SPG11-HSP is the commonest form of complicated autosomal recessive hereditary spastic paraplegia. Pozner et al. review current knowledge of SPG11, focusing on clinical and cellular aspects of the pathology, and consider how impairment of the autophagy-lysosomal machinery may link neurodevelopmental and neurodegenerative phenotypes.
Keywords: SPG11, hereditary spastic paraplegia, neurodevelopment, neurodegeneration, autophagy
Abstract
Hereditary spastic paraplegia (HSP) is a heterogeneous group of rare motor neuron disorders characterized by progressive weakness and spasticity of the lower limbs. HSP type 11 (SPG11-HSP) is linked to pathogenic variants in the SPG11 gene and it represents the most frequent form of complex autosomal recessive HSP. The majority of SPG11-HSP patients exhibit additional neurological symptoms such as cognitive decline, thin corpus callosum, and peripheral neuropathy. Yet, the mechanisms of SPG11-linked spectrum diseases are largely unknown. Recent findings indicate that spatacsin, the 280 kDa protein encoded by SPG11, may impact the autophagy-lysosomal machinery. In this update, we summarize the current knowledge of SPG11-HSP. In addition to clinical symptoms and differential diagnosis, our work aims to link the different clinical manifestations with the respective structural abnormalities and cellular in vitro phenotypes. Moreover, we describe the impact of localization and function of spatacsin in different neuronal systems. Ultimately, we propose a model in which spatacsin bridges between neurodevelopmental and neurodegenerative phenotypes of SPG11-linked disorders.
Introduction
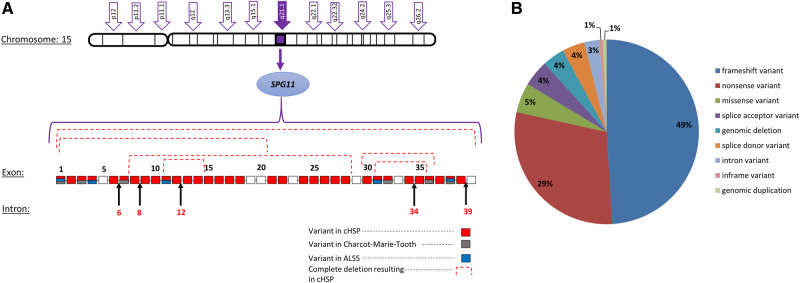
The human SPG11 gene is located on chromosome 15q21.1 and spans an 8-kb region that encompasses 40 exons encoding spatacsin (Fig. 1A). More than 100 pathogenic variants in SPG11 have been identified throughout the gene without any evidence for clustering in mutational ‘hot spots’ (Denora et al., 2016). The vast majority of SPG11 mutations are frameshift or nonsense mutations, suggesting truncation of the protein encoded by SPG11 and in all likelihood, loss of function (Fig. 1B).
Figure 1.
Genetic heterogeneity in SPG11-HSP. (A) Schematic representation of the SPG11 gene. The specific pathogenic variants are represented in different colours, indicating the specific neurological disorder. (B) Pie chart representing the distribution of SPG11 mutations. Data retrieved from ClinVar and Locus Specific Mutation Databases. cHSP = complicated HSP.
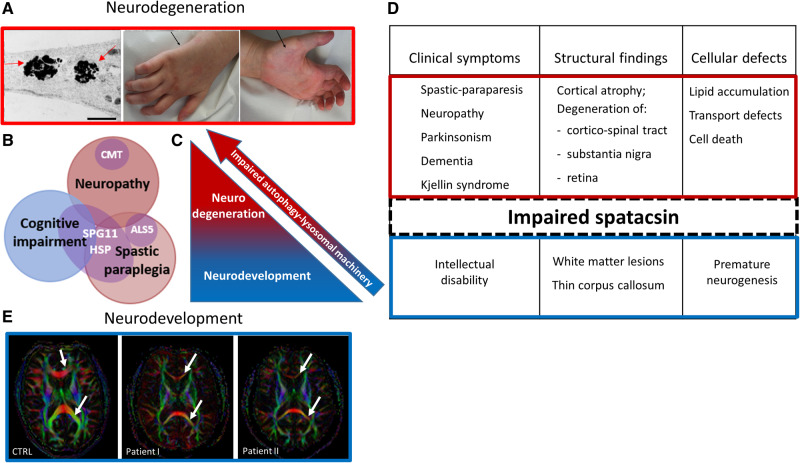
The molecular aspects of spatacsin have predominantly been obtained by studying its interaction partners in non-neuronal cells. However, because of the prominent involvement of the central and peripheral nervous systems in hereditary spastic paraplegia type 11 (SPG11-HSP), this update focuses on data from neuronal models in correlation with clinical phenotypes of neurodevelopment and degeneration (Fig. 2).
Figure 2.
Spatacsin as a mediator of the two distinct stages of SPG11-HSP pathogenesis. (A) Representation of neurodegenerative pathology in SPG11-HSP patients. Membranous inclusions (indicated by the arrows) are accumulated in the neurites of induced pluripotent stem cell-derived cortical neurons [left; adapted from Pozner et al. (2018)]. Distal neuropathy in SPG11-HSP characterized by amyotrophy of intrinsic hand muscles (arrows) and by claw hands (middle and right). (B) Heterogeneity and clinical overlap of SPG11-spectrum disorders. (C and D) Schematic representation of spatacsin-linked pathology. According to the proposed mechanism, impaired spatacsin leads to the failure of autophagy-lysosomal machinery, causing neurodevelopmental defects (blue) and over time, resulting in neurodegeneration (red). (E) Representation of neurodevelopmental pathology in SPG11-HSP patients. High-field diffusion tensor imaging (DTI) with calculation of axial fractional anisotropy maps demonstrating the thin corpus callosum as the major phenotypic hallmark of SPG11-HSP compared to controls (3 T MRI). Upper and lower arrows indicate genu and splenum of the corpus callosum, respectively, which are of significantly smaller diameter in SPG11-HSP. Scale bar = 1 μm.
SPG11-HSP
Bi-allelic pathogenic variants in SPG11 most frequently cause complicated HSP (Fig. 1B). They are also the most common cause of complicated autosomal recessive HSP, followed by pathogenic variants in SPG15, now known as ZFYVE26. Patients with SPG11-HSP show progressive spasticity and paraparesis as predominant symptoms. Additional symptoms can be classified according to CNS or peripheral nervous system (PNS) involvement. CNS-related symptoms comprise cognitive deterioration, parkinsonism, psychosis, and visual impairments, whereas PNS-linked symptoms include neuropathy and sphincter disturbance; other signs such as peripheral lymphoedema and obesity are also often present (Winner et al., 2004; Hehr et al., 2007; de Bot et al., 2013; Schule et al., 2016; Faber et al., 2018a).
Within the clinical spectrum of SPG11 mutations, no clear-cut genotype-phenotype correlation exists to explain the specific functional implications of single mutations depending on their localization within the gene.
Differential diagnosis of SPG11-HSP
As the observed symptoms and age at onset are highly variable in overall HSP, the differential diagnosis is broad and has been discussed in detail elsewhere (Fink, 2014). In turn, SPG11-HSP presents as an early-onset motor neuron disease with complicating symptoms that may steer the differential diagnosis.
Unlike other complicated types of HSP, SPG11-HSP frequently presents with parkinsonism in addition to the upper motor neuron phenotype, i.e. as a ‘pallido-pyramidal syndrome’ (PPS). The PPS comprises different multisystem neurodegenerative disorders. It may be caused by several types of genetic Parkinson’s disease, by genetic neurometabolic disorders, by neurodegeneration with brain iron accumulation (NBIA), other rare neurogenetic diseases, and by atypical Parkinson’s disease (Tranchant et al., 2017). Similar to SPG11-HSP, most of the mechanisms in PPS are linked to lysosomal pathology and may present during neurodevelopment and neurodegeneration (Ysselstein et al., 2019). α-Synuclein pathology is observed in atypical Parkinson’s disease and in some genetic causes of PPS, including Kufor-Rakeb syndrome and Gaucher’s disease. Whether α-synucleinopathy is also present in SPG11-HSP remains to be determined.
Mild cases of cerebral palsy may also present as lower limb spasticity along with additional neurological symptoms (Ashwal et al., 2004). Differential diagnosis relies on a history of pregnancy and delivery, cerebral imaging studies, asymmetry of symptoms and rate of progression.
Mutations in SPG11 have also been found in cases of early-onset autosomal recessive amyotrophic lateral sclerosis (termed ALS5) (Orlacchio et al., 2010). However, the clinical classification distinguishing ‘HSP with neuropathy’ from ALS constitutes a spectrum, highlighting the need for exact and standardized clinical characterizations of patients for the future genotype-phenotype correlations (Fig. 2B and E).
In the case series describing patients with autosomal recessive Charcot-Marie-Tooth (ARCMT) disease, presenting with pure lower motor neuron phenotype, SPG11 mutations were found in 43% of the patients, appearing as a significant cause for the disease (Montecchiani et al., 2016). None of these patients exhibited signs of spasticity, retinal disease or cerebellar dysfunction (Montecchiani et al., 2016). In other types of HSP, the shared vulnerability of both corticospinal motor neurons and of spinal motor neurons is rarely observed. Thus, SPG11-HSP should be routinely tested in ARCMT disease. Of note, biallelic pathogenic variants in ZFYVE26/SPG15 (encoding spastizin) and SPG48 (now known as AP5Z1; encoding ZNF267) cause autosomal recessive complicated HSP. According to the published case reports, these are clinically undistinguishable from SPG11 but are observed at lower frequencies (Erfanian Omidvar et al., 2019). As a result of the limited number of reports, it remains unclear whether the clinical spectra of SPG11, ZFYVE26/SPG15 and AP5Z1/SPG48 are truly identical or whether there are predominant symptoms in any of the three entities (Pensato et al., 2014). Because of the lack of genotype-specific symptoms, the genetic work-up of autosomal recessive HSP with complicating symptoms should include the analysis of ZFYVE26/SPG15 and AP5Z1/SPG48 genes in SPG11-negative cases.
Clinical and morphological characteristics of impaired neurodevelopment in SPG11-HSP
In comparison to pure HSP, complex SPG11-HSP starts earlier in life, with a peak age of diagnosis in the second decade of life (Winner et al., 2004; Schule et al., 2016). The clinical manifestation encompasses mostly subtle childhood-onset neurodevelopmental deficits, culminating in a broad range of symptoms, from subtle learning difficulties to severe intellectual disability (Siri et al., 2010). Many families report poor school performance preceding gait abnormalities (Faber et al., 2016). Because of the wide range of phenotypes, many patients remain undiagnosed during early childhood when motor symptoms remain mild or are not yet present.
On a structural level, a thin corpus callosum is the major phenotypic hallmark of the disorder (Fig. 2E). Most patients with a thin corpus callosum show a hyperintensity of the forceps minor of the corpus callosum, which has been termed the ‘ears of the lynx’ sign (Riverol et al., 2009; Pascual et al., 2019). Moreover, transcranial magnetic stimulation reveals impairment of transcallosal inhibition, reflecting functional impairments of cortico-cortical projections (Winner et al., 2006).
It remains unclear whether the thin corpus callosum phenotype is a result of congenital thinning/hypoplasia or whether it is related to progressive atrophy (Nakamura et al., 1995; Teive et al., 2001). Several reports support the possibility of congenital processes, due to unchanged thickness of the thin corpus callosum during follow-up examinations (Nakamura et al., 1995; Teive et al., 2001; Sperfeld et al., 2004). Others suggest that the thin corpus callosum is due to neurodegeneration, occurring as a secondary process to the gliosis in white matter (Casali et al., 2004; Hourani et al., 2009). The main challenge of addressing this question is the frequent delay of genetic diagnosis until motor symptoms occur. As the clinical observations may not be sufficient to determine the nature of the thin corpus callosum, experimental models will be needed. Experiments to date support the idea of a neurodevelopmental origin of a thin corpus callosum that worsens over time due to the degeneration of projection neurons. Thus, the premature neurogenesis observed in an SPG11-HSP patient-derived induced pluripotent stem cell (iPSC) 2D and 3D neuronal model, combined with reduced neuronal complexity, may account for the potential vulnerability of the projection neurons, resulting in a thin corpus callosum (Perez-Branguli et al., 2014, 2019). Moreover, one SPG11 in vivo murine model exhibited thinning of the corpus callosum after only 4 months, prior to general brain atrophy (Branchu et al., 2017).
In addition to a thin corpus callosum, mild to severe enlargement of the lateral ventricles has been observed in the majority of patients (Pensato et al., 2014). White matter lesions in the frontal, occipital, temporal and periventricular regions were found in a subset of patients (Winner et al., 2004; Stevanin et al., 2007; Denora et al., 2016; da Graca et al., 2018; Faber et al., 2018a). Additional longitudinal clinical evaluation revealed differences between grey and white matter involvement. Thus, while the cortex and the spinal cord progressively deteriorated, diffusion tensor imaging (DTI) revealed only static involvement of the white matter (Faber et al., 2018a). This observation suggests a neurodevelopmental origin of white matter impairments, linking it to imaging results found in leukodystrophies. Yet, there is no evidence of defects in the myelin sheath-forming cells, oligodendrocytes and Schwann cells in SPG11-HSP, suggesting that the white matter deficits may result from a ‘dying back’ of the axons.
There is also indirect evidence for neurodevelopmental deficits in SPG15-HSP: age at onset occurs as early as in SPG11 and thinning of the corpus callosum and periventricular white matter hyperintensity along with cognitive impairment is present (Pensato et al., 2014). However, these developmental abnormalities were not observed in a Zfyve26/Spg15 knock-out mouse model and should therefore be further assessed in the future, in different models (Khundadze et al., 2013).
Taken together, the currently available data suggest that progressive cognitive decline in SPG11-HSP patients is at least partially of neurodevelopmental origin.
Clinical and morphological characteristics of neurodegeneration in SPG11-HSP
The progressive nature of SPG11-HSP, combined with observations of severe motor neuron degeneration in patients, render neurodegeneration a prominent characteristic of the disease (Winner et al., 2004). The ongoing neurodegeneration affects both the CNS and PNS.
The predominant SPG11-HSP symptoms of spasticity and paraplegia are attributed to axonal degeneration in the corticospinal tract and peripheral nerves (Winner et al., 2004). Indeed, clinical reports affirm that progressive upper and lower motor neuron degeneration occurs in SPG11-HSP (Stevanin et al., 2008; Orlacchio et al., 2010; Iskender et al., 2015; Denora et al., 2016). The severity of the reduction of spinal cord volume in SPG11-HSP patients correlates with the duration of the disease.
The frequently observed motor neuropathy is remarkably similar to that observed in slowly progressive ALS (Faber et al., 2018a). In addition to clinical similarities with ALS, neurons from the medulla oblongata and spinal cord of SPG11-HSP patients accumulate lysosome-like structures (Denora et al., 2016). These clinical and neuropathological findings are in agreement with reports of juvenile ALS caused by SPG11 mutations (Orlacchio et al., 2010; Daoud et al., 2012).
Aside from motor neuron degeneration, initial imaging studies identified increasing grey matter atrophy in the basal ganglia, the thalamus, and the precentral gyri (Hehr et al., 2007; Franca et al., 2012). Recent findings support a more widespread involvement of grey matter in the disease, including the amygdala, red nucleus and nucleus accumbens, as well as the superior temporal sulcus, cingulum, parahippocampal, parietal and paracentral regions (Faber et al., 2018a). The extent of cortical atrophy renders it challenging to associate the damage to a distinct region. However, it may account for the progressive nature of the cognitive decline occurring in SPG11-HSP. Indeed, dementia has previously been reported in a number of SPG11-HSP patients (Fraidakis et al., 2016; Faber et al., 2018a).
A clinical study on a large patient cohort consisting of 22 SPG11 mutation carriers, reported degeneration of the substantia nigra and reduced dopamine transporter density as common findings in SPG11-HSP (Faber et al., 2018b). The latter was indicative of a disruption of presynaptic dopaminergic pathways even in patients without extrapyramidal motor symptoms (Faber et al., 2018b). These observations are in line with reports of parkinsonism in SPG11-HSP patients, partly as a presenting symptom and partly with a positive l-DOPA response (Anheim et al., 2009; Paisan-Ruiz et al., 2010; Guidubaldi et al., 2011; Vanderver et al., 2012; Wijemanne et al., 2015).
Interestingly, central retinal degeneration has also been reported in SPG11-HSP, adding a neuro-ophthalmological aspect termed Kjellin syndrome to the complex phenotype of the disease (Orlen et al., 2009). In this case, retinal impairment becomes evident after the onset of spastic paraplegia (Puech et al., 2011).
All of these clinical symptoms of progressive neurodegeneration have also been described in ZFYVE26/SPG15 and partly also in AP5Z1/SPG48 patients, further emphasizing the large clinical overlap of these related entities (Hanein et al., 2008; Kara et al., 2016). The fact that, unlike in SPG11-HSP, pathogenic variants of ZFYVE26/SPG15 were not detected in a case series on patients with autosomal recessive pure neuropathy without pyramidal symptoms, may be at least partially explained by the low frequency of ZFYVE26/SPG15 mutations (Montecchiani et al., 2016).
Spatacsin: spatio-temporal expression and function
To understand the cellular dysfunction involved in the pathogenesis of the neurodevelopmental and neurodegenerative features of SPG11-HSP, we focus on spatacsin, the protein encoded by SPG11.
Spatacsin is a large protein (2443 amino acids; ∼280 kDa), which is highly conserved across vertebrates. Human spatacsin shares 85%, 76% and 73% sequence identity with its homologues in dogs, mice and rats, respectively (Stevanin et al., 2008). According to in silico analyses, spatacsin is predicted to contain a short-coiled coil domain, a glycosyl hydroxylase F1 signature, a leucine zipper, a Myb domain and four transmembrane domains (Paisan-Ruiz et al., 2008). Spatacsin is associated with other proteins linked to HSP, i.e. spastizin (encoded by ZFYVE26/SPG15) and the fifth adaptor protein complex (AP-5), which is localized to the late endosomal compartment (Slabicki et al., 2010; Hirst et al., 2011, 2013; Murmu et al., 2011; Chang et al., 2014).
Expression of spatacsin in the developing and adult brain
Existing data on the localization of spatacsin are scarce and inconsistent. The major reason for reported discrepancies in the protein expression is a lack of specific antibodies. The development of alternative techniques to reliably detect the protein expression of spatacsin is crucial to enable the study of its temporal and spatial expression, function and interpretation of disease phenotypes.
The available data from different experimental species indicate a widespread expression of spatacsin in the CNS during development. In vivo rodent studies demonstrate strong and ubiquitous expression of spatacsin during embryonic development in the brain, spinal cord and in regions outside the CNS of rat and mouse models (Stevanin et al., 2007; Murmu et al., 2011; Perez-Branguli et al., 2014). Similarly, SPG11 mRNA is highly expressed in zebrafish throughout embryogenesis, from the one-cell stage to at least 3 days of development (Southgate et al., 2010; Martin et al., 2012).
In adults, studies regarding spatacsin expression have mostly been confined to the CNS because of its prominent involvement in SPG11-HSP. The first study to examine spatacsin expression in the brain indicated strong mRNA expression in the human cerebral cortex and cerebellum (Stevanin et al., 2007). In the mouse cortex, spatacsin expression was restricted to neurons and was not present in astrocytes (Perez-Branguli et al., 2014). Overall, in the CNS, regions with a high density of neurons tend to exhibit the strongest expression of spatacsin as it is expressed mainly in neuronal cells.
On a subcellular level, spatacsin was detected in axons and dendrites of both mouse cortical neurons and human embryonic stem cell (HUES6)-derived cortical neurons (Perez-Branguli et al., 2014). In both neuronal models, spatacsin was found in the cytosol and it co-localized with microtubules, actin, and synaptic markers (Perez-Branguli et al., 2014). In non-neuronal models (e.g. fibroblasts or HeLa cells; Hirst et al., 2011, 2013, 2015) spatacsin co-localized to late endosomes/lysosomes suggesting a role in vesicular trafficking.
Role of spatacsin during development
The first evidence for the functional involvement of spatacsin in neurodevelopment originated from experiments with embryonic zebrafish. Morpholino knock-down of SPG11 led to developmental defects, which included locomotor abnormalities, aberrant branching of spinal cord motor neurons at the neuromuscular junction (Martin et al., 2012) and impaired neuronal differentiation (Southgate et al., 2010). Similarly, a recent Spg11 knock-out mouse model exhibited early-onset cognitive and motor defects which were linked to a failure of lysosomal lipid clearance, and subsequent lysosomal accumulation of lipids in neurons (Branchu et al., 2017).
In humans, a major step towards understanding human neurodevelopment resulted from an investigation of iPSC-derived neural progenitor cells (NPCs) from SPG11-HSP patients. SPG11-NPCs exhibited cell cycle alterations of reduced S and G2/M phases, pointing towards decreased neural proliferation (Mishra et al., 2016). The underlying mechanism was attributed to impairments of the Wnt/β-catenin signalling pathway, which has a prominent role during neural development (Clevers, 2006). In SPG11-NPCs, over-activation of GSK3β resulted in a reduction of β-catenin levels, which in turn compromised cellular proliferation (Mishra et al., 2016). A follow-up study revealed an increased asymmetric division rate of patients’ NPCs as the underlying cause of the observed proliferation deficits in cerebral organoids (Perez-Branguli et al., 2019). SPG11-NPCs undergo premature neurogenesis resulting in a smaller size of SPG11 cerebral organoids (Perez-Branguli et al., 2019). In both experimental models, these neurodevelopmental defects were rescued upon application of tideglusib, a specific GSK3 inhibitor (Mishra et al., 2016; Perez-Branguli et al., 2019).
Role of spatacsin during neurodegeneration
To date, two different Spg11 knock-out mouse models have been published. While in the first model (Varga et al., 2015) spatacsin was disrupted by inserting a gene-trap cassette in the first intron of Spg11, the recent model (Branchu et al., 2017) was generated by inserting a stop codon in exon 32 of the gene. Both models exhibit neurodegenerative features, including a neuronal loss in the motor cortex and the cerebellum (Varga et al., 2015; Branchu et al., 2017). However, the latter (Branchu et al., 2017) exhibits early symptoms and more extensive signs of neurodegeneration, e.g. lower motor neurons, muscular atrophy, and memory deficits. The differences are consistent with the vast heterogeneity reported in human phenotypes. However, the different strategies of gene knock-out may at least partially account for this difference.
A follow-up study using the recent mouse model (Branchu et al., 2017) identified gangliosides as the lipid type accumulating in the lysosomes, resulting in neurodegeneration that was rescued by inhibition of ganglioside synthesis (Boutry et al., 2018). Similarly, the inhibition of ganglioside synthesis by miglustat improved the motor phenotype of the SPG11 zebrafish model (Boutry et al., 2018).
Additional evaluation of cellular models derived from the Spg11 knock-out mouse (Branchu et al., 2017) linked the loss of spatacsin to lysosomal cholesterol accumulation, eventually leading to reduced level of cholesterol in the plasma membrane, resulting in impaired calcium homeostasis (Boutry et al., 2019). Both cellular phenotypes—altered cholesterol distribution and calcium dysregulation—are known to be tightly linked to neurodegeneration (Mattson, 2007; Zundorf and Reiser, 2011; Arenas et al., 2017).
Further insights into spatacsin-linked neurodegeneration were obtained from in vitro measurements of axonal transport. Anterograde axonal trafficking of synaptophysin-positive vesicles was significantly reduced in SPG11-patient-derived neurons, as well as in SPG11-silenced mouse cortical neurons (Perez-Branguli et al., 2014). The observation of axonopathy was supported by the downregulation of axonal genes, decreased neuritic complexity and membranous inclusions within the axons (Perez-Branguli et al., 2014). Defects in neuritic complexity were also present in a model of patient iPSC-derived cortical neurons and in CRISPR-Cas9-mediated SPG11 knock-out lines (Pozner et al., 2018). SPG11-HSP neuronal cells also exhibited increased cell death and accumulation of membranous inclusions, all of which were rescued by the application of the previously mentioned GSK3 inhibitor, tideglusib (Pozner et al., 2018). Thus, GSK3 inhibition may constitute a first therapeutic target in SPG11 related disorders.
Impaired autophagy-lysosomal machinery: linking neurodevelopmental and neurodegenerative phenotypes
The most prominent defects observed in different models of SPG11-HSP are connected to autophagy (Ebrahimi-Fakhari et al., 2016). Neuropathological studies revealed ubiquitin and p62 positive granules in medulla oblongata and spinal cord neurons of SPG11-HSP patients (Denora et al., 2016) and an increase in p62 protein is usually correlated with a reduction in autophagy (Zatloukal et al., 2002). Correspondingly, both SPG11 mousee models exhibited autophagic defects (Varga et al., 2015; Branchu et al., 2017).
The mechanism underlying this phenomenon can be attributed to defects in the autophagic lysosomal reformation (ALR) process, which is crucial for lysosomal homeostasis during autophagy (Yu et al., 2010). Spatacsin, together with its interaction partner spastizin, has been implicated in ALR initiation (Chang et al., 2014). ALR impairment might also explain the observations of increased autophagosome accumulation and lysosomal enlargement frequently reported in SPG11 models (Chang et al., 2014; Renvoise et al., 2014; Varga et al., 2015). Interestingly, zebrafish oocyte model with mutated homologue of ZFYVE26/SPG15, named souffle (suf) accumulated immature secretory precursors due to the lack of separated clathrin-coated buds (Kanagaraj et al., 2014). Thus, a general failure of secretory vesicle formation mechanism may be the underlying cause of the observed ALR impairment.
The observed lysosomal aberration and subsequent lipid accumulation may be caused by spatacsin’s role in intracellular cargo trafficking, mediated through its interaction with spastizin and AP-5. AP-5, in turn, has been implicated in the endosome to trans-Golgi network recycling of the cation independent mannose-6-phosphate receptor (CIMPR), a protein crucial for targeting lysosomal enzymes to the lysosomes (Hirst et al., 2018). Indeed, blocking of the endosome to trans-Golgi network transport resulted in lipid accumulation reminiscent of SPG11-associated neurodegenerative processes (Lin et al., 2018; Eising et al., 2019).
Dysfunction of the autophagy-lysosomal machinery may mediate the pathogenic effects of SPG11 mutations in both neurodevelopment and neurodegeneration (Fig. 2C). As neurons are post-mitotic cells, they are highly dependent on the process of autophagy for proper function and are therefore extremely vulnerable to an aberration of this process. Interestingly, an altered lysosomal function has also been reported in pure types of hereditary spastic paraplegia, including SPAST, ATL1 and REEP1 (Allison et al., 2017). Lysosomal pathology has also been implicated in most of the corresponding mechanisms of PPS (Ysselstein et al., 2019). Overall, the damage is cumulative and the detrimental consequences of the impairment become evident with the progression of the disease.
Conclusions
In SPG11-HSP the neurodevelopmental origins of the disorder are evident in the juvenile stage of a patient’s life and, over time, give rise to neurodegeneration.
Pathogenic variants in the SPG11 gene encoding spatacsin are the most common cause of complicated autosomal recessive HSP, accounting for ∼20% of cases with complicated HSP. Existing in vivo and in vitro models (Tables 1 and 2) link the protein function to development and neurodegeneration through its role in the lipid clearance process, which is mediated by a proper function of autophagic-lysosomal machinery (Fig. 2C and D). However, many questions regarding the exact localization and function of the protein remain unclear, mainly as a result of the difficulty in detecting the protein and limited knowledge of its structure. The development of alternative techniques to reliably detect the protein expression of spatacsin is crucial to enable an analysis of its temporal and spatial expression, determine its function and interpret disease phenotypes.
Table 1.
Genetic models of SPG11-related pathology
| Species | Genetic modification/mutation | Cellular phenotype | Affected cells | Affected regions | Thin corpus callosum | Cognitive impairment | Motor impairment | Neuro- developmental defects | Neuro- degenerative defects | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | Cre-loxP system: exon 32 | Lysosomal lipid accumulation, dystrophic axons, impaired calcium homeostasis | Cortical neurons, spinal motor neurons | Motor cortex, cerebellum, hippocampus, cortico-spinal tract | Yes | Yes | Yes (early onset) | Yes | Yes | Branchu et al., 2017; Boutry et al., 2018, 2019 |
| Gene trap cassette: intron 1 | Autophagy-lysosomal impairments | Cortical neurons, Purkinje cells | Motor cortex, cerebellum | No | ND | Yes (late onset) | No | Yes | Varga et al., 2015 | |
| Zebrafish | Morpholino knock-down: exon 26–intron 26 junction | Axonal growth defect, lipid accumulation | Spinal motor neurons | Hindbrain | – | ND | Yes (early onset) | Yes | Yes | Martin et al., 2012; Boutry et al., 2018 |
| Morpholino knock-down: exon 2–intron 2 and exon 4–intron 4 junctions | Impaired neuronal differentiation | Spinal motor neurons | Brain ventricles, hindbrain, eyes | – | ND | Yes (early onset) | Yes | No | Southgate et al., 2010 | |
| Human nerve biopsies | Frameshift, nonsense and splice mutations | Hypomyelinization of large nerve fibres, axonal accumulation of membranous material | Sensoryneurons | Peripheral nervous system | – | – | Yes | – | Yes | Hehr et al., 2007 |
| Human post- mortem tissue | Nonsense mutations | Intracytoplasmic granular lysosome-like structures | Spinal motor neurons | Cortex, white matter, motor tracts in medulla oblongata and spinal cord | Yes | Yes | Yes | Yes | Yes | Denora et al., 2016 |
ND = no data.
Table 2.
Human cellular models of SPG11-related pathology
| Cell type | Patients, n | Observed phenotype | Neuro- developmental defects | Neuro- degenerative defects | References |
|---|---|---|---|---|---|
| Fibroblasts | ND | Lysosomal abnormality | – | – | Renvoise et al., 2014 |
| 8 | No significant alteration in autophagic/lysosomal markers | – | – | Kara et al., 2016 | |
| Patient fibroblasts and spatacsin-depleted HeLa cells | ND | Autophagic lysosomal reformation (ALR) defects | No | Yes | Chang et al., 2014 |
| 3 | Autophagy defects | – | – | Vantaggiato et al., 2019 | |
| Patient iPSC-derived NPCs and neurospheres | 3a | Proliferation defect | Yes | No | Mishra et al., 2016 |
| Patient iPSC-derived neurospheres | 3a | Premature neurogenesis | Yes | No | Perez-Branguli et al., 2019 |
| Patient iPSC-derived cortical neurons | 3a | Axonal pathology and vesicle trafficking defects. | No | Yes | Perez-Branguli et al., 2014 |
| 3a | Neurite impairment, increased cell death | No | Yes | Pozner et al., 2018 | |
| Patient iPSC-derived organoids | 3a | Smaller organoid size | Yes | No | Perez-Branguli et al., 2019 |
| 2 | Lipid metabolism | Yes | Yes | Boutry et al., 2018 |
iPSC = induced pluripotent stem cells; ND = no data; NPC = neural progenitor cells.
Indicates the same patients.
The majority of molecular experimental data stem from established knowledge of spatacsin’s association with the AP-5 complex and spastizin (Hirst et al., 2013, 2015, 2018). However, the fact that AP-5 knock-out did not affect the localization of spatacsin suggests that it might have other, AP-5 independent, functions (Hirst et al., 2013). This notion is supported by interaction of Drosophila SPG11 and ZFYVE26/SPG15 orthologues exclusively with Rab7 and Rab26 (Gillingham et al., 2014). As flies lack functional AP-5 complex, this interaction may shed light on alternative, vesicular trafficking associated pathways independent of the AP-5 complex. In addition, it has been shown that while mutations in ZFYVE26/SPG15, encoding spastizin, reduce the stability of spatacsin, mutations in SPG11 have no effect on spastizin level (Vantaggiato et al., 2019). Thus, although both proteins are involved in ALR, only ZFYVE26/SPG15 mutations lead to defects in the fusion between autophagosomes and endosomes (Vantaggiato et al., 2019). Therefore, while dysfunction or lack of spatacsin may impair the autophagy-lysosomal machinery through disruption of either AP-5 complex or spastizin, it is likely that spatacsin acts through additional, distinct pathways.
It is important to add that the majority of data on the molecular function of spatacsin come from studies conducted on fibroblasts, HeLa cells, and animal models. Human neuronal models are essential in order to provide missing links regarding the function of the protein in patients. Recent advances in iPSC-derived 3D models may serve as a useful means of further studying the protein and its function in an environment that recapitulates human cellular organization. Advances in genome editing techniques can be of further benefit, by reconciling the issue of variability in the human patient-derived models. A combination of these methods provides an opportunity to determine the spatio-temporal localization of spatacsin and its mechanistic involvement in the pathogenesis of the disease.
Acknowledgements
This work is dedicated to our patients and in particular to the memory of Anne Wahlig.
Funding
T.P., M.R., J.W., and B.W. were funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 270949263/GRK2162. This work was supported by the German Federal Ministry of Education and Research funded treatHSP consortium (01GM1905B to B.W. and M.R. and grants 01GQ113, 01GM1520A, 01EK1609B to B.W.), and by the Bavarian Ministry of Education and Culture, Science and the Arts within the framework of the Bavarian Network for studying brain cell interactions: ForInter. Additional support came from the Tom Wahlig Stiftung, granted to T.P. and M.R.
Competing interests
The authors report no competing interests
References
- Allison R, Edgar JR, Pearson G, Rizo T, Newton T, Gunther S, et al. Defects in ER-endosome contacts impact lysosome function in hereditary spastic paraplegia. J Cell Biol 2017; 216: 1337–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anheim M, Lagier-Tourenne C, Stevanin G, Fleury M, Durr A, Namer IJ, et al. SPG11 spastic paraplegia. A new cause of juvenile parkinsonism. J Neurol 2009; 256: 104–8. [DOI] [PubMed] [Google Scholar]
- Arenas F, Garcia-Ruiz C, Fernandez-Checa JC.. Intracellular cholesterol trafficking and impact in neurodegeneration. Front Mol Neurosci 2017; 10: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M, et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2004; 62: 851–63. [DOI] [PubMed] [Google Scholar]
- Boutry M, Branchu J, Lustremant C, Pujol C, Pernelle J, Matusiak R, et al. Inhibition of lysosome membrane recycling causes accumulation of gangliosides that contribute to neurodegeneration. Cell Rep 2018; 23: 3813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M, Pierga A, Matusiak R, Branchu J, Houllegatte M, Ibrahim Y, et al. Loss of spatacsin impairs cholesterol trafficking and calcium homeostasis. Commun Biol 2019; 2: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchu J, Boutry M, Sourd L, Depp M, Leone C, Corriger A, et al. Loss of spatacsin function alters lysosomal lipid clearance leading to upper and lower motor neuron degeneration. Neurobiol Dis 2017; 102: 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali C, Valente EM, Bertini E, Montagna G, Criscuolo C, De Michele G, et al. Clinical and genetic studies in hereditary spastic paraplegia with thin corpus callosum. Neurology 2004; 62: 262–8. [DOI] [PubMed] [Google Scholar]
- Chang J, Lee S, Blackstone C.. Spastic paraplegia proteins spastizin and spatacsin mediate autophagic lysosome reformation. J Clin Invest 2014; 124: 5249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006; 127: 469–80. [DOI] [PubMed] [Google Scholar]
- da Graca FF, de Rezende TJR, Vasconcellos LFR, Pedroso JL, Barsottini OGP, Franca MC Jr,. Neuroimaging in hereditary spastic paraplegias: current use and future perspectives. Front Neurol 2018; 9: 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud H, Zhou S, Noreau A, Sabbagh M, Belzil V, Dionne-Laporte A, et al. Exome sequencing reveals SPG11 mutations causing juvenile ALS. Neurobiol Aging 2012; 33: 839 e5–9. [DOI] [PubMed] [Google Scholar]
- de Bot ST, Burggraaff RC, Herkert JC, Schelhaas HJ, Post B, Diekstra A, et al. Rapidly deteriorating course in Dutch hereditary spastic paraplegia type 11 patients. Eur J Hum Genet 2013; 21: 1312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denora PS, Smets K, Zolfanelli F, Ceuterick-de Groote C, Casali C, Deconinck T, et al. Motor neuron degeneration in spastic paraplegia 11 mimics amyotrophic lateral sclerosis lesions. Brain 2016; 139: 1723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Saffari A, Wahlster L, Lu J, Byrne S, Hoffmann GF, et al. Congenital disorders of autophagy: an emerging novel class of inborn errors of neuro-metabolism. Brain 2016; 139: 317–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising S, Thiele L, Frohlich F.. A systematic approach to identify recycling endocytic cargo depending on the GARP complex. eLife 2019; 8: e42837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfanian Omidvar M, Torkamandi S, Rezaei S, Alipoor B, Omrani MD, Darvish H.. Genotype-phenotype associations in hereditary spastic paraplegia: a systematic review and meta-analysis on 13,570 patients. J Neurol 2019. doi: 10.1007/s00415-019-09633-1. [DOI] [PubMed] [Google Scholar]
- Faber I, Branco LMT, Franca Junior MC.. Cognitive dysfunction in hereditary spastic paraplegias and other motor neuron disorders. Dement Neuropsychol 2016; 10: 276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber I, Martinez ARM, de Rezende TJR, Martins CR Jr, Martins MP, Lourenco CM, et al. SPG11 mutations cause widespread white matter and basal ganglia abnormalities, but restricted cortical damage. NeuroImage Clin 2018a; 19: 848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber I, Martinez ARM, Martins CR Jr, Maia ML, Souza JP, Lourenco CM, et al. SPG11-related parkinsonism: clinical profile, molecular imaging and l-dopa response. Mov Disord 2018b; 33: 1650–6. [DOI] [PubMed] [Google Scholar]
- Fink JK. Hereditary spastic paraplegia: clinical principles and genetic advances. Semin Neurol 2014; 34: 293–305. [DOI] [PubMed] [Google Scholar]
- Fraidakis MJ, Brunetti M, Blackstone C, Filippi M, Chio A.. Novel compound heterozygous spatacsin mutations in a Greek kindred with hereditary spastic paraplegia SPG11 and dementia. Neurodegener Dis 2016; 16: 373–81. [DOI] [PubMed] [Google Scholar]
- Franca MC Jr, Yasuda CL, Pereira FR, D’Abreu A, Lopes-Ramos CM, Rosa MV, et al. White and grey matter abnormalities in patients with SPG11 mutations. J Neurol Neurosurg Psychiatry 2012; 83: 828–33. [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Sinka R, Torres IL, Lilley KS, Munro S.. Toward a comprehensive map of the effectors of rab GTPases. Dev Cell 2014; 31: 358–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidubaldi A, Piano C, Santorelli FM, Silvestri G, Petracca M, Tessa A, et al. Novel mutations in SPG11 cause hereditary spastic paraplegia associated with early-onset levodopa-responsive Parkinsonism. Mov Disord 2011; 26: 553–6. [DOI] [PubMed] [Google Scholar]
- Hanein S, Martin E, Boukhris A, Byrne P, Goizet C, Hamri A, et al. Identification of the SPG15 gene, encoding spastizin, as a frequent cause of complicated autosomal-recessive spastic paraplegia, including Kjellin syndrome. Am J Hum Genet 2008; 82: 992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehr U, Bauer P, Winner B, Schule R, Olmez A, Koehler W, et al. Long-term course and mutational spectrum of spatacsin-linked spastic paraplegia. Ann Neurol 2007; 62: 656–65. [DOI] [PubMed] [Google Scholar]
- Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MN, Dacks JB, et al. The fifth adaptor protein complex. PLoS Biol 2011; 9: e1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Borner GH, Edgar J, Hein MY, Mann M, Buchholz F, et al. Interaction between AP-5 and the hereditary spastic paraplegia proteins SPG11 and SPG15. MBoC 2013; 24: 2558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Edgar JR, Esteves T, Darios F, Madeo M, Chang J, et al. Loss of AP-5 results in accumulation of aberrant endolysosomes: defining a new type of lysosomal storage disease. Hum Mol Genet 2015; 24: 4984–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Itzhak DN, Antrobus R, Borner GHH, Robinson MS.. Role of the AP-5 adaptor protein complex in late endosome-to-Golgi retrieval. PLoS Biol 2018; 16: e2004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourani R, El-Hajj T, Barada WH, Hourani M, Yamout BI.. MR imaging findings in autosomal recessive hereditary spastic paraplegia. AJNR Am J Neuroradiol 2009; 30: 936–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskender C, Kartal E, Akcimen F, Kocoglu C, Ozoguz A, Kotan D, et al. Turkish families with juvenile motor neuron disease broaden the phenotypic spectrum of SPG11. Neurol Genet 2015; 1: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagaraj P, Gautier-Stein A, Riedel D, Schomburg C, Cerda J, Vollack N, et al. Souffle/Spastizin controls secretory vesicle maturation during zebrafish oogenesis. PLoS Genet 2014; 10: e1004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara E, Tucci A, Manzoni C, Lynch DS, Elpidorou M, Bettencourt C, et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain 2016; 139: 1904–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundadze M, Kollmann K, Koch N, Biskup C, Nietzsche S, Zimmer G, et al. A hereditary spastic paraplegia mouse model supports a role of ZFYVE26/SPASTIZIN for the endolysosomal system. PLoS Genet 2013; 9: e1003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Lee PT, Chen K, Mao D, Tan KL, Zuo Z, et al. Phospholipase PLA2G6, a Parkinsonism-associated gene, affects Vps26 and Vps35, retromer function, and ceramide levels, similar to alpha-synuclein gain. Cell Met 2018; 28: 605–18 e6. [DOI] [PubMed] [Google Scholar]
- Martin E, Yanicostas C, Rastetter A, Alavi Naini SM, Maouedj A, Kabashi E, et al. Spatacsin and spastizin act in the same pathway required for proper spinal motor neuron axon outgrowth in zebrafish. Neurobiol Dis 2012; 48: 299–308. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell 2007; 6: 337–50. [DOI] [PubMed] [Google Scholar]
- Mishra HK, Prots I, Havlicek S, Kohl Z, Perez-Branguli F, Boerstler T, et al. GSK3ss-dependent dysregulation of neurodevelopment in SPG11-patient induced pluripotent stem cell model. Ann Neurol 2016; 79: 826–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecchiani C, Pedace L, Lo Giudice T, Casella A, Mearini M, Gaudiello F, et al. ALS5/SPG11/KIAA1840 mutations cause autosomal recessive axonal Charcot-Marie-Tooth disease. Brain 2016; 139: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murmu RP, Martin E, Rastetter A, Esteves T, Muriel MP, El Hachimi KH, et al. Cellular distribution and subcellular localization of spatacsin and spastizin, two proteins involved in hereditary spastic paraplegia. Mol Cell Neurosci 2011; 47: 191–202. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Izumi K, Umehara F, Kuriyama M, Hokezu Y, Nakagawa M, et al. Familial spastic paraplegia with mental impairment and thin corpus callosum. J Neurol Sci 1995; 131: 35–42. [DOI] [PubMed] [Google Scholar]
- Orlacchio A, Babalini C, Borreca A, Patrono C, Massa R, Basaran S, et al. SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain 2010; 133: 591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlen H, Melberg A, Raininko R, Kumlien E, Entesarian M, Soderberg P, et al. SPG11 mutations cause Kjellin syndrome, a hereditary spastic paraplegia with thin corpus callosum and central retinal degeneration. Am J Med Genet 2009; 150B: 984–92. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E, et al. Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov Disord 2010; 25: 1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Nath P, Wood NW, Singleton A, Houlden H.. Clinical heterogeneity and genotype-phenotype correlations in hereditary spastic paraplegia because of Spatacsin mutations (SPG11). Eur J Neurol 2008; 15: 1065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B, de Bot ST, Daniels MR, Franca MC Jr, Toro C, Riverol M, et al. “Ears of the Lynx” MRI sign is associated with SPG11 and SPG15 hereditary spastic paraplegia. AJNR Am J Neuroradiol 2019; 40: 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensato V, Castellotti B, Gellera C, Pareyson D, Ciano C, Nanetti L, et al. Overlapping phenotypes in complex spastic paraplegias SPG11, SPG15, SPG35 and SPG48. Brain 2014; 137: 1907–20. [DOI] [PubMed] [Google Scholar]
- Perez-Branguli F, Buchsbaum IY, Pozner T, Regensburger M, Fan W, Schray A, et al. Human SPG11 cerebral organoids reveal cortical neurogenesis impairment. Hum Mol Genet 2019; 28: 961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Branguli F, Mishra HK, Prots I, Havlicek S, Kohl Z, Saul D, et al. Dysfunction of spatacsin leads to axonal pathology in SPG11-linked hereditary spastic paraplegia. Hum Mol Genet 2014; 23: 4859–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozner T, Schray A, Regensburger M, Lie DC, Schlotzer-Schrehardt U, Winkler J, et al. Tideglusib rescues neurite pathology of SPG11 iPSC derived cortical neurons. Front Neurosci 2018; 12: 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puech B, Lacour A, Stevanin G, Sautiere BG, Devos D, Depienne C, et al. Kjellin syndrome: long-term neuro-ophthalmologic follow-up and novel mutations in the SPG11 gene. Ophthalmology 2011; 118: 564–73. [DOI] [PubMed] [Google Scholar]
- Renvoise B, Chang J, Singh R, Yonekawa S, FitzGibbon EJ, Mankodi A, et al. Lysosomal abnormalities in hereditary spastic paraplegia types SPG15 and SPG11. Ann Clin Transl Neurol 2014; 1: 379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riverol M, Samaranch L, Pascual B, Pastor P, Irigoyen J, Pastor MA, et al. Forceps minor region signal abnormality “ears of the lynx”: an early MRI finding in spastic paraparesis with thin corpus callosum and mutations in the spatacsin gene (SPG11) on chromosome 15. J Neuroimaging 2009; 19: 52–60. [DOI] [PubMed] [Google Scholar]
- Schule R, Wiethoff S, Martus P, Karle KN, Otto S, Klebe S, et al. Hereditary spastic paraplegia: clinicogenetic lessons from 608 patients. Ann Neurol 2016; 79: 646–58. [DOI] [PubMed] [Google Scholar]
- Siri L, Battaglia FM, Tessa A, Rossi A, Rocco MD, Facchinetti S, et al. Cognitive profile in spastic paraplegia with thin corpus callosum and mutations in SPG11. Neuropediatrics 2010; 41: 35–8. [DOI] [PubMed] [Google Scholar]
- Slabicki M, Theis M, Krastev DB, Samsonov S, Mundwiller E, Junqueira M, et al. A genome-scale DNA repair RNAi screen identifies SPG48 as a novel gene associated with hereditary spastic paraplegia. PLoS Biol 2010; 8: e1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate L, Dafou D, Hoyle J, Li N, Kinning E, Critchley P, et al. Novel SPG11 mutations in Asian kindreds and disruption of spatacs in function in the zebrafish. Neurogenetics 2010; 11: 379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperfeld AD, Kassubek J, Crosby AH, Winner B, Ludolph AC, Uttner I, et al. Complicated hereditary spastic paraplegia with thin corpus callosum: variation of phenotypic expression over time. J Neurol 2004; 251: 1285–7. [DOI] [PubMed] [Google Scholar]
- Stevanin G, Azzedine H, Denora P, Boukhris A, Tazir M, Lossos A, et al. Mutations in SPG11 are frequent in autosomal recessive spastic paraplegia with thin corpus callosum, cognitive decline and lower motor neuron degeneration. Brain 2008; 131: 772–84. [DOI] [PubMed] [Google Scholar]
- Stevanin G, Santorelli FM, Azzedine H, Coutinho P, Chomilier J, Denora PS, et al. Mutations in SPG11, encoding spatacsin, are a major cause of spastic paraplegia with thin corpus callosum. Nat Genet 2007; 39: 366–72. [DOI] [PubMed] [Google Scholar]
- Teive HA, Iwamoto FM, Della Coletta MV, Camargo CH, Bezerra RD, Minguetti G, et al. Hereditary spastic paraplegia associated with thin corpus callosum. Arq Neuro-Psiquiatr 2001; 59: 790–2. [DOI] [PubMed] [Google Scholar]
- Tranchant C, Koob M, Anheim M.. Parkinsonian-pyramidal syndromes: a systematic review. Parkinsonism Relat Disord 2017; 39: 4–16. [DOI] [PubMed] [Google Scholar]
- Vanderver A, Tonduti D, Auerbach S, Schmidt JL, Parikh S, Gowans GC, et al. Neurotransmitter abnormalities and response to supplementation in SPG11. Mol Genet Metab 2012; 107: 229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantaggiato C, Panzeri E, Castelli M, Citterio A, Arnoldi A, Santorelli FM, et al. ZFYVE26/SPASTIZIN and SPG11/SPATACSIN mutations in hereditary spastic paraplegia types AR-SPG15 and AR-SPG11 have different effects on autophagy and endocytosis. Autophagy 2019; 15: 34–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga RE, Khundadze M, Damme M, Nietzsche S, Hoffmann B, Stauber T, et al. In vivo evidence for lysosome depletion and impaired autophagic clearance in hereditary spastic paraplegia type SPG11. PLoS Genet 2015; 11: e1005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijemanne S, Shulman JM, Jimenez-Shahed J, Curry D, Jankovic J.. SPG11 mutations associated with a complex phenotype resembling dopa-responsive dystonia. Mov Disord Clin Pract 2015; 2: 149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Gross C, Uyanik G, Schulte-Mattler W, Lurding R, Marienhagen J, et al. Thin corpus callosum and amyotrophy in spastic paraplegia–case report and review of literature. Clin Neurol Neurosurg 2006; 108: 692–8. [DOI] [PubMed] [Google Scholar]
- Winner B, Uyanik G, Gross C, Lange M, Schulte-Mattler W, Schuierer G, et al. Clinical progression and genetic analysis in hereditary spastic paraplegia with thin corpus callosum in spastic gait gene 11 (SPG11). Arch Neurol 2004; 61: 117–21. [DOI] [PubMed] [Google Scholar]
- Ysselstein D, Shulman JM, Krainc D.. Emerging links between pediatric lysosomal storage diseases and adult parkinsonism. Mov Disord 2019; 34: 614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 2010; 465: 942–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, et al. p62 is a common component of cytoplasmic inclusions in protein aggregation diseases. Am J Pathol 2002; 160: 255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundorf G, Reiser G.. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal 2011; 14: 1275–88. [DOI] [PMC free article] [PubMed] [Google Scholar]