Abstract
Background.
Flavin mononucleotide (FMN), released from damaged mitochondrial complex I during hypothermic liver perfusion, has been shown to be predictive of 90-day graft loss. Normothermic machine perfusion (NMP) and normothermic regional perfusion (NRP) are used for organ reconditioning and quality assessment before transplantation. This pilot study aimed to investigate the changes of FMN levels during normothermic reperfusion of kidneys, livers, and lungs and examine whether FMN could serve as a biomarker to predict posttransplant allograft quality.
Methods.
FMN concentrations, in perfusates collected during NMP of kidneys, abdominal NRP, and ex vivo lung perfusion, were measured using fluorescence spectrometry and correlated to the available perfusion parameters and clinical outcomes.
Results.
Among 7 transplanted kidneys out of the 11 kidneys that underwent NMP, FMN levels at 60 minutes of NMP were significantly higher in the allografts that developed delayed graft function and primary nonfunction (P = 0.02). Fifteen livers from 23 circulatory death donors that underwent NRP were deemed suitable for transplantation. Their FMN levels at 30 minutes of NRP were significantly lower than those not procured for transplantation (P = 0.004). In contrast, little FMN was released during the 8 lung perfusions.
Conclusions.
This proof of concept study suggested that FMN in the perfusates of kidney NMP has the potential to predict posttransplant renal function, whereas FMN at 30 minutes of NRP predicts whether a liver would be accepted for transplantation. More work is required to validate the role of FMN as a putative biomarker to facilitate safe and reliable decision-making before embarking on transplantation.
INTRODUCTION
For all solid organ transplantation, there is a discrepancy between number of patients on the waitling lists and level of transplant activity. Despite this organ shortage, a significant number of the available donor organs are not accepted for transplantation. For example, from April 2018 to March 2019, only 84% of the kidneys, 62% of livers, 25% of pancreases, 26% of hearts, and 18% of lungs offered for donation in the United Kingdom were transplanted into recipients.1 Organs from extended-criteria donors (ECD), such as donors of older age and donation after circulatory death (DCD), have been increasingly considered for transplant. However, these organs are known to be associated with higher risks of poor posttransplant outcomes. To increase organ utilization rates, alternative methods of organ preservation and assessment have been explored in the last decade, including both, ex situ normothermic machine perfusion (NMP) and normothermic regional perfusion (NRP) in DCD donors. NMP revives donor organs before transplantation, providing an opportunity to assess ECD organs.2–4 NRP aims to reverse warm ischemic injury and so improve allograft quality in the DCD setting.5
Comprehensive evaluation of allograft quality is of vital importance to minimize unnecessary organ discard and optimize donor-recipient matching. However, as of yet, there has been no successfully validated scoring system or biomarker that is in widespread clinical use to facilitate transplant teams’ decision-making process before implantation.6–9 The lack of objective measurement leaves transplant surgeons to accept organs based on their clinical experience. There is a need for robust and reliable assessment techniques with rapidly obtainable results in a routine setting.
Muller et al6 used the release of flavin mononucleotide (FMN) from liver into the hypothermic oxygenated perfusion solution as a surrogate marker for ischemia-reperfusion injury (IRI). They demonstrated that a real-time fluorometric reading of FMN levels in the acellular perfusate after 30 minutes of hypothermic perfusion had a strong correlation with posttransplant hospital stay and cumulative complications and was predictive of 90-day graft loss.
FMN, a small molecule bound to the mitochondrial complex I (C-I), oxidizes NADH and generates electrons for ubiquinone reduction.10 During ischemia, the noncovalent bond between the reduced FMN and mitochondrial C-I becomes weaker.11 Upon reperfusion, succinate-supported reverse electron transfer drives FMN dissociation and generates reactive oxygen species.12 The loss of FMN results in a decline of C-I activity and thus mitochondrial dysfunction.11,12
During NMP and NRP, the restoration of perfusion to ischemic organs inevitably causes IRI and FMN release from the damaged mitochondria. Perfusion approaches provide a unique opportunity to objectively evaluate potential organs before implantation. This study retrospectively analyzed FMN levels in perfusate samples collected during NMP (kidneys and lungs) and abdominal NRP (livers) by the research groups in the network of the National Institute for Health Research Blood and Transplant Research Unit in Organ Donation and Transplantation between Cambridge and Newcastle Universities. We aimed to investigate the changes of FMN levels during normothermic reperfusion of kidneys, livers, and lungs and examine whether FMN level in perfusates collected during reperfusion could serve as a biomarker to predict posttransplant allograft quality.
MATERIALS AND METHODS
FMN Measurement Calibration
Riboflavin 5′-monophosphate sodium salt hydrate (Sigma-Aldrick, United States) was dissolved in 0.9% sodium chloride to make a series of FMN solutions with concentrations ranging from 12.5 to 625.0 ng/mL. Two hundred microliters of each solution in triplicates were loaded into a black nonbinding 96-well microplate (Greiner Bio-one, Austria) at room temperature under dimmed light. The microplate was analyzed by fluorescence spectroscopy (Infinite 200 PRO, Switzerland). A monochrome light with excitation wavelength of 450 nm was used and the fluorescence with wavelength of 525 nm emitted by the FMN was recorded with 100% gain. The fluorescence readings in artificial unit (AU) were displayed in an Excel spreadsheet produced by the spectrometer. This experiment was performed 3 times. The average fluorescence readings of the FMN solutions were plotted against their known concentrations to generate a standard curve. The same steps were repeated for the FMN solutions that were stored at 4°C for 1 day and 7 days and at –80°C for 28 days, to assess whether cold storage alters FMN concnentration.
Organ Perfusion Studies
A series of organ perfusion studies were carried out at Cambridge and Newcastle Universities under the research governance infrastructure established by the Blood and Transplant Research Unit. During these studies, as detailed next, perfusate samples were prospectively collected from NMP for kidneys and lungs and NRP for livers in DCD donors. All of the perfusate samples taken were centrifuged immediately after collection. The supernatants were stored at –80°C until analysis.
Normothermic Kidney Perfusion
Eleven single kidneys that were turned down by all UK transplant centers were recruited for the NMP project by the Cambridge group. Apart from 1 pair that were declined by all of the UK transplant centers because of old donor age, the others were all turned down because of poor in situ perfusion. These kidneys were perfused with oxygenated red blood cell–based solution on the NMP circuit for 60 minutes at 35.0°C–36.0°C, according to the previously established protocol.2 Perfusate samples were collected just before and after 60 minutes of NMP, and the perfusion-related parameters, including lactate, potassium, venous pH, and urine output, were recorded. The quality assessment score (QAS), based on macroscopic appearance, mean renal blood flow, and total urine output, was performed at the end of NMP.2 Seven of these kidneys were implanted into recipients. The posttransplant outcomes, such as complications, hospital stay, and follow-up creatinine, were collected from the hospital electronic database. The Remuzzi score of these kidneys was assessed by a consultant pathologist using wedge biopsies taken at the end of cold storage.
Abdominal Normothermic Regional Perfusion
Abdominal NRP was undertaken in 23 DCD donors by the Cambridge group. Following verification of death at least 5 minutes after asystole, a normothermic circulation of oxygenated donor blood was restored to the abdominal organs using an extracorporeal membrane oxygenation circuit. During the 2-hour NRP, abdominal organs were monitored closely, and blood samples were taken every 30 minutes for blood gas and biochemical analysis, as per the protocol published previously.5 A total of 15 livers were accepted for clinical transplantation. FMN levels in the blood samples collected before and at 30, 60, 90, and 120 minutes of NRP were measured. The recipients’ characteristics and posttransplant biochemical results, complications, and outcomes were retrieved. The model for early allograft function (MEAF) score was calculated based on the maximum alanine aminotransferase and international normalized ratio within the first 3 days posttransplant and bilirubin level on the third-day posttransplant.13
Normothermic Lung Perfusion
Eight single lungs that were turned down for transplantation were assessed using ex situ normothermic perfusion by the Newcastle group. Along with the physiological monitoring, the acellular perfusate samples were collected every 30 mintues during perfusion. The samples taken before and at 30, 60, and 90 minutes of the perfusion were used for FMN measurement. As this project was designed for research purposes only, none of these lungs were transplanted.
Ethics
All of the projects had ethical approval granted by regional or national research ethic committees (16/NE/0230 and 15/EE/0175).
FMN Concentration Measurement
After thawing, 200 μL of each samples in triplicates were loaded into the microplate and analyzed by the fluorescence spectrometry using the same settings detailed previously. The fluorescence readings in AU were correlated with the perfusion parameters and clinical data when appropriate.
Statistical Analysis
Shapiro-Wilk test of normality was performed for each set of data. Continuous variables with normal distribution were reported as mean ± SD and non-Gaussian data as median and interquatile range (IQR). To compare the means of continuous variables, 2-sided paired and independent t-tests were used when appropriate. Correlation of the fluorescence readings and other perfusion parameters with posttransplant outcomes were calculated using the Pearson correlation coefficient (r). Sensitivity and specificity of the predictive value of FMN was analyzed using receiver-operating characteristic curve analysis. Binary logistic regression was used to assess the predictive value of FMN for mortality. P values of <0.05 were considered as statistically significant. All statistical analyses were undertaken using the GraphPad Prism 8.0 and IBM SPSS Statistics version 24 64-bit edition.
RESULTS
Fluorescence Spectrometry Measurement of FMN
The fluorescence readings had a linear relationship with the FMN concentrations within the range from 12.5 to 625.0 ng/mL (Figure 1A). These readings did not change significantly after the samples had been stored at 4°C for up to 7 days and –80°C for up to 28 days (Figure 1B). Therefore, FMN levels in the perfusate solutions remain stable during –80°C storage. It is feasible and reliable to measure FMN levels in the perfusate samples collected previously and stored in –80°C freezer.
FIGURE 1.
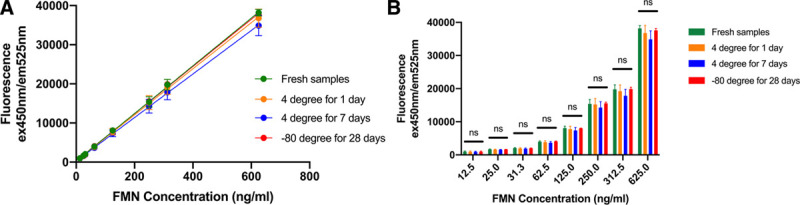
A, Concentration curves of FMN generated by fluorescence spectrometry. B, Comparison of the FMN fluorescence readings of fresh samples, samples stored at 4°C for 1 d and 7 d, and at –80°C for 28 d (n = 3).
Normothermic Kidney Perfusion
The 11 kidneys recruited were from 7 DCD donors (4 males and 3 females) with age of 51.9 ± 18.2 years (Table 1). The mean terminal creatinine level was 68.1 ± 20.4 μmol/L. These kidneys were procured in the standard fashion with cold flush after explantation and preserved with static cold storage. The warm ischemic time and cold ischemic time before the onset of NMP were 14.4 ± 2.7 minutes and 11.1 ± 5.6 hours, respectively. After 1 hour of NMP, the FMN levels in the perfusion circuits increased significantly from 3359 ± 2309 AU to 7815 ± 3386 AU (P = 0.005; 95% confidence interval [CI], 1683-7231 AU) (Figure 2A). However, it had no correlation with QAS assessed at the end of NMP (r = –0.23; P = 0.5) (Figure 2B). The perfusion parameters and QAS of these 11 kidneys were also summarized in Table 1.
Table 1.
Donor characteristics and perfusion-related details of the normothermic kidney perfusion project
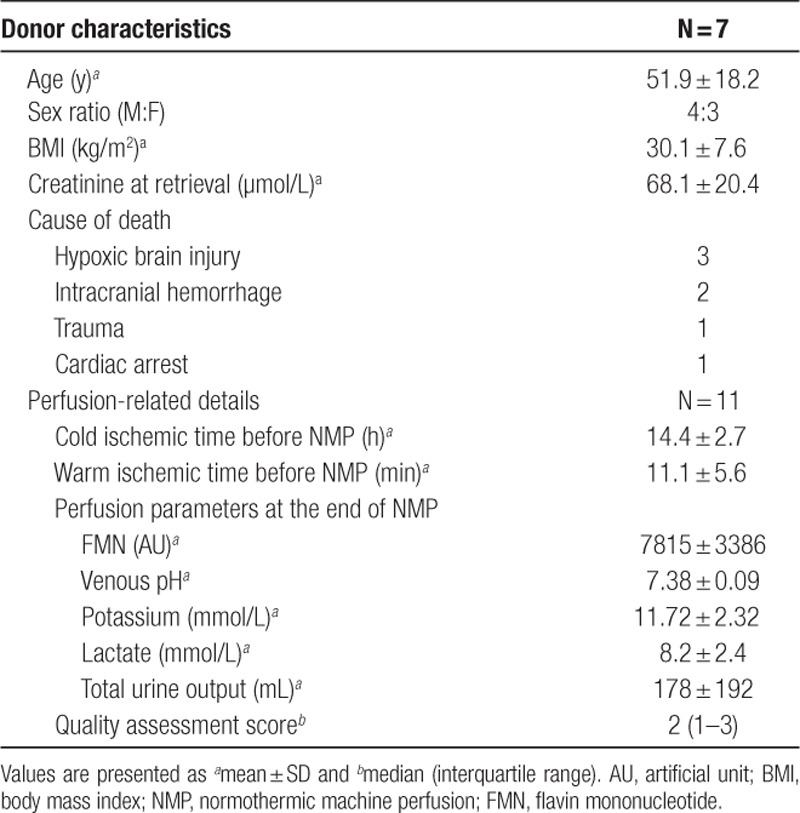
FIGURE 2.
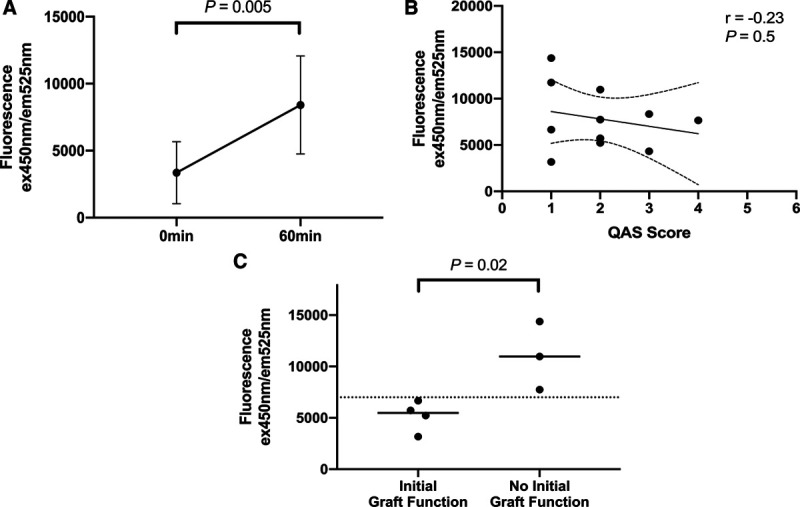
A, The FMN fluorescence readings of the perfusates taken before and at 60 min of normothermic machine perfusion of kidneys (n = 11). B, Correlation of the FMN fluorescence readings in the perfusates at 60 min of NMP with QAS (n = 11). C, Comparison of the FMN levels at 60 min of NMP in the kidneys that had initial function (n = 4) to those of the ones that did not (n = 3). FMN, flavin mononucleotide; NMP, normothermic machine perfusion; QAS, quality assessment score.
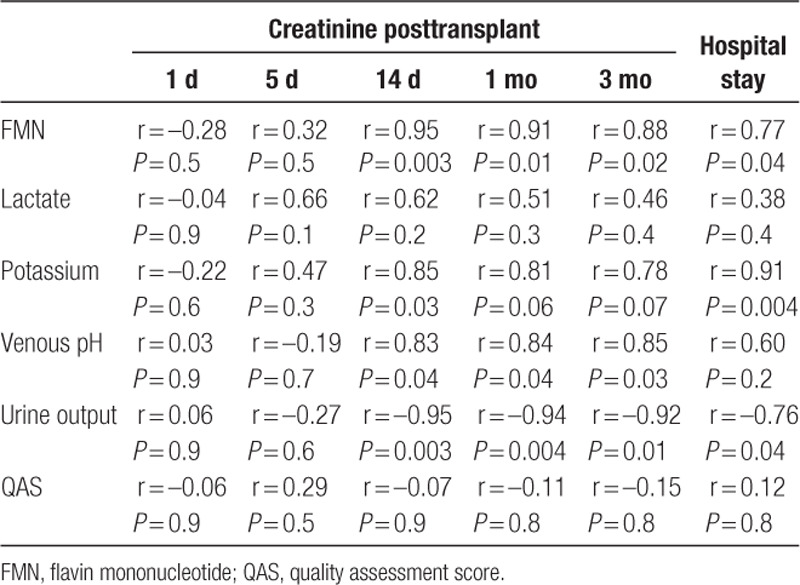
Seven out of the 11 kidneys were deemed suitable for clinical transplantation. All of them had both QAS and Remuzzi socres <3. The 7 recipients (6 males and 1 female) were 47.4 ± 11.5 years old (Table 2). After transplantation, 1 (14.3%) of these 7 kidneys developed delayed graft function and 2 (28.6%) developed primary nonfunction. These 3 kidneys had significantly higher FMN levels at 60 minutes of NMP than the other 4 which had initial graft function (11 029 ± 3315 AU versus 5201 ± 1474 AU; P = 0.02; 95% CI, –1141 to –10 515 AU) (Figure 2C). None of the recipients developed acute rejection. One patient passed away with a functioning allograft on posttransplant day 10, because of myocardial infarction. All of the other patients were followed up for at least 3 months by the transplant team. Among the parameters that were measured at the end of NMP, FMN, potassium level, and urine output were correlated with the recipients’ hospital stay; and FMN, venous pH, and urine output were strongly correlated with their creatinine on 14 days, 1 month, and 3 months posttransplant (Table 3).
Table 2.
Recipient characteristics and posttransplant complications and renal function of the normothermic kidney perfusion project
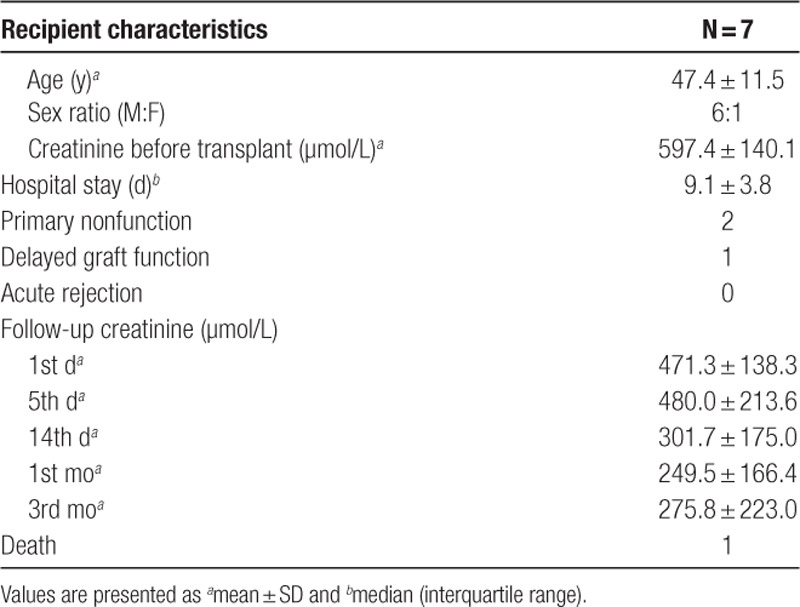
Table 3.
Correlation of the perfusion parameters at the end of normothermic kidney perfusion with the recipients’ hospital stay and renal function posttransplant
Abdominal Normothermic Regional Perfusion
Twenty-three circulatory death donors (17 males and 6 females) aged 50.2 ± 13.9 years underwent NRP, after a median of 13 minutes (IQR, 10–22 min) agonal phase and 15.3 ± 2.9 minutes of asystolic duration (Table 4). During 2 hours of abdominal NRP, FMN levels in the perfusion circuits first peaked at 30 minutes, then reduced at 60 minutes and stabilized afterwards (Figure 3A). The FMN fluorescence reading at 30 minutes of NRP was significantly higher than that of the readings at baseline (27 504 ± 1516 AU versus 20 797 ± 10 759 AU; P = 0.008; 95% CI, –1738 to –10 069 AU). For the 15 livers that were accepted for clinical transplantation, their FMN fluorescence readings at 30 minutes were significantly lower than those of the 8 livers declined (19 628 ± 7456 AU versus 33 368 ± 12 798 AU; P = 0.004; 95% CI, 4802-22 677 AU) (Figure 3B). Higher FMN level at 30 minutes of NRP was predictive of livers being declined for transplantation (area under the receiver-operating characteristic curve [AUC] was 0.85; 95% CI, 0.67-1.00).
Table 4.
Donor characteristics and procurement details of the abdominal normothermic regional perfusion project
FIGURE 3.
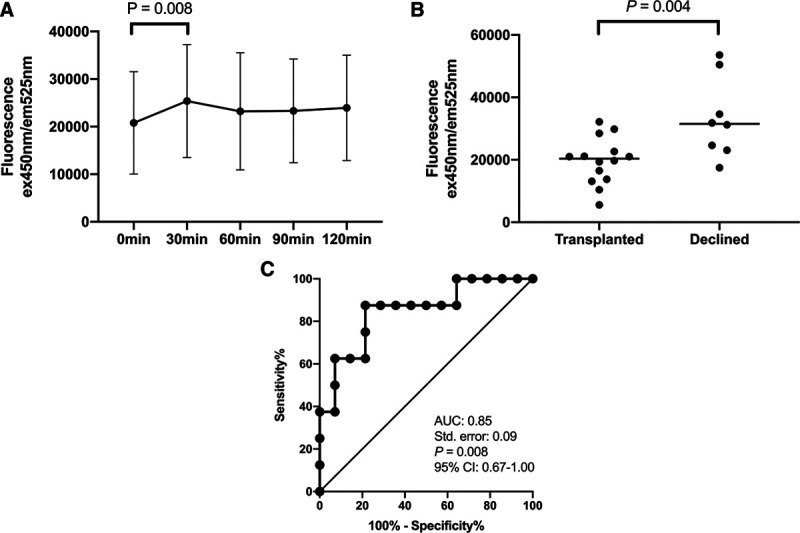
A, The changes in the FMN fluorescence readings during the 2 h of abdominal normothermic regional perfusion. B, Comparison of the FMN levels at 30 min of NRP in the livers that were accepted for transplantation (n = 15) to those of the ones that were declined (n = 8). C, ROC analysis of FMN levels at 30 min of NRP on prediction of livers being accepted for transplantation after NRP. AUC, area under the receiver-operating characteristic curve; CI, confidence interval; FMN, flavin mononucleotide; NRP, normothermic regional perfusion. ROC, receiver-operating characteristic curve.
Table 5 listed the characteristics and posttransplant biochemical results, comoplications, and hospital stay. One patient died on posttransplant day 34 because of graft-versus-host disease. No other severe complications, graft loss, or mortality were reported. FMN reading at 30 minutes of NRP was not correlated with neither lactate (r = –0.16; P = 0.6) or international normalized ratio at 24 hours (r = 0.17; P = 0.6), or peak alanine aminotransferase within 7 days posttransplant (r = –0.08; P = 0.8), nor MEAF score (r = –0.18; P = 0.5). In this cohort, neither FMN reading (odds ratio, 1.00; P = 0.4) nor MEAF score (odds ratio, 1.96; P = 0.4) was predictive of mortality.
Table 5.
Recipient characteristics and posttransplant outcomes of the abdominal normothermic regional perfusion project
Normothermic Lung Perfusion
Eight single lungs from 5 donors were included in this study. The donor ages ranged from 22 to 63 years (median, 52 y; IQR, 40–56 y). Although they represented a heterogenous group of donors with their lungs declined for different reasons, they uniformly release little FMN into the perfusate. The perfusate FMN fluorescence reading was 1149 ± 35 AU before perfusion and gradually increased as the perfusion went on. The FMN fluorescence reading was 1197 ± 37 AU at 30 minutes, 1208 ± 49 AU at 60 minutes, and 1241 ± 53 AU at 90 minutes of normothermic perfusion. Although the FMN levels at 60 and 90 minutes were significantly higher than the baseline level (P values of 0.003 and 0.02, respectively), the differences in the readings were very small.
DISCUSSION
This novel study is the first to examine FMN fluorescence readings in the perfusate samples collected during the NMP and regional perfusion projects of 3 different types of solid organs. The findings demonstrated that FMN release during postischemic reperfusion was consistent with clinical judgment of liver allograft quality and associated with posttransplant renal function in kidney transplantation. FMN measurement is simple and quick to perform, and fluorescence spectrometry is widely available in the biochemistry laboratories in most secondary healthcare facilites. In addition, we have shown that it is a robust molecule whose concentration does not alter after cold storage. If proven and validated as a reliable marker of allograft quality in larger cohorts, it would provide an objective measure to inform decisions about whether or not to transplant an ECD organ during machine perfusion or NRP.
NMP technology has been adopted by many transplant centers to assess potential ECD kidneys, which would otherwise be discarded.2,3,14 For example, in the NMP project done by the Cambridge group, the 7 kidneys would not have been transplanted, if they had not been assessed during NMP. However, ECD kidneys are associated with poorer posttransplant outcomes; as in this project, 3 out of the 7 transplanted kidneys did not have initial function. FMN fluorescence readings in the perfusates at 60 minutes of NMP, albeit a small cohort size, seemed to be able to differentiate kidneys that had initial function from those that did not. Higher release of FMN into perfusates during NMP suggests more extensive mitochondrial dysfunction. The kidney, as the organ with the second highest mitochondrial content, relies on energy produced by adequate amount of functioning mitochondria. Therefore, these kidneys that lost more FMN were expected to have reduced initial function posttransplant. Although the FMN level had no correlation with QAS, in this cohort of kidneys with low QAS, FMN was demonstrated to have better predictive value for posttransplant renal function than QAS. More work is required to validate the predictive power of FMN in larger cohorts, but FMN is a promising biomarker with potential to improve decision-making during NMP.
Muller et al6 demonstrated that with hypothermic oxygenated perfusion after cold storage that livers from DCD donors can be safely implanted in selected recipients with short-term and mediun-term graft survivals similar to that of the standard critieria livers. This work reflects an isolated organ perfusion system.
A period of in situ normothermic perfusion in DCD donors before organ extraction has also been suggested to reverse the deleterious effect of warm ischemia on solid organs.5 During NRP, retrieval surgeons have the opportunity to fully inspect and assess the organs and prepare for procurement without undue pressure to minimize warm ischemic time. The findings that the livers declined for transplantation had higher FMN fluorescence readings at 30 minutes of NRP suggested that more FMN was released from livers perceived as poor quality based on surgeons’ clinical experience. However, during NRP, other abdominal organs also contribute FMN to the perfusion circuit. This might be the reason that the FMN level at 30 minutes of NRP was not associated with posttransplant liver function, in contrast with the findings reported in the isolated circuit by Muller et al.6 Interestingly, the FMN levels in the NRP circuit reduced after reaching a peak at about 30 minutes. One possible explanation was that NRP for longer than 30 minutes partially repaired the IRI-induced mitochondrial damage and thus reduced mitochondrial dysfunction in the donor organs. Further investigation is required to explore this speculation.
In contrast to kidneys and livers, lungs did not release much FMN during NMP, and the amounts released were consistent despite the drastic difference in donor characteristics. Lungs have strikingly low-metabolic activity, reflected in a far smaller mitochondrial mass, compared with metabolically very active organs such as kidneys and livers. In addition, the presence of oxygen in the partially inflated lungs may ameliorate or even abolish any true ischemia during static cold storage.
This study is limited by the small sample sizes, its retrospective nature, and the number of available perfusion and posttransplant parameters. In the future, the exact concentration of FMN in perfusate samples, assessed by the point-of-care fluorescence spectrometry, will be confirmed using high-performance liquid chromatography mass spectrometry. It is also unclear how the preexisting FMN in the donor’s blood and the packed red blood cells used for blood-based perfusion influence the release of FMN from damaged mitochondrial C-I. In future organ reperfusion projects, one could consider the use of hemoglobin-based oxygen carriers or other acellular perfusates to minimize the background FMN level.
In conclusion, FMN analysis is quick to perform, widely accessible, and consistent in spite of cold storage. Its concentration during kidney NMP, as a point-of-care measurement, has the potential to predict posttransplant renal function and facilitate safe and reliable assessment of kidneys before transplantation. A more detailed examination of the association between FMN levels in the perfusates and posttransplant outcomes in larger cohorts is required to confirm and validate the role of FMN as a putative biomarker of allograft quatliy.
ACKNOWLEDGMENTS
The research was funded by the NIHR BTRU in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the author(s) and not necessarily those of the NIHR, the Department of Health, and Social Care or NHSBT. The authors are grateful to the research staff in the BTRU and theater teams at the Cambridge University Hospitals and Newcastle upon Tyne Hospitals for their contribution to the perfusion projects. We thank all the organ donors and their families for their donation and support to organ transplantation and research. We would like to extend our sincere gratutide to Prof. Philipp Dutkowski and his team in Zurich, Switzerland, for sharing with us their knowledge and experience with FMN as a biomarker.
Footnotes
Published online 21 August, 2020.
The research was funded by the National Institute for Health Research (NIHR) Blood and Transplant Research Unit (BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT).
The authors declare no conflicts of interests.
L.W. and J.H.D. contributed to the conception and design of the study. E.T., L.B., T.L.P., S.A.H., M.L.N., C.J.E.W., C.W., A.J.F., and S.A. contributed to sample collection, data acquisition, and interpretation. L.W. performed the experiments and statistical analysis, and wrote the article. All authors reviewed and revised the article and approved the final version of the article. All authors agree to be accountable for all aspects of the work and resolve any questions related to the accuracy of the article.
REFERENCES
- 1.NHS: Blood and Transplant. Annual Activity Report. Available at https://www.odt.nhs.uk/statistics-and-reports/annual-activity-report/. Accessed April 2020.
- 2.Hosgood SA, Thompson E, Moore T, et al. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br J Surg. 2018; 105:388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hameed AM, Lu DB, Patrick E, et al. Brief normothermic machine perfusion rejuvenates discarded human kidneys. Transplant Direct. 2019; 5:e502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreasson AS, Dark JH, Fisher AJ. Ex vivo lung perfusion in clinical lung transplantation–state of the art. Eur J Cardiothorac Surg. 2014; 46:779–788 [DOI] [PubMed] [Google Scholar]
- 5.Oniscu GC, Randle LV, Muiesan P, et al. In situ normothermic regional perfusion for controlled donation after circulatory death–the United Kingdom experience. Am J Transplant. 2014; 14:2846–2854 [DOI] [PubMed] [Google Scholar]
- 6.Muller X, Schlegel A, Kron P, et al. Novel real-time prediction of liver graft function during hypothermic oxygenated machine perfusion before liver transplantation. Ann Surg. 2019; 270:783–790 [DOI] [PubMed] [Google Scholar]
- 7.Moeckli B, Sun P, Lazeyras F, et al. Evaluation of donor kidneys prior to transplantation: an update of current and emerging methods. Transpl Int. 2019; 32:459–469 [DOI] [PubMed] [Google Scholar]
- 8.Guy AJ, Nath J, Cobbold M, et al. Metabolomic analysis of perfusate during hypothermic machine perfusion of human cadaveric kidneys. Transplantation. 2015; 99:754–759 [DOI] [PubMed] [Google Scholar]
- 9.Aleksova N, Alba AC, Molinero VM, et al. Risk prediction models for survival after heart transplantation: A systematic review. Am J Transplant. 2020; 20:1137–1151 [DOI] [PubMed] [Google Scholar]
- 10.Hirst J. Mitochondrial complex I. Annu Rev Biochem. 2013; 82:551–575 [DOI] [PubMed] [Google Scholar]
- 11.Kahl A, Stepanova A, Konrad C, et al. Critical role of flavin and glutathione in complex i-mediated bioenergetic failure in brain ischemia/reperfusion injury. Stroke. 2018; 49:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepanova A, Sosunov S, Niatsetskaya Z, et al. Redox-dependent loss of flavin by mitochondrial complex I in brain ischemia/reperfusion injury. Antioxid Redox Signal. 2019; 31:608–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pareja E, Cortes M, Hervás D, et al. A score model for the continuous grading of early allograft dysfunction severity. Liver Transpl. 2015; 21:38–46 [DOI] [PubMed] [Google Scholar]
- 14.Kabagambe SK, Palma IP, Smolin Y, et al. Combined ex vivo hypothermic and normothermic perfusion for assessment of high-risk deceased donor human kidneys for transplantation. Transplantation. 2019; 103:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]


