Abstract
Background:
Previous studies showed difference results about the effect of nurse in improvement of the colonoscopy detection rate. This meta-analysis aims to investigate whether nurse participation during colonoscopy can help in improving the detection rate of polyps and adenomas.
Methods:
Original studies in English were searched from the MEDLINE database, PubMed, Web of Science, and the Cochrane Library database. Randomized control trials (RCT) comparing colonoscopy with and without nurse participation for the detection of colorectal polyps and adenomas were identified. A meta-analysis was performed using Revman 5.3 software.
Results:
A total of 2268 patients from 4 RCTs were included in this meta-analysis. Outcomes of colonoscopy with nurse participation were compared with those of colonoscopy without nurse participation. The results showed that nurses’ participation during colonoscopy could significantly increase both, polyp detection rate and adenoma detection rate.
Conclusion:
Nurse assistance during colonoscopy can help improve the rate of detection of polyps and adenomas.
Keywords: adenoma detection, colonoscopy, Meta-analysis, nurse, polyp detection
1. Introduction
Colorectal cancer has a high rate of mortality.[1] Colonoscopy is a useful tool for early detection and removal of precancerous lesions of the colorectal tract and then might significantly reduce the incidence and mortality from colorectal cancer.[2,3] However, several factors might influence the rate of early detection of colorectal lesions during colonoscopy.[4] Previous studies suggested that an additional observer (including nurses or trainees) participating in the observation during colonoscopy might increase the rate of detection of colorectal lesions.[5,6]
In recent years, nurses have played an important role in endoscopy procedures.[7] Several trials reported that nurse participation during colonoscopy might increase the rate of detection of colorectal lesions.[5,8–10] A meta-analysis including 3 randomized controlled trials (RCTs) was published in 2016, and the study evaluated the impact of nurses’ presence during colonoscopy, on the rate of detection of polyps and adenomas. The results indicated that nurses’ participation during colonoscopy can increase the adenoma detection rate (ADR) with a high risk of bias, while there was no impact on the polyp detection rate (PDR) and advanced lesions detection rate.[11] Hence, to provide the latest evidence and minimize the potential bias, an updated meta-analysis was conducted to further investigate the impact of a nursing assistant during colonoscopy on the detection rate of colorectal polyps.
2. Methods
2.1. Search methods and study selection
This meta-analysis included RCTs published from 1996 to December 31, 2019, which compared the outcomes with regard to detection rate of colorectal polyps and adenomas between colonoscopies with nurse participation and those performed by the colonoscopist alone. The articles were searched in the MEDLINE database, PubMed, Web of Science, and the Cochrane Library database using the following search terms: “colonoscopy,” “nurse,” “polyp,” “adenoma” combined with “randomized trials.” The reference lists of the included trials were also reviewed to identify additional publications. Two observers independently and blindly identified the studies for inclusion and extracted the data from each study. All analyses were based on previously published studies; thus, ethical approval and patient consent were not required.
2.2. Inclusion and exclusion criteria
All titles and abstracts generated from the search were screened for inclusion further selection was conducted by obtaining full texts of identified articles to determine whether they met inclusion criteria. Inclusion criteria were a priori delineated using the PICO statement as follows:
P: inpatients undergoing colonoscopy for any indication;
I: nurse participation (with colonoscopist);
C: no nurse participation (colonoscopist alone); and
O: colonoscopy lesions detection rate including ADR, PDR and advanced lesion detection rate.
Studies were included if they met the following criteria: published as full article RCTs comparing the outcomes between colonoscopies with nurse participation and those performed by the colonoscopists alone. Only studies published in English were included. Retrospective trials and duplicate publications were excluded. Publications without data for retrieval and unpublished trials were also excluded. All articles retrieved from the search were screened independently by 2 reviewers (AL and HC).
2.3. Data extraction and outcomes
Data including the trial name, year of publication, sample size, the country in which the study was conducted, study design, characters of colonoscopists and nurses, patients’ age, and indications for colonoscopy were extracted from the included studies by 2 authors, (AL and YL) independently and blindly. The PDR, ADR and advanced adenoma or cancer detection rate were also extracted for this meta-analysis. The adenoma detection rate (ADR) was defined as the percentage of colonoscopies in which at least one histologically proven adenoma was detected. The polyp detection rate (PDR) was defined as the percentage of colonoscopies in which at least one polyp was detected. The advanced lesion was defined as a lesion >10 mm in diameter, lesions with a villous component, or lesions with high-grade dysplasia or early cancer. The advanced lesions detection rate was defined as the percentage of colonoscopies in which at least one advanced lesion was detected. Any disagreements were resolved through discussion with a third reviewer (HC).
2.4. Statistical analysis
We used the Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK) to perform this meta-analysis. P < .05 was considered statistically significant. The dichotomous variables were analyzed using risk ratio (RR) with a 95% confidence interval (CI). For continuous outcomes, standard mean differences (SMD) with 95% confidence intervals (95% CIs) were used to analyze the data. The results of this meta-analysis are indicated by forest plots. The pooled outcomes were analyzed using the fixed effects model and the random-effects model. The extent of heterogeneity was classified as low, moderate, or high for I2 values of 25%, 50%, and 75% respectively. Heterogeneity was considered statistically significant when P was <.05, and the random-effects model results were reported. The sensitivity analysis was performed by excluding 1 study at a time and the changes in statistical results were observed.
2.5. Methodological quality assessment and Publication bias analysis
The methodological quality of the included studies was assessed using the Cochrane Collaboration's risk of bias tool via Revman 5.3 software. The criterion of the methodological quality was set as low, high, or unclear risk of bias. Two authors (AL and HW) evaluated the methodological quality independently, and any disagreements were resolved through discussion with a third reviewer (HC).
3. Results
3.1. Characteristics of included studies
As shown in Figure 1, 4 RCTs with 2316 patients met the criteria for inclusion in this meta-analysis.[8–10,12] These studies were published between 2011 and 2018. Two studies were from Korea, 1 from the USA, and 1 from China. The sample sizes ranged between 383 and 844 patients. The indications for colonoscopy were mainly screening colonoscopy, while 1 trial included all patients undergoing colonoscopy during the study period. The age of included patients varied between the trials; 3 trials included the patients aged above 50 years and 1 trial included patients aged 40 to 70 years. The experience of the colonoscopist and nurses varied between the trials; only 1 trial performed a subgroup analysis on the experience of nurses. The regimens for bowel preparation were stated in all trials; while 3 trials used the 4-liter polyethylene glycol solution (PEG) regimen, the fourth trial used the 3-liter PEG regimen. All trials reported the ADR and advance lesions detection rate; however, only 3 trials reported the PDR. The above findings indicate that factors that might influence the detection rate of colorectal lesions were designed differently in the protocols and a methodological bias might exist. The characteristics of the including studies were shown in Table 1.
Figure 1.

Flow diagram of included and excluded studies.
Table 1.
Characteristics of the including studies.

3.2. Clinical outcomes
Three trials presented data on PDR. A moderate heterogeneity related to PDR was found between trials; however, no statistical significance was detected (χ2 = 3.59, df = 2, P = .17, I2 = 44%). In the fixed models, there was a significant difference in the PDR between the single observation group and dual observation group (RR, 1.16; 95% CI, 1.05, 1.27; Z = 2.97; P = .003; Fig. 2A). There was no heterogeneity between the trials with respect to ADR (χ2 = 1.87, df = 3, P = .60; I2 = 0%). In the fixed models, there was a significant difference in the ADR between the single observation group and dual observation group (OR, 1.19; 95% CI, 1.07, 1.32; Z = 3.29; P = .001; Fig, 2B). Three trials provided information about the advanced lesions detection rate. There was no heterogeneity between the trials in terms of advanced lesions detection rate (χ2 = 0.79, df = 2, P = .67; I2 = 0%). In the fixed models, there was no significant difference in the advanced lesions detection rate between the single observation group and dual observation group (RR, 1.33; 95% CI, 0.95, 1.87; Z = 1.65; P = .10; Fig. 2C).
Figure 2.

Forest plot of the clinical outcomes between single observation group and dual observation group. A. PDR, B. ADR, C. advanced lesions detection rate.
3.3. Sensitivity analysis
We evaluated the impact of each study on the overall meta-analysis by excluding one study at a time, and the exclusion of any single study made no significant difference. Thus, this sensitivity analysis suggested that the above results of the meta-analysis were stable. However, because only 4 RCTs were included, more detailed stratification comparisons could not be made, which could affect the stability of this meta-analysis to some extent.
3.4. Methodological quality and publication bias
Following the instructions of Cochrane Collaboration, the risk of bias from each study was analyzed (Fig. 3). All 4 trials had a low risk of bias in random sequence generation, and 2 trials showed unclear risks of bias in allocation concealment. Thus, a selection bias might exist among the trials. The aim of the included trials was to compare the colorectal lesions detection rate between 2 groups of observers; thus, the observers were aware of the groups that they joined, and blinding of the observers was not possible. However, the experience of colonoscopists and nurses might have influenced the detection rate; thus, a performance bias and detection bias might exist, while the attrition bias and reporting bias were high. Finally, the publication bias was shown using a funnel plot, and a minimal bias was observed (Fig. 4). Hence, the included trials were of moderate quality with minimal publication bias.
Figure 3.

Summary of risk of bias assessment. A, Risk of bias graph. B, Risk of bias summary.
Figure 4.
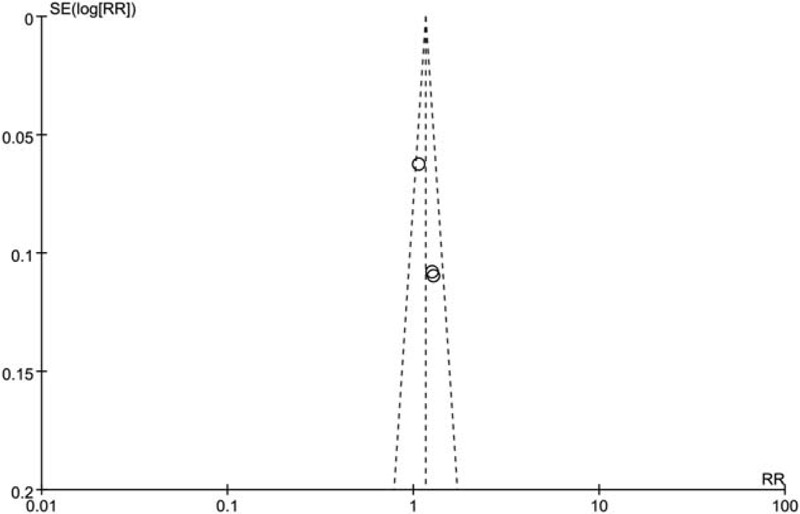
Funnel plot evaluating publication bias. Diagonal lines indicate 95%. CI. Trials within these boundaries indicate minimal publication bias.
4. Discussion
PDR and ADR are important indices to assess the quality of colonoscopy, as they predict the risk of interval cancer after colonoscopy. However, the PDR and ADR are influenced by various factors, such as bowel preparation, the experience of the colonoscopists, and new techniques of colonoscopy. Colonoscopy performance varies between endoscopists, and several methods have been investigated to improve the quality.
In recent years, some studies suggested that an additional observer might improve the quality of colonoscopy. A previous meta-analysis including 3 RCTs was published in 2016.[11] Their results showed that nurse participation during colonoscopy was found to be associated with a higher PDR, ADR, and advanced lesions detection rate; however, a statistically significant difference was only detected in terms of ADR. In this previous study, only 2 trials with 1174 cases (599 cases in nurse participation group and 575 colonoscopist alone group) were included in the meta-analysis of PDR, thus a potential selection bias might exist. Our meta-analysis was in line with this previous study, we added another study with 585 patients in this meta-analysis, and the updated results show that nurses’ participation during colonoscopy could significantly increase both PDR and ADR but not the advanced lesions detection rate. In addition, according to the included studies, the total number of polyps detected was higher when the nurse participated in colonoscopy observation. Hence, a potential selection bias might be decreased in our study, and our results demonstrated that the nurse as a second observer could help colonoscopists to increase the quality of the colonoscopy examination in terms of colorectal polyp and adenoma detection.
Other factors that might influence the detection rate were the size and shape of the polyp and adenoma. Previous studies indicated that the miss rate was higher when polyps were less than 10 mm in size. Although advanced neoplasia was found more frequently in large polyps, about 20% to 30% of all advanced histopathology was seen in polyps less than 10 mm.[13] Thus, it is very important to detect the small size lesions. All included studies reported that nurse involvement during colonoscopy did not impact the outcomes in terms of the size and shape of the lesions detected. Well-designed studies are necessary to determine whether dual observation could impact the detection of small-sized or depressed lesions.
However, there are some limitations to this meta-analysis. First, the regimens of bowel preparation were different between the trials, and the quality of bowel preparation is a critical factor that can influence the colorectal lesion detection rate. However, the bowel preparation quality was not reported in some studies; this is a risk for underestimation of the lesion detection rate during colonoscopy.[14] Second, the experience of colonoscopists and nurses were different between the trials; the colorectal lesion detection rate might also be influenced by this factor.[15] Third, newer techniques of colonoscopy, such as narrowband image and linked color image, might increase the lesion detection rate on colonoscopy.[16–18] Colonoscopists could use these techniques. However, no trial on the usefulness of these new techniques has been reported. Fourth, other factors such as withdrawal time and sedation administered were inconsistent or not reported.[19,20] Fifth, our results shown that no statistically significant difference of the advanced lesions detection rate was found. Due to the small sample size of each trial and small number of advanced lesions, type II error appears to be quite likely exist. Finally, most trials are from Eastern countries. Studies on races in other parts of the world might be needed for further research.[21]
In conclusion, the meta-analysis indicated that nurses as additional observers during a colonoscopy can improve the PDR and ADR. Further large-scale, high-quality, multicenter trials using a standard protocol are required to confirm the above results.
Author contributions
Analysis and interpretation of data: AH.L, HS.W, YJ.L.
Conception and design: AH.L, HL.C and YN.L.
Critical revision of the manuscript for important intellectual content: AH.L, HL.C and YN.L.
Data collection: YJ.L, JK.W, LP.F, and YN.L.
Drafting of the manuscript and prepared figures: AH.L, HL.C and HS.W.
Conceptualization: Aihong Liu, Yanan Liu.
Conception of the work, analysis the data and writing the manuscript: Shuhong Yan.
Data curation: Yijia Lin, Liping Fu.
Formal analysis: Aihong Liu, Huashe Wang.
Software: Aihong Liu, Huashe Wang, Yanan Liu.
Writing – original draft: Aihong Liu, Huashe Wang, Yanan Liu.
Writing – review & editing: Aihong Liu, Huashe Wang, Yanan Liu.
Footnotes
Abbreviations: ADR = adenoma detection rate, CI = confidence interval, PDR = polyp detection rate.
How to cite this article: Liu A, Wang H, Lin Y, Fu L, Liu Y, Yan S, Chen H. Gastrointestinal endoscopy nurse assistance during colonoscopy and polyp detection: A PRISMA-compliant meta-analysis of randomized control trials. Medicine. 2020;99:34(e21278).
AL and HW contributed equally to this work and are co-first authors.
This work was supported by grants from the Guangzhou Science and Technology Project (grant no. 201803010040) and the National Key Clinical Discipline.
All authors reviewed the manuscript.
The work was approved by the Ethics Committee of the Sixth Affiliated Hospital, Sun Yat-sen University and has been performed in accordance with the Declaration of Helsinki.
Informed consent was obtained from all individual participants included in the study included in the studies considered for this meta-analysis.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- [2].Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- [3].Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut 2018;67:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fayad NF, Kahi CJ. Colonoscopy quality assessment. Gastrointest Endosc Clin N Am 2015;25:373–86. [DOI] [PubMed] [Google Scholar]
- [5].Dellon ES, Lippmann QK, Sandler RS, et al. Gastrointestinal endoscopy nurse experience and polyp detection during screening colonoscopy. Clin Gastroenterol Hepatol 2008;6:1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Qayed E, Shea L, Goebel S, et al. Association of trainee participation with adenoma and polyp detection rates. World J Gastrointest Endosc 2017;9:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tucker D, Scrymgeour G, Marshall B. Toward developing a nurse endoscopist role in New Zealand. Gastroenterol Nurs 2017;40:128–33. [DOI] [PubMed] [Google Scholar]
- [8].Lee CK, Park DI, Lee SH, et al. Participation by experienced endoscopy nurses increases the detection rate of colon polyps during a screening colonoscopy: a multicenter, prospective, randomized study. Gastrointest Endosc 2011;74:1094–102. [DOI] [PubMed] [Google Scholar]
- [9].Kim TS, Park DI, Lee DY, et al. Endoscopy nurse participation may increase the polyp detection rate by second-year fellows during screening colonoscopies. Gut Liver 2012;6:344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aslanian HR, Shieh FK, Chan FW, et al. Nurse observation during colonoscopy increases polyp detection: a randomized prospective study. Am J Gastroenterol 2013;108:166–72. [DOI] [PubMed] [Google Scholar]
- [11].Xu L, Zhang Y, Song H, et al. Nurse participation in colonoscopy observation versus the colonoscopist alone for polyp and adenoma detection: a meta-analysis of randomized, controlled trials. Gastroenterol Res Pract 2016;2016:7631981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang W, Xu L, Bao Z, et al. Differences with experienced nurse assistance during colonoscopy in detecting polyp and adenoma: a randomized clinical trial. Int J Colorectal Dis 2018;33:561–6. [DOI] [PubMed] [Google Scholar]
- [13].Turner KO, Genta RM, Sonnenberg A. Lesions of all types exist in colon polyps of all sizes. Am J Gastroenterol 2018;113:303–6. [DOI] [PubMed] [Google Scholar]
- [14].Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline - update 2019. Endoscopy 2019;51:775–94. [DOI] [PubMed] [Google Scholar]
- [15].Crockett SD, Gourevitch RA, Morris M, et al. Endoscopist factors that influence serrated polyp detection: a multicenter study. Endoscopy 2018;50:984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Leung WK, Guo CG, Ko MKL, et al. Linked color imaging versus narrow-band imaging for colorectal polyp detection: a prospective randomized tandem colonoscopy study. Gastrointest Endosc 2020;91:104–12 e105. [DOI] [PubMed] [Google Scholar]
- [17].Castaneda D, Popov VB, Verheyen E, et al. New technologies improve adenoma detection rate, adenoma miss rate, and polyp detection rate: a systematic review and meta-analysis. Gastrointest Endosc 2018;88:209–22 e211. [DOI] [PubMed] [Google Scholar]
- [18].Jrebi NY, Hefty M, Jalouta T, et al. High-definition colonoscopy increases adenoma detection rate. Surg Endosc 2017;31:78–84. [DOI] [PubMed] [Google Scholar]
- [19].Chen CW, Chiu CT, Su MY, et al. Factors associated with polyp detection during colonoscopy: a retrospective observational study. Kaohsiung J Med Sci 2019;35:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kashiwagi K, Inoue N, Yoshida T, et al. Polyp detection rate in transverse and sigmoid colon significantly increases with longer withdrawal time during screening colonoscopy. PLoS One 2017;12:e0174155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lieberman DA, Williams JL, Holub JL, et al. Race, ethnicity, and sex affect risk for polyps >9 mm in average-risk individuals. Gastroenterology 2014;147:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


