
2.4. Quality assessment
The 5-point Jadad scale was adopted to evaluate the quality of the RCTs; it consisted of three sections, namely, randomization, masking and accountability. Studies with scores ≥3 were assessed as being of high quality.[28]
The Newcastle-Ottawa Scale (NOS, 9 points) was adopted to determine the cohort study quality; it contained three sections, namely, selection, comparability and exposure. Studies with scores between 8 and 9 were assessed as being of high quality; studies with scores between 6-7 were assessed as being of medium quality.[29]
The grading of recommendations assessment, development and evaluation (GRADE) system was use to explore the quality of the data; it consisted of five sections, namely, inconsistency, indirectness, imprecision, risk of bias and publication bias. Data were assessed as being of high, medium, low or very low quality.[30]
2.5. Statistical analysis
Stata 14.0 (Stata Corp) and Review Manager 5.3 (The Nordic Cochrane Center) were utilized to perform this meta-analysis. To analyze the OS, LCSS and PFS, hazard ratios (HRs, HRs > 1 favored the CRT arm) and 95% confidence interval (CIs) were applied. HR, OSR, LCSSR, LCR, PFSR were extracted directly from the included articles and indirectly from Kaplan–Meier curves, pursuant to the methods proposed by Tierney et al.[31] Risk ratios (RRs) and their 95% CIs were used for AEs (RRs > 1 favored the CRT arm) as well as the OSR, LCSSR, LCR, and PFSR (RR > 1 favored the SBRT arm). To clarify whether the results would change by region, sex, age, stage, PS, histology, CRT type, SBRT fraction dose, biologically effective dose (BED) of SBRT, medically inoperable rate, treatment time or study design, subgroup analyses of OS, LCSS and PFS were performed. We utilized the χ2 test and I2 statistic to assess heterogeneity. A P < .1 (on the χ2 test) or I2 > 50% reflected evident heterogeneity and a random-effects model was used; otherwise the fixed-effects model was used. Begg rank correlation[32] and Egger linear regression tests[33] were utilized to assess publication bias; P < .05 indicated statistical significance.
3. Results
3.1. Search results and study quality assessment
On the last search on September 3, 2019, 11,110 potentially qualified studies were initially identified. After strict screening, 17 studies (2 RCTs,[9,11] 2 prospective cohorts[13,14] and 13 retrospective cohorts[15–27]) involving 17,973 patients (SBRT, 7395; CRT, 10,578) were included in this analysis (Fig. 1). In total, 8/17 studies included all medically inoperable patients, 12/17 studies analyzed the reasons patients were deemed inoperable. Table 1 summarizes the basic characteristics and chief evaluation indexes of the involved articles. Of the 17 articles, 10 were high quality and 7 were medium quality (Supplementary Digital Content, Table 2. The GRADE approach indicated that the evidence was all in the low- and very low-quality categories (Supplementary Digital Content, Table 3.
Figure 1.
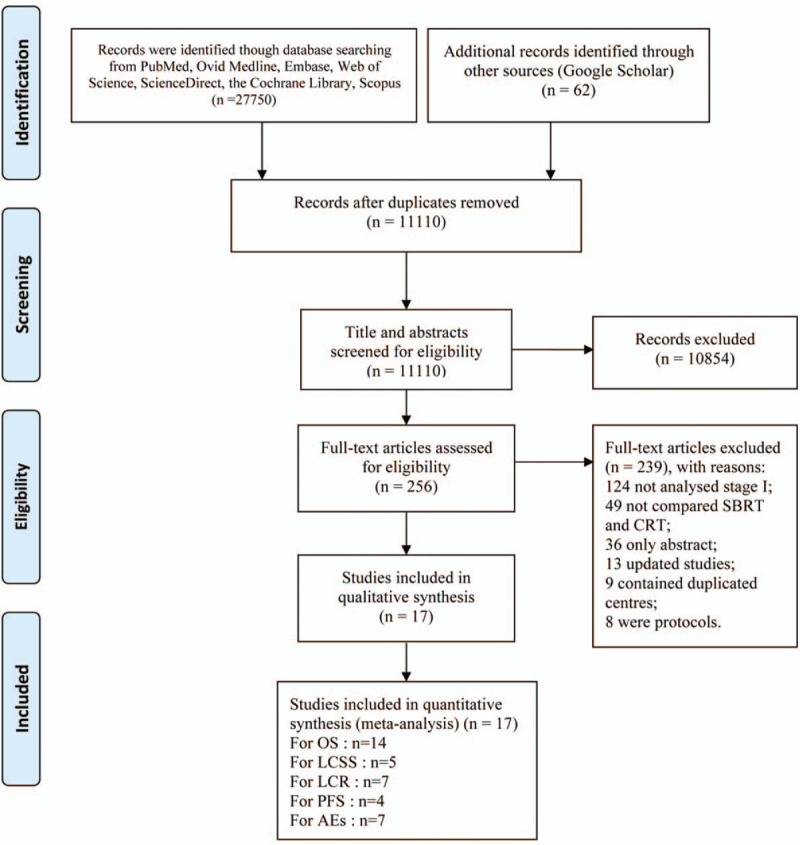
Flow chart of study selection.
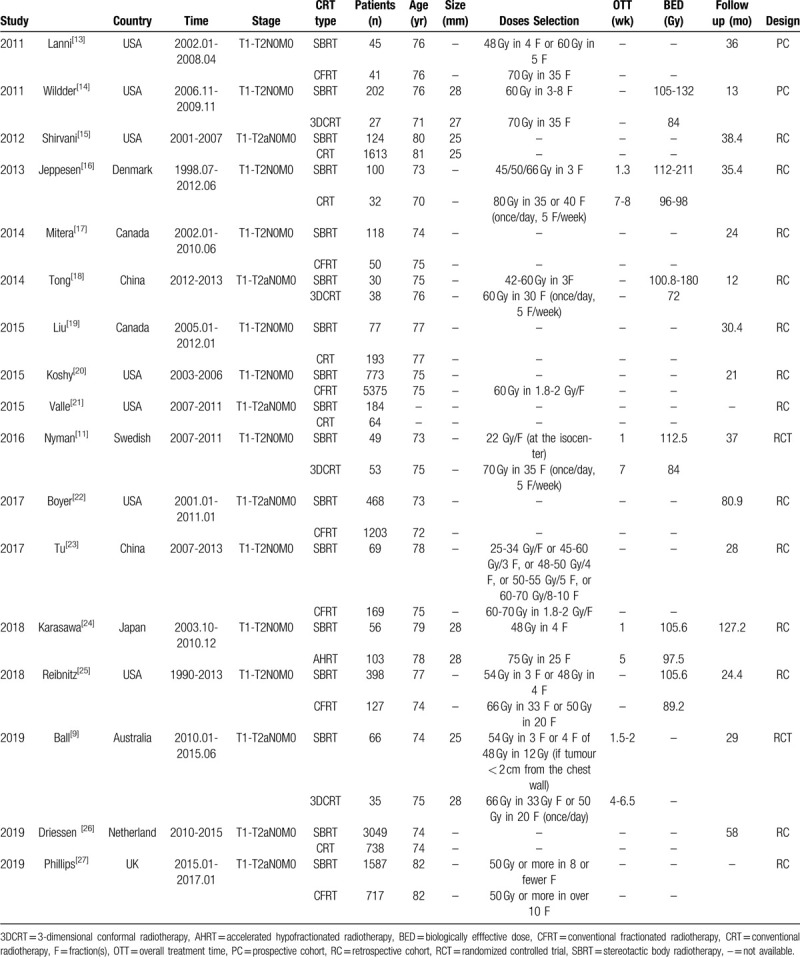
Table 1.
Characteristics of included studies.
3.1.1. Antitumor efficacy
Fourteen articles focused on OS (heterogeneity: P = .29, I2 = 15%). SBRT showed significant superiority in OS (HR: 0.66 [0.62-0.70], P < .00001) compared with CRT (Fig. 2A). The subgroup analyses revealed that the 1-year OSR (86.23% vs 77.80%; RR: 1.10 [0.97-1.26], P = .1387), 2-year OSR (69.26% vs 53.76%; RR: 1.28 [1.02-1.60], P = .0274), 3-year OSR (54.73% vs 39.50%; RR: 1.38 [1.02-1.85], P = .0317), 4-year OSR (40.36% vs 27.47%; RR: 1.48 [0.99-2.21], P = .0493), and 5-year OSR (29.30% vs 27.47%; RR: 1.45 [0.88-2.39], P = .1368) were higher in the SBRT group than in the CRT group (Fig. 3A, Fig. 4A).
Figure 2.
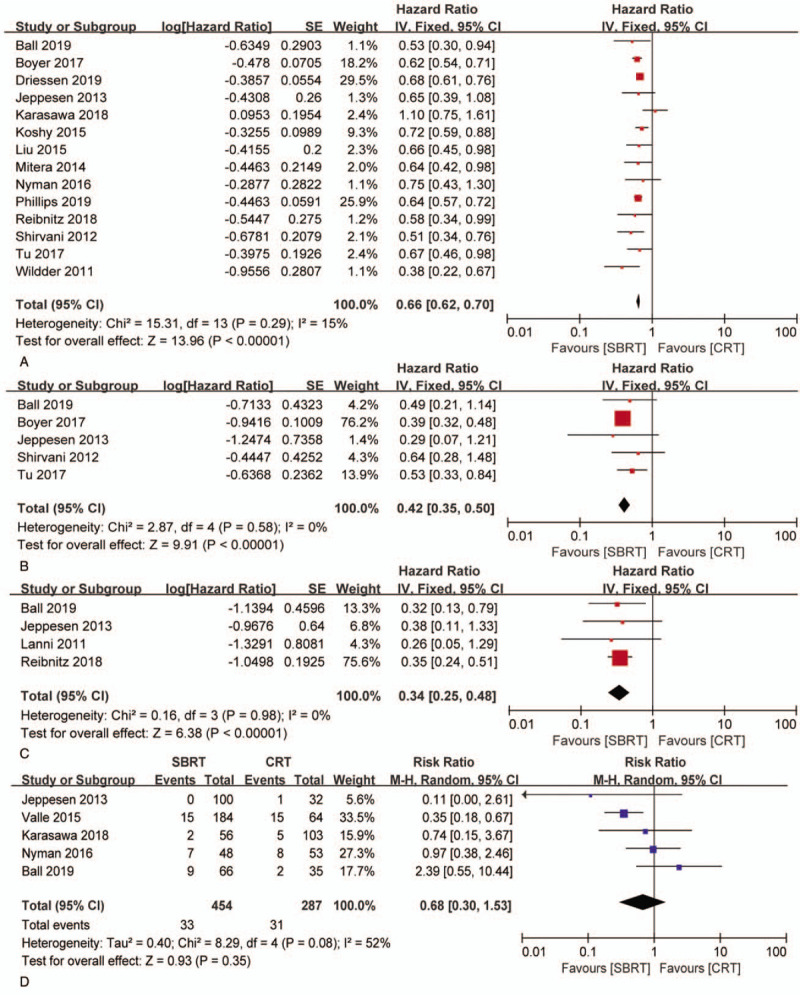
Forest plot of HRs for OS (A), LCSS (B), PFS (C) and forest plots of RRs for grade 3–5 AEs (D) associated with SBRT versus CRT.
Figure 3.
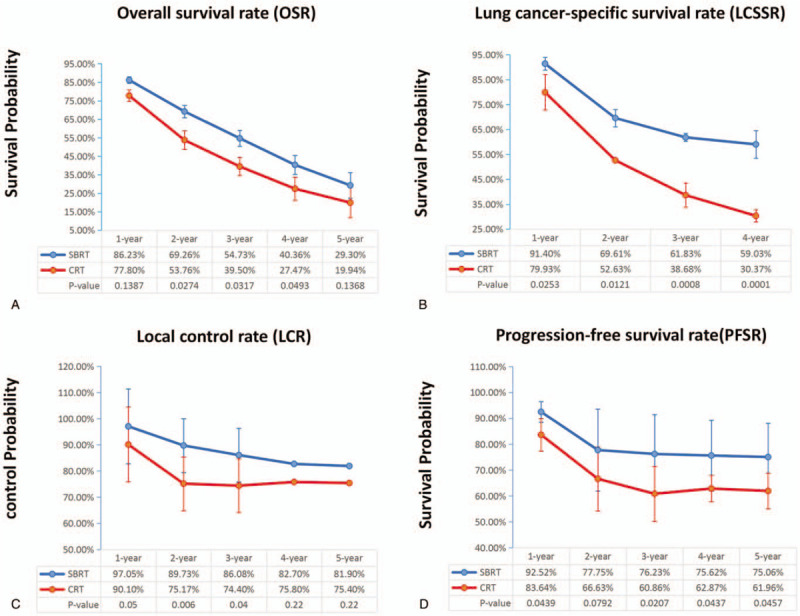
Broken-line graphs of OSR (A), LCSSR (B), LCR (C) and PFSR (D) associated with SBRT vs CRT.
Figure 4.
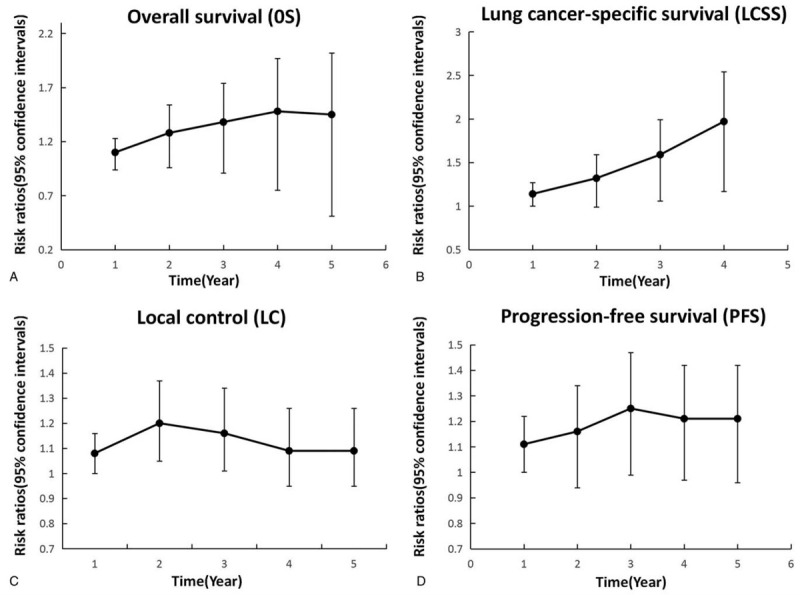
Broken-line graphs of RRs for OSR (A), LCSSR (B), LCR (C) and PFSR (D) associated with SBRT vs CRT.
Five articles evaluated LCSS (heterogeneity: P = .58, I2 = 0). SBRT showed significant superiority in LCSS (HR: 0.42 [0.35-0.50], P < .00001) compared with CRT (Fig. 2B). The subgroup analyses showed that the 1-year LCSSR (91.40% vs 79.93%; RR: 1.14 [1.01-1.28], P = .0253), 2-year LCSSR (69.61% vs 52.63%; RR: 1.32 [1.05-1.65], P = .0121), 3-year LCSSR (61.83% vs 38.68%; RR: 1.59 [1.19-2.12], P = .0008), and 4-year LCSSR (59.03% vs 30.37%; RR: 1.97 [1.40-2.77], P < .0001) were higher in the SBRT group than in the CRT group (Figs. 3B and 4B).
Seven articles evaluated LCR (heterogeneity: P = .61, I2 = 0). SBRT showed significant superiority in LCR (RR: 1.12 [1.06-1.19], P < .00001) compared with CRT. The subgroup analyses demonstrated that the 1-year LCR (97% vs 90%; RR: 1.08 [1.00-1.16], P = .05), 2-year LCSSR (90% vs 75%; RR: 1.20 [1.05-1.37], p = 0.006), 3-year LCSSR (86% vs 74%; RR: 1.16 [1.01-1.34], P = .04), and 4-year LCSSR (83% vs 76%; RR: 1.09 [0.95-1. 26], P = .22) were higher in the SBRT group than in the CRT group (Figs. 3C and F4C).
Four articles compared PFS (heterogeneity: P = .98, I2 = 0). SBRT showed significant superiority in PFS (HR: 0.34 [.25-0.48], P < .00001) compared with CRT (Fig. 2C). The subgroup analyses showed that the 1-year PFSR (92.52% vs 83.64%; RR: 1.11 [1.00-1.22], P = .0439), 2-year PFSR (77.75% vs 66.63%; RR: 1.16 [0.98-1.38], P = .0792), 3-year PFSR (76.23% vs 60.86%; RR: 1.25 [1.03-1.51], P = .0207), 4-year PFSR (75.62% vs 62.87%; RR: 1.21 [1.00-1.45], P = .0437), and 5-year PFSR (75.06% vs 61.96%; RR: 1.21 [1.00-1.46], P = .0457) were higher in the SBRT group than in the CRT group (Figs. 3D and 4D).
3.1.2. Adverse effects
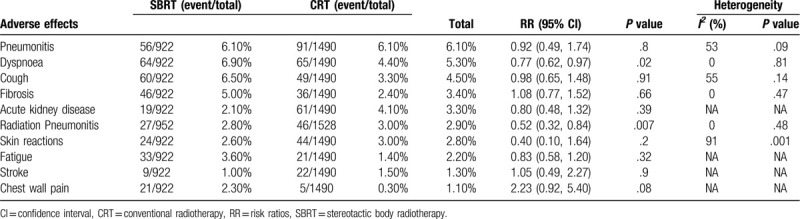
Six articles compared all-grade AEs[9,11,13,20,22,23] (Supplementary Digital Content, Table 4). The 10 most commonly reported AEs in all grades were pneumonitis, dyspnea, cough, fibrosis, acute kidney disease, radiation pneumonitis, skin reactions, fatigue, and chest wall pain (Table 2). SBRT was associated with a significantly lower risk of dyspnea (RR: 0.77 [0.62-0.97], P = .02), radiation pneumonitis (RR: 0.52 [0.32-0.84], P = .0007) and esophagitis (RR: 0.30 [0.12-0.74], P = .009) than CRT; no significant difference was found in the other AEs.
Table 2.
Top 10 adverse effects (all grades) associated with SBRT versus CRT.
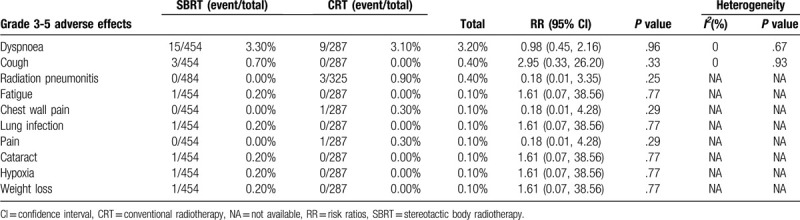
Five articles referred to grade 3–5 AEs,[9,11,13,20,23] in which SBRT was associated with no obvious difference (RR: 0.68 [0.30-1.53], P = .35) when compared with CRT (heterogeneity: P = .08, I2 = 52%, Fig. 2D) (Supplementary Digital Content, Table 5). Dyspnea, cough, radiation pneumonitis, fatigue, chest wall pain, lung infection, pain, cataracts, hypoxia and weight loss were the 10 most commonly reported grade 3–5 AEs induced by SBRT and CRT (Table 3), among which we observed no significant differences.
Table 3.
Top 10 adverse effects (grade 3-5) associated with SBRT versus CRT.
3.1.3. Subgroup analysis
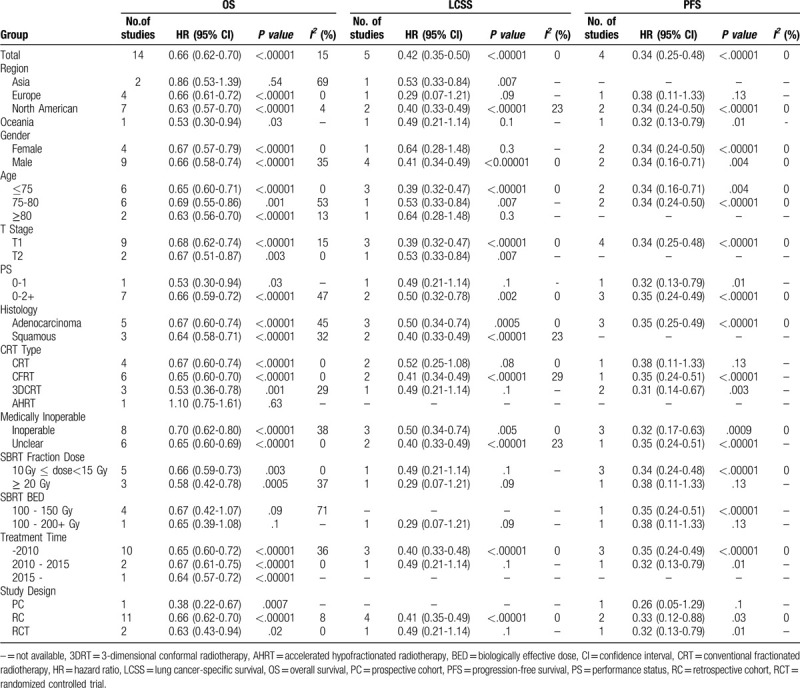
Subgroup analyses for OS, LCSS and PFS were performed in order to explore whether the better efficacy with SBRT existed among subgroups stratified by the following variables: region, sex, age, stage, PS, histology, study design, CRT type, SBRT fraction dose, BED of SBRT, medically inoperable rate, and treatment time. The vast majority of subgroup differences in OS, LCSS and PFS between the SBRT and CRT groups were significant (Table 4).
Table 4.
Subgroup analysis for overall survival, lung cancer-specific survival and progression-free survival.
3.1.4. Sensitivity analysis
We assessed the stability and sensitivity of these results by evaluating the impact of each article on the pooled results. The OS (Supplementary Digital Content, Fig. 1A), LCSS (Supplementary Digital Content, Fig. 1B) and PFS (Supplementary Digital Content, Fig. 1C) results were stable.
3.1.5. Publication bias
We concluded that no publication bias existed in the evaluation of OS (Supplementary Digital Content, Fig. 2A), LCSS (Supplementary Digital Content, Fig. 2B) or PFS (Supplementary Digital Content, Fig. 2C), according to the P values from Egger test (OS: 0.702; LCSS: 0.464; PFS: 0.375) and Begg test (OS: 0.101; LCSS: 0.308; PFS: 0.806).
4. Discussion
Early-stage NSCLC accounts for approximately 10% to 20% of new NSCLC diagnoses, and the figure is continually rising due to the implementation of the new NSCLC screening guidelines.[3] SBRT has improved substantially during the past decade, and it has supplanted CRT and gained popularity among early-stage inoperable NSCLC patients.[34] However, several debates still exist concerning whether SBRT can completely replace CRT. This meta-analysis is the first to directly compare SBRT with CRT in patients with inoperable stage I NSCLC. The outcomes revealed that the patients who were administered SBRT had superior OS, LCSS, LCR and PFS when compared with the patients who received CRT. In addition, the subgroup results were also statistically significant and in favor of better survival with SBRT. The analyses also demonstrated that the SBRT group had a significantly lower risk of dyspnea, radiation pneumonitis and esophagitis among all-grade AEs, though there was no apparent difference in the rate of grade 3–5 AEs between the SBRT and CRT groups.
The main benefit of SBRT treatment is the significantly better survival (OS, LCSS, LCR and PFS). Compared with CRT, SBRT had obvious advantages in OSR-4y, LCSSR-4y, LCR-3y and PFSR-5y in our study. Navarro-Martin et al[35] found that the 3-year OSR was 66%. In addition, Timmerman et al[36] showed the 5-year OSR and 5-year PFSR with SBRT were 40.0% and 25.5%, respectively, in the RTOG 0236 Trial. However, the SPACE trial[9] indicated no apparent difference in local control or OS after directly comparing SBRT and CRT. We speculated that the superior survival with SBRT could be due to many reasons. First, SBRT delivers high doses (e.g., 3 fractions of 15–22 Gy) of radiation that precisely and directly target tumors for ablation, while CRT is based on a protracted treatment with minimal 1.8 to 2.0 dose-per-fraction sizes.[37] Additionally, the most common SBRT dose-fractionation schedules provide a BED of at least 100 Gy to achieve antitumor efficacy, while the BED of CRT usually reaches 80 Gy or less, which is not high enough to completely kill all the tumor cells in the gross target volume, resulting in a lower rate of long-term local control.[38] Finally, with highly accurate doses delivered during minimal courses of therapy, SBRT prevents tissues within the irradiation volume from radiation injury to some extent. CRT involves 6 to 7 weeks of daily radiation; as these doses are above the radiation tolerance, the accumulated dose injuries inevitably lead to some degree of lung fibrosis.[39] Our subgroup analyses showed that SBRT led to better survival than CRT, conventional fractionated radiotherapy (CFRT) and 3D conformal radiotherapy (3DCRT), though not in the subgroup of patients who received accelerated hypofractionated radiotherapy (AHRT). We also found that the patients with a performance status score of 0-1, patients aged ≤75 years, males, patients with squamous histology and patients who received an SBRT fraction dose ≥20 Gy were predicted to have relatively better survival. Additionally, residence in Asian or European regions, age between 75 and 80, and a fraction dose of 10 to 15 Gy were also likely to influence the outcomes. In terms of AHRT subgroup results, we surmised that several schedules with an adequately high BED of fractionated and accelerated CRT could achieve tumor control rates similar to those achieved with SBRT. The differences in the SPACE trial concerning survival may be attributed to the lower BED in the SBRT group with the 3 prescribed fractions of 45 Gy. However, a worse performance status (24% PS > 2) of the patients treated in the SPACE trial cannot be ignored. As van Baardwijk et al stated, the high BED in SBRT could also be considered “overkill.”[40] Overall, SBRT appears to lead to better survival in patients with inoperable stage I NSCLC.
Our meta-analysis demonstrated that the risk of toxicity was low in both the SBRT and CRT groups. Our subgroup analyses indicated that SBRT induced significantly lower rates of dyspnea, radiation pneumonitis and esophagitis in all-grade AEs while there were no significant difference in grade 3–5. For the statistical difference, we suspected the difference might because the special courses and doses of SBRT and CRT treatment. Both BED are rather “safe”, there were rarely treatment-related deaths and the majority of the AEs were mostly grade 1–2. This may illustrate the low correlation in grade 3–5 AEs. For SBRT, higher fraction dose and less fractions meant more of the ray was precisely concentrated on the tumor regions with less harm to normal structures and tissues, which effectively promoted a declined probability and quantity of AEs. Additionally, the suitable BED effectively meant that latent radiotherapy damage and lung fibrosis could be avoided during a shorter treatment time. While CRT with lower BED struggles to get enough efficacy to kill tumor cells in the gross target volume via a longer course treatment involving 6 to 7 weeks of daily radiation. Accumulated dose injuries inevitably lead to some grade 1–2 AEs. In the published literature, it has been reported that tumors located in the central lung may enhance the risks associated with SBRT due to the potential damage to the mediastinal tissues; for instance, there may be an increased risk of pleural effusion, pneumonitis and lung capacity reduction. In addition, related rare AEs are tracheobronchial injury, esophageal ulcer and myeleterosis.[41–45] When the tumors are near the brachial plexus nerve, severe damage could result in neuropathic pain and brachial palsy.[46] For tumors near the chest wall, the latent complications are rib fractures and chest wall pain[47]; therefore, the SBRT dose should be limited to 30 to 35 Gy. SBRT seems to be acceptable for patients with tumors located in the peripheral lung but away from chest wall; however, radiation pneumonitis is the most common complication in these patients.[36] It is accepted that, in SBRT, 4 to 10 fractions are safe and effective, yet 3 fractions with 54 to 60 Gy pose a risk that needs to be avoided.[48] The prospective RTOG 0813 study reported that no serious toxicities occurred at the maximum tolerated dose (50 Gy) in 5-fraction regimens.[49] However, particular attention should be paid to tumors abutting the bronchial tree and esophagus to avoid severe toxicity. In summary, compared with CRT, SBRT is a safer treatment strategy.
Several limitations exist in the present meta-analysis. First and foremost, the articles we included were all in the English language; therefore, there may have been language bias. Additionally, while most of the 17 articles were of medium- and high- quality, the 2 RCTs included in the analysis could have weakened the study conclusions. Moreover, attention should be paid to the significant heterogeneity that existed in the analyses of all-grade and grades 3–5 AEs, which might have reduced the reliability of the results. Furthermore, we obtained the data pertaining to grades 3–5 AE mainly from 2 RCTs, which may have led to representativeness bias. Finally, the CRT types and the treatment doses were diverse, which perhaps increased the heterogeneity and decreased the quality, although subgroup analyses were conducted. Accordingly, we suggest that determining the suitable dose for tumors in each anatomical region should be considered in future studies.
5. Conclusions
Our study illustrated that SBRT tends to lead to better survival (OS, LCSS, LCR and PFS) and carries lower risks of dyspnea, radiation pneumonitis and esophagitis than CRT for patients with inoperable stage I NSCLC. The subgroup analyses indicated that a 0-1 patient performance status and suitable BED predicted an improved prognosis. Given the inherent limitations of the present study, the conclusions need to be confirmed in more large-scale high-quality RCTs.
Acknowledgments
The authors thank Professor Jinhua Peng, MD (The second affiliated hospital of Nanchang University) for his statistical advice and professor Jichun Liu, MD, PhD (The second affiliated hospital of Nanchang University) for his data collection. The authors also thank the support from National Natural Science Foundation of China (NSFC).
Author contributions
Can Li had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acquisition, analysis, or interpretation of data: All authors.
Concept and design: All authors.
Critical revision of the manuscript for important intellectual content: Can Li, Li Wang, Qian Wu, Jiani Zhao, Fengming Yi and Jianjun Xu.
Drafting of the manuscript: Can Li and Wenxiong Zhang.
Statistical analysis: Can Li and Wenxiong Zhang.
Supervision: Yiping Wei and Wenxiong Zhang.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 3DCRT = 3D conformal radiotherapy, AEs = adverse effects, AHRT = accelerated hypofractionated radiotherapy, BED = biologically effective dose, CFRT = conventional fractionated radiotherapy, CI = confidence interval, CRT = conventional radiotherapy, GRADE = the grading of recommendations assessment, development and evaluation system, HR = hazard ratio, LCR = local control rate, LCSS = lung cancer-specific survival, LCSSR = lung cancer-specific survival rate, NOS = Newcastle-Ottawa Scale, NSCLC = non-small cell lung cancer, OS = overall survival, OSR = overall survival rate, PFS = progression-free survival, PFSR = progression-free survival rate, PRISMA = preferred reporting items for systematic review and meta-analysis, RR = risk ratio, RCT = randomized controlled trial, SBRT = stereotactic body radiotherapy.
How to cite this article: Li C, Wang L, Wu Q, Zhao J, Yi F, Xu J, Wei Y, Zhang W. A meta-analysis comparing stereotactic body radiotherapy vs conventional radiotherapy in inoperable stage I non-small cell lung cancer. Medicine. 2020;99:34(e21715).
All analyses were based on previously published studies, and hence no ethical approval and patient consent were required.
All data generated or analyzed during this study are included in this manuscript and its Additional files.
This study was supported by National Natural Science Foundation of China (NSFC), number of grants (81560345), Natural Science Foundation of Jiangxi Province (Grant number: 20181BAB215027). Role of the Funding: The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [3].Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1–21. [DOI] [PubMed] [Google Scholar]
- [5].Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193–9. [DOI] [PubMed] [Google Scholar]
- [6].Leduc C, Antoni D, Charloux A, et al. Comorbidities in the management of patients with lung cancer. Eur Respir J 2017;49:1601721. [DOI] [PubMed] [Google Scholar]
- [7].Dosoretz DE, Katin MJ, Blitzer PH, et al. Medically inoperable lung carcinoma: the role of radiation therapy. Semin Radiat Oncol 1996;6:98–104. [DOI] [PubMed] [Google Scholar]
- [8].Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer 2005;103:2118–27. [DOI] [PubMed] [Google Scholar]
- [9].Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494–503. [DOI] [PubMed] [Google Scholar]
- [10].Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol 2009;91:307–13. [DOI] [PubMed] [Google Scholar]
- [11].Nyman J, Hallqvist A, Lund JÅ, et al. SPACE-A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1–8. [DOI] [PubMed] [Google Scholar]
- [12].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2016;354:g7647. [DOI] [PubMed] [Google Scholar]
- [13].Lanni TB, Jr, Grills IS, Kestin LL, et al. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol 2011;34:494–8. [DOI] [PubMed] [Google Scholar]
- [14].Widder J, Postmus D, Ubbels JF, et al. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e291–7. [DOI] [PubMed] [Google Scholar]
- [15].Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jeppesen SS, Schytte T, Jensen HR, et al. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552–8. [DOI] [PubMed] [Google Scholar]
- [17].Mitera G, Swaminath A, Rudoler D, et al. Cost-effectiveness analysis comparing conventional versus stereotactic body radiotherapy for surgically ineligible stage I non-small-cell lung cancer. J Oncol Pract 2014;10:e130–6. [DOI] [PubMed] [Google Scholar]
- [18].Tong AN, Yan P, Yuan GH, et al. Advantages of CyberKnife for inoperable stage I peripheral non-small-cell lung cancer compared to three-dimensional conformal radiotherapy. Mol Clin Oncol 2015;3:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu H-W, Gabos Z, Ghosh S, et al. Outcomes in stage I non-small cell lung cancer following the introduction of stereotactic body radiotherapy in Alberta-A population-based study. Radiother Oncol 2015;117:71–6. [DOI] [PubMed] [Google Scholar]
- [20].Koshy M, Malik R, Mahmood U, et al. Stereotactic body radiotherapy and treatment at a high volume facility is associated with improved survival in patients with inoperable stage I non-small cell lung cancer. Radiother Oncol 2015;114:148–54. [DOI] [PubMed] [Google Scholar]
- [21].Valle LF, Jagsi R, Bobiak SN, et al. Variation in definitive therapy for localized non-small cell lung cancer among national comprehensive cancer network institutions. Int J Radiat Oncol Biol Phys 2016;94:360–7. [DOI] [PubMed] [Google Scholar]
- [22].Boyer MJ, Williams CD, Harpole DH, et al. Improved survival of stage I non-small cell lung cancer: a VA central cancer registry analysis. J Thorac Oncol 2017;12:1814–23. [DOI] [PubMed] [Google Scholar]
- [23].Tu C-Y, Hsia T-C, Fang H-Y, et al. A population-based study of the effectiveness of stereotactic ablative radiotherapy versus conventional fractionated radiotherapy for clinical stage I non-small cell lung cancer patients. Radiol Oncol 2018;52:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Karasawa K, Hayakawa S, Machitori Y, et al. Accelerated hypofractionated radiotherapy versus stereotactic body radiotherapy for the treatment of stage i nonsmall cell lung cancer-a single institution experience with long-term follow-up. Technol Cancer Res Treat 2018;17:1533033818806318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].von Reibnitz D, Shaikh F, Wu AJ, et al. Stereotactic body radiation therapy (SBRT) improves local control and overall survival compared to conventionally fractionated radiation for stage I non-small cell lung cancer (NSCLC). Acta Oncol 2018;57:1567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Driessen E, Detillon D, Bootsma G, et al. Population-based patterns of treatment and survival for patients with stage I and II non-small cell lung cancer aged 65-74 years and 75 years or older. J Geriatr Oncol 2019;10:547–54. [DOI] [PubMed] [Google Scholar]
- [27].Phillips I, Sandhu S, Luchtenborg M, et al. Stereotactic ablative body radiotherapy versus radical radiotherapy: comparing real-world outcomes in stage I lung cancer. Clin Oncol 2019;31:681–7. [DOI] [PubMed] [Google Scholar]
- [28].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [29].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- [30].Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–2. [DOI] [PubMed] [Google Scholar]
- [31].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [33].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Navarro-Martin A, Aso S, Cacicedo J, et al. Phase II trial of SBRT for stage I NSCLC: survival, local control, and lung function at 36 months. J Thorac Oncol 2016;11:1101–11. [DOI] [PubMed] [Google Scholar]
- [36].Timmerman RD, Hu C, Michalski JM, et al. Long-term results of stereotactic body radiation therapy in medically inoperable stage I non-small cell lung cancer. JAMA Oncol 2018;4:1287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Folkert MR, Timmerman RD. Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT). Adv Drug Deliv Rev 2017;109:3–14. [DOI] [PubMed] [Google Scholar]
- [38].Fang LC, Komaki R, Allen P, et al. Comparison of outcomes for patients with medically inoperable Stage I non-small-cell lung cancer treated with two-dimensional vs. three-dimensional radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:108–16. [DOI] [PubMed] [Google Scholar]
- [39].Giuliani ME, Bezjak A. Alternatives to surgery in early stage disease-stereotactic body radiotherapy. Transl Lung Cancer Res 2013;2:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Baardwijk A, Tome WA, Van Elmpt W, et al. Is high-dose stereotactic body radiotherapy (SBRT) for stage I non-small cell lung cancer (NSCLC) overkill?. A systematic review. Radiother Oncol 2012;105:145–9. [DOI] [PubMed] [Google Scholar]
- [41].Chang JY, Bezjak A, Mornex F. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol 2015;10:577–85. [DOI] [PubMed] [Google Scholar]
- [42].Park HS, Harder EM, Mancini BR, et al. Central versus peripheral tumor location: influence on survival, local control, and toxicity following stereotactic body radiotherapy for primary non-small-cell lung cancer. J Thorac Oncol 2015;10:832–7. [DOI] [PubMed] [Google Scholar]
- [43].Roach MC, Robinson CG, DeWees TA, et al. Stereotactic body radiation therapy for central early-stage NSCLC: results of a prospective phase I/II Trial. J Thorac Oncol 2018;13:1727–32. [DOI] [PubMed] [Google Scholar]
- [44].Rowe BP, Boffa DJ, Wilson LD, et al. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol 2012;7:1394–9. [DOI] [PubMed] [Google Scholar]
- [45].Sio TT, Mohindra P, Yu NY, et al. The search for optimal stereotactic body radiotherapy dose in inoperable, centrally located non-small-cell lung cancer continues. J Clin Oncol 2019;37:2697–9. [DOI] [PubMed] [Google Scholar]
- [46].Roach MC, Videtic GMM, Bradley JD. Treatment of peripheral nonsmall cell lung carcinoma with stereotactic body radiation therapy. J Thorac Oncol 2015;10:1261–7. [DOI] [PubMed] [Google Scholar]
- [47].Voroney JP, Hope A, Dahele MR, et al. Chest wall pain and rib fracture after stereotactic radiotherapy for peripheral non-small cell lung cancer. J Thorac Oncol 2009;4:1035–7. [DOI] [PubMed] [Google Scholar]
- [48].Adebahr S, Hechtner M, Schrader N, et al. Early impact of pulmonary fractionated stereotactic body radiotherapy on quality of life:benefit for patients with low initial scores (STRIPE Trial). J Thorac Oncol 2019;14:408–19. [DOI] [PubMed] [Google Scholar]
- [49].Bezjak A, Paulus R, Gaspar LE, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG oncology/RTOG 0813 trial. J Clin Oncol 2019;37:1316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


