Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, critical illness, respiratory failure
Abstract
Objectives:
Differences in mortality rates previously reported in critically ill patients with coronavirus disease 2019 have increased the need for additional data on mortality and risk factors for death. We conducted this study to describe length of stay, mortality, and risk factors associated with in-hospital mortality in mechanically ventilated patients with coronavirus disease 2019.
Design:
Observational study.
Setting:
Two urban, academic referral hospitals in Indianapolis, Indiana.
Patients or Subjects:
Participants were critically ill patients 18 years old and older, admitted with coronavirus disease 2019 between March 1, 2020, and April 27, 2020.
Interventions:
None.
Measurements and Main Results:
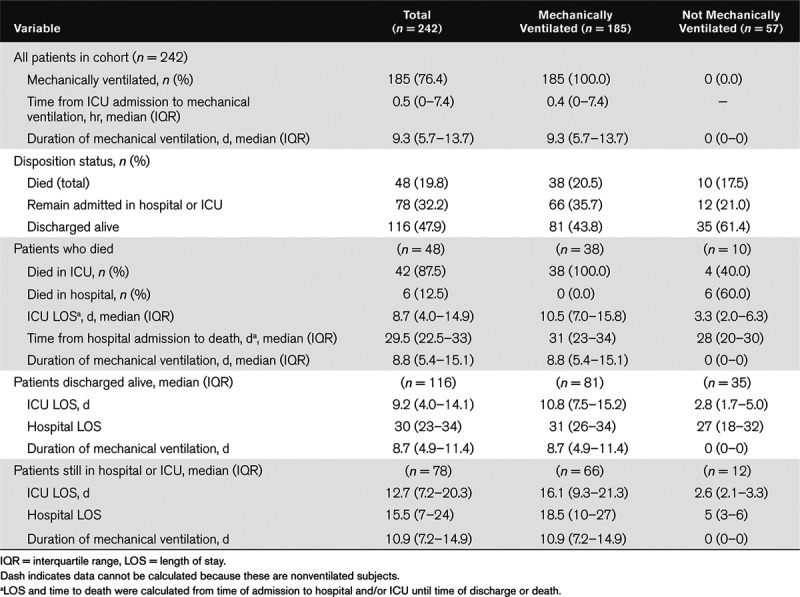
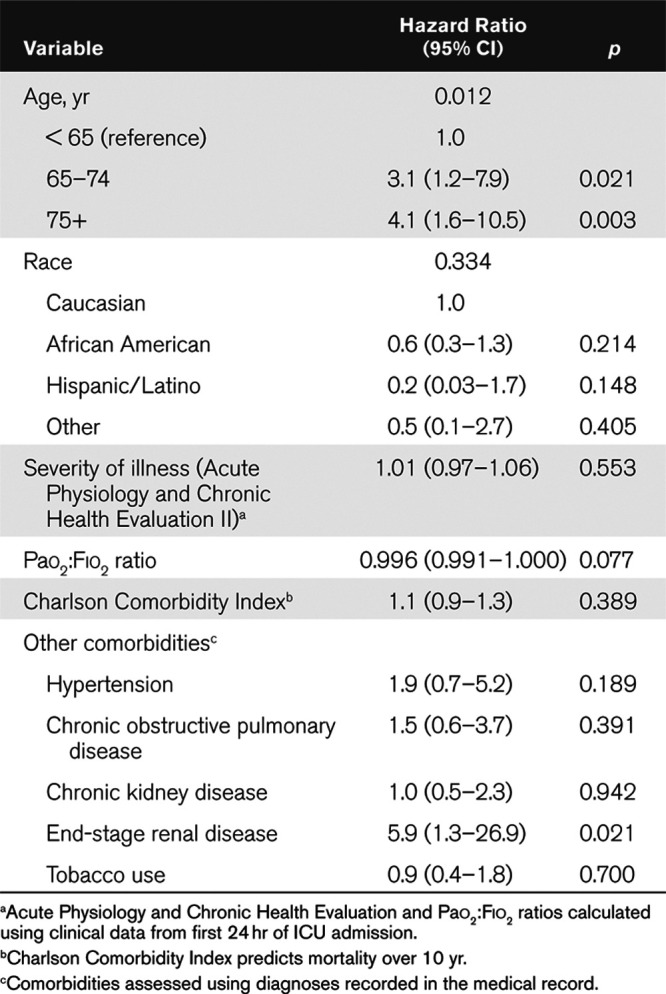
Outcomes included in-hospital mortality, duration of mechanical ventilation, and length of stay. A total of 242 patients were included with mean age of 59.6 years (sd, 15.5 yr), 41.7% female and 45% African American. Mortality in the overall cohort was 19.8% and 20.5% in the mechanically ventilated subset. Patients who died were older compared with those that survived (deceased: mean age, 72.8 yr [sd, 10.6 yr] vs patients discharged alive: 54.3 yr [sd, 14.8 yr]; p < 0.001 vs still hospitalized: 59.5 yr [sd, 14.4 yr]; p < 0.001) and had more comorbidities compared with those that survived (deceased: 2 [0.5–3] vs survived: 1 [interquartile range, 0–1]; p = 0.001 vs still hospitalized: 1 [interquartile range, 0–2]; p = 0.015). Older age and end-stage renal disease were associated with increased hazard of in-hospital mortality: age 65–74 years (hazard ratio, 3.1 yr; 95% CI, 1.2–7.9 yr), age 75+ (hazard ratio, 4.1 yr; 95% CI, 1.6–10.5 yr), and end-stage renal disease (hazard ratio, 5.9 yr; 95% CI, 1.3–26.9 yr). The overall median duration of mechanical ventilation was 9.3 days (interquartile range, 5.7–13.7 d), and median ICU length of stay in those that died was 8.7 days (interquartile range, 4.0–14.9 d), compared with 9.2 days (interquartile range, 4.0–14.0 d) in those discharged alive, and 12.7 days (interquartile range, 7.2–20.3 d) in those still remaining hospitalized.
Conclusions:
We found mortality rates in mechanically ventilated patients with coronavirus disease 2019 to be lower than some previously reported with longer lengths of stay.
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also known as coronavirus disease 2019 (COVID-19), has created a global public health emergency (1) due to its rapid spread and significant morbidity and mortality. More than 11 million cases of COVID-19 have been identified worldwide (1), and as of July 10, 2020, there have been 3.1 million confirmed cases and over 130,000 deaths in the United States (2). Older individuals and patients with cardiovascular disease, chronic lung disease, hypertension, and diabetes are at particular risk for death from COVID-19, often due to the development of severe acute respiratory distress syndrome (ARDS) (3–5).
The mortality of critically ill mechanically ventilated patients with COVID-19 was reported as high as 88–97% (3, 5–8). A recent study reported mortality rates of 30.9%, but whether these low rates are seen at other centers is not known (9).
Further data on mortality and length of stay are needed to advance the global understanding of outcomes in critically ill COVID-19 patients. We carried out this analysis with the primary aim to describe the length of stay, mortality, and risk factors associated with death in ICU patients with COVID-19 infection.
MATERIALS AND METHODS
This observational study was conducted at two large urban academic centers affiliated with Indiana University School of Medicine: Indiana University Health Methodist Hospital, an 802-bed quaternary care referral center, and Eskenazi Health, a 336-bed safety net hospital. All consecutive patients admitted to the ICUs with a positive result by SARS-CoV-2 nasopharyngeal swab polymerase chain reaction test from March 1, 2020, to April 27, 2020, were included. Patients under the age of 18 were excluded. Clinical outcomes were collected until April 29, 2020. The primary outcomes were in-hospital mortality, duration of mechanical ventilation, and length of ICU and hospital stay. The study received approval from the local Institutional Review Board.
Data Collection
Data were extracted from hospital electronic medical systems (Cerner PowerChart, Epic Health Systems) and entered directly into a REDCap database. Records were randomly audited to reduce the risk of measurement error or bias. Data included patient demographics, comorbidities, Acute Physiology and Chronic Health Evaluation (APACHE) II score (10), vital signs and laboratory results (from first 24 hr of ICU admission), including SARS-CoV-2 test results, and dates of admission, discharge, and death. APACHE II was calculated using values from the first 24 hours of admission to the ICU. If the patient was readmitted during the study follow-up period, laboratory data from the initial ICU admission was used. For patients transferred to the two hospitals, data collection began at time of ICU admission to our system.
Statistical Analysis
Demographic and clinical characteristics were compared between patients who were discharged alive, those still admitted at the end of the follow-up period, and those who died in the hospital using analysis of variance (normal data) and Wilcoxon rank-sum tests (skewed data) for continuous outcomes or Fisher exact test for categorical variables. For significant variables, we explored pairwise comparisons and adjusted for multiple comparisons using the stepdown Bonferroni method. Summary statistics were provided for mechanically ventilated patients and those who were not ventilated. Variables with significant group differences in univariate analysis (with the exception of insurance as it was collinear with age) were included in a cause-specific Cox’s proportional hazards model to identify risk factors associated with in-hospital mortality. For this model, the event of interest was time from ICU admission to death with a competing event as time to discharge. Patients still in the hospital were censored.
RESULTS
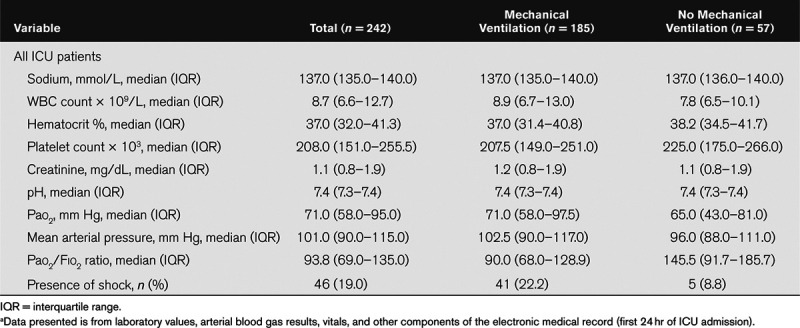
Six-hundred forty-four consecutive patients with COVID-19 were admitted from March 1, 2020, to April 29, 2020. Two-hundred forty-two were ICU admissions (including 2% [5/242] transferred from other hospitals) and comprised the study cohort (Figure E1 in the Supplemental Digital Content http://links.lww.com/CCX/A283). Demographics and clinical characteristics for the cohort are presented in Table 1. The mean age of the cohort was 59.6 years (sd, 15.5 yr), 41.7% were female and 45% African American. Hypertension (61.6%), obesity (56.4%), diabetes mellitus (43%), and tobacco use (26.9%) were the most frequent comorbid conditions. The median APACHE II score calculated using values from the first 24 hours of ICU admission was 19 (interquartile range [IQR], 13–26), and median Charlson Comorbidity Index (11) was 1 (IQR, 0–2). The mean Pao2:Fio2 ratio for the cohort was 116.6 (sd, 77.6). Table 2 provides additional laboratory and hemodynamic characteristics of the cohort by mechanical ventilation status.
Table 1.
Characteristics of ICU Patients With Coronavirus Disease 2019 (n = 242)
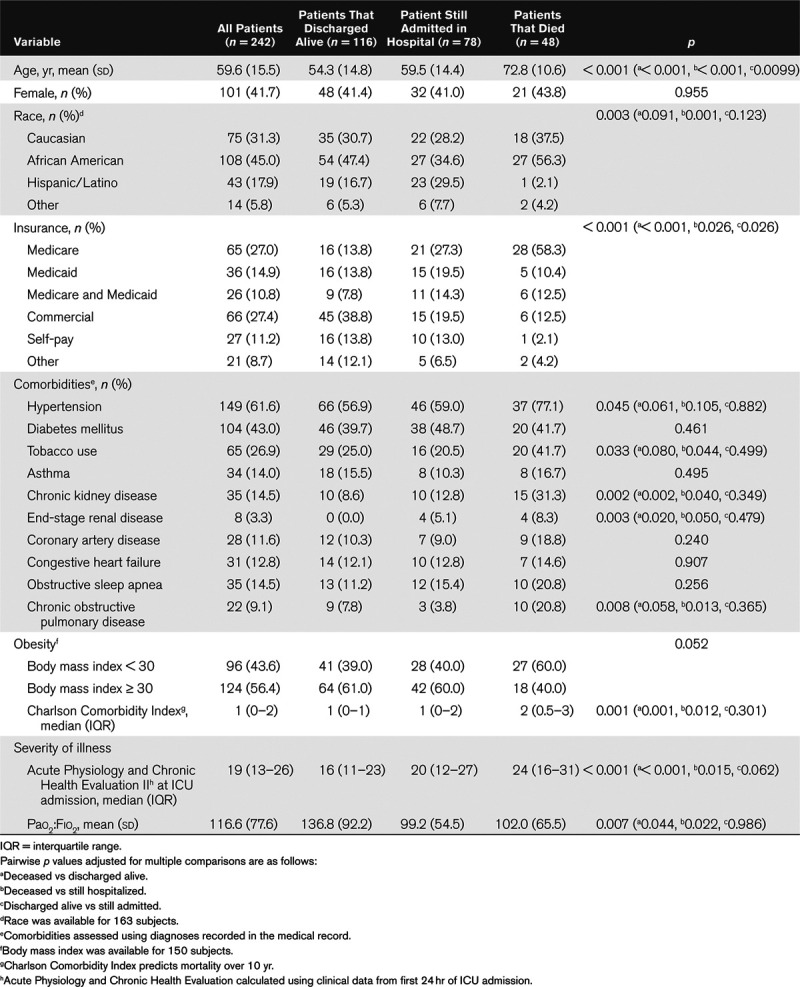
Table 2.
Laboratory Values, Oxygenation, and Hemodynamic Characteristics of Patients Admitted with Novel Coronavirus on Day of ICU Admissiona
Differences in Characteristics Between Patients That Died, Survived, or Remained Admitted
Table 1 compares clinical characteristics and comorbidities of patients who died, discharged alive, or were still admitted at the end of the study period, including pairwise p values adjusted for multiple comparisons. Patients who died were older compared with those that survived (deceased: mean age, 72.8 yr [sd, 10.6 yr] vs patients discharged alive: 54.3 yr [sd, 14.8 yr]; p < 0.001 and vs still hospitalized: 59.5 yr [sd, 14.4 yr]; p < 0.001). Patients that died also had more comorbidities as assessed by the Charlson Comorbidity Index compared with patients who were alive (deceased: 2 [0.5–3] vs survived: 1 [IQR, 0–1]; p = 0.001 vs still hospitalized: 1 [IQR, 0–2]; p = 0.015). Median APACHE II scores were higher in patients that died (24 [IQR, 16–31]) versus those discharged alive (16 [IQR, 11–23]; p < 0.001) or those still in the hospital (20 [IQR, 12–27]; p = 0.015).
Mortality and Length of Stay Outcomes
Mechanical ventilation occurred in 76.4% of patients (185/242) in the overall cohort (Table 3). Mortality in the overall cohort was 19.8% (48/242) and 20.5% (38/185) among mechanically ventilated patients. There were no differences in mortality rates between the two health centers. The median duration of mechanical ventilation in the overall cohort was 9.3 days (IQR, –5.7 to 13.7 d). In patients that died, median ICU length of stay was 8.7 days (IQR, 4.0–14.9 d), compared with 9.2 days (IQR, 4.0–14.0 d) in those discharged alive, and 12.7 days (IQR, 7.2–20.3 d) in those still admitted at the end of the follow-up period (Table 3).
Table 3.
Mortality Outcomes and Length of Stay for ICU Patients With Coronavirus Disease 2019 (n = 242)
Risk Factors Associated With Mortality
Older age and end-stage renal disease (ESRD) were associated with increased hazard of in-hospital mortality: age 65–74 years (hazard ratio [HR], 3.1 yr; 95% CI, 1.2–7.9 yr; p = 0.021), age 75+ (HR, 4.1 yr; 95% CI, 1.6–10.5 yr; p = 0.003) compared with those younger than age 65, and ESRD (HR, 5.9; 95% CI, 1.3–26.9; p = 0.021). In our cause-specific Cox’s proportional hazard model, race, Charlson Comorbidity Index, and severity of illness by APACHE II were not significantly associated with increased odds of hospital mortality (Table 4).
Table 4.
Cause-Specific Cox’s Proportional Hazards Model for Death With Discharge As a Competing Risk (n = 242)
DISCUSSION
In this study, we report a mortality rate of approximately 20% in mechanically ventilated COVID-19 patients, lower than recently published reports (3, 5–9). Furthermore, the mortality in our cohort is even lower than reported 28-day mortality of 35–46% for mild to severe ARDS (12).
Our mortality results are likely due to the surge in COVID-19 patients occurring later in Indiana than in other places, providing us the opportunity to learn from experiences at other centers. In addition, our medical system was not highly stressed. Due to a lack of strain on ventilator resources, clinicians at our hospitals elected to intubate patients early, as evidenced by 76% of the COVID-19-related ICU admissions receiving invasive ventilation. In contrast to other centers, we saw a relatively rapid but brief increase in COVID-19 ICU cases beginning in mid-March, peaking in early April (Figure E2 in the Supplemental Digital Content http://links.lww.com/CCX/A283), and subsequently declining. Near the peak, only 58% of ICU beds and 27% of ventilators in the state were being used. This flattening of the curve can be attributed to a lower population density and early implementation of social distancing restrictions, allowing the medical system to effectively deal with the COVID-19 surge. This relationship between medical system capacity, the number of patients, and outcomes have been well described in the context of pandemic influenza (13, 14).
Our study is smaller compared with other cohorts limiting our ability to draw conclusions about mortality risk factors, although, like others, we found older age to be a significant risk factor, while patients identified as African American or Hispanic did not have increased risk of in-hospital mortality. We did not identify specific treatments, which led to a decreased mortality in our ventilated COVID-19 patients. Such work will require more granular analysis. However, we believe that sites able to prepare resources without getting overwhelmed with disease surges will show similar experiences. Our cumulative experience at Indiana University counterbalances prior published reports leading to the public perception that ventilatory support in COVID-19 patients frequently results in death, causing some to question the utility of mechanical ventilation in these patients.
CONCLUSIONS
In conclusion, we found lower mortality among mechanically ventilated COVID-19 patients than recently reported by others. This highlights that clinical experience with COVID-19 will vary widely across the country, which may be due to differences in public health practices, local medical practices, or resource availability. Ascertainment of comprehensive and accurate statistics will be important when developing policies and guidelines regarding the COVID-19 pandemic (15).
ACKNOWLEDGMENTS
We would like to thank our patients, caregivers, healthcare team, clinical partners, and members of Team Vitality for making this work possible.
Supplementary Material
Footnotes
This work was performed at Indiana University Health Methodist Hospital and Eskenazi Health.
Drs. Perkins, Gao, and Khan are supported through National Institutes of Health (NIH)-National Institute on Aging (NIA) R01 AG 055391, R01 AG 052493, and National Heart, Lung, and Blood Institute R01 HL131730. Dr. Twigg is supported through NIH-NIA U01 AG060900. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.World Health Organization: Rolling Updates on Coronavirus Disease (Covid-19). 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed July 10, 2020
- 2.Johns Hopkins Coronavirus Resource Center. 2020. Available at: https://coronavirus.Jhu.Edu/map.Html. Accessed July 10, 2020
- 3.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA. 2020; 323:1545–1546 [DOI] [PubMed] [Google Scholar]
- 5.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020 May 26. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE-Acute Physiology and Chronic Health Evaluation: A physiologically based classification system. Crit Care Med. 1981; 9:591–597 [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Sax FL, MacKenzie CR, et al. Assessing illness severity: Does clinical judgment work? J Chronic Dis. 1986; 39:439–452 [DOI] [PubMed] [Google Scholar]
- 12.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 13.Qualls N, Levitt A, Kanade N, et al. ; CDC Community Mitigation Guidelines Work Group. Community mitigation guidelines to prevent pandemic influenza - United States, 2017. MMWR Recomm Rep. 2017; 66:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin PJ, Gebbie EN, Qureshi K. Can the health-care system meet the challenge of pandemic flu? Planning, ethical, and workforce considerations. Public Health Rep. 2007; 122:573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce N, Vandenbroucke JP, VanderWeele TJ, et al. Accurate statistics on COVID-19 are essential for policy guidance and decisions. Am J Public Health. 2020; 110:949–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


