Abstract
Study design:
Standardized, two-part laboratory protocol with an interim observational period.
Background:
Fluctuations in limb volume degrade prosthesis fit and require users to accommodate changes using management strategies, such as donning or doffing prosthetic socks.
Objectives:
To examine how activities and self-reported outcomes relate to daily changes in residual limb fluid volume and volume accommodation.
Methods:
Participants were classified as “accommodators” or “non-accommodators,” based on self-report prosthetic sock use. Participants’ residual limb fluid volume change was measured using a custom bioimpedance analyzer and a standardized in-laboratory activity protocol. Self-report health outcomes were assessed with the Socket Comfort Score and Prosthesis Evaluation Questionnaire. Activity was monitored while participants left the laboratory for at least 3 hours. They then returned to repeat the bioimpedance test protocol.
Results:
Twenty-nine people were enrolled. Morning-to-afternoon percent limb fluid volume change per hour was not strongly correlated to percent time weight-bearing or to self-report outcomes. As a group, non-accommodators (n=15) spent more time with their prosthesis doffed and reported better outcomes than accommodators.
Conclusions:
Factors other than time weight–bearing may contribute to morning-to-afternoon limb fluid volume changes and reported satisfaction with the prosthesis among trans-tibial prosthesis users. Temporary doffing may be a more effective and satisfying accommodation method than sock addition.
Keywords: Amputation stumps, artificial limbs, bioimpedance, outcome assessment, residual limb volume, socket fit, volume accommodation
Background
The quality of socket fit is commonly acknowledged by both patients and prosthetists as the most important aspect of a prosthesis.1,2,3 Socket fit is influenced by changes in the residual limb, most notably limb volume.3 Trans-tibial sockets oversized by as little as 1.0% have been shown to induce clinically detectable changes in socket fit.4
Activity may accentuate limb volume loss.5 Pressures and shear stresses applied to residual limb soft tissues during ambulation may drive fluid out of the residuum, decreasing limb volume. Thus, users who spend more time weight-bearing, i.e. standing and walking, would be expected to experience greater limb volume losses over the day than people who spend less time weight-bearing.
Prosthesis users who routinely gain or lose fluid volume are instructed by practitioners to adjust their prosthesis, typically by adding or removing socks, when they feel a change in socket fit. Users who spend much time weight-bearing would be expected to perform more sock accommodations compared with people who spend relatively little time weight-bearing. Those users who experience limb volume changes and do not make said changes would be expected to report reduced satisfaction, comfort, and perceived mobility compared to those who accommodate volume changes and maintain quality of their socket fit.
The purpose of this research was to explore if trans-tibial prosthesis users’ morning-to-afternoon fluid volume change was associated with percentage time weight-bearing and user-reported satisfaction, comfort, and perceived mobility. We also evaluated if persons who accommodated to daily limb volume change by adjusting sock thickness spent more time weight-bearing and reported greater satisfaction, comfort, and perceived mobility than those who did not accommodate.
Methods
Study design
A standardized, two-part laboratory protocol with an interim observational period was conducted to assess the correlation of residual limb fluid volume change and volume accommodation with prosthesis users’ health-related function, health, and quality-of-life. Residual limb fluid volume, activity, and self-report health evaluations of people with unilateral, trans-tibial amputation were collected during a single, three-part assessment (i.e., morning test session, between-sessions unrestricted activity, and afternoon test session) performed on a single day. Data were collected from October 2013 to June 2014. All procedures were approved by a University of Washington institutional review board and all participants provided informed consent.
Participants
Volunteers with trans-tibial amputation were recruited to be in this study. Individuals were recruited from regional prosthetics and orthotics clinics using posted flyers. Inclusion criteria included age 18 years or older, at least 9 months post-amputation with a stable residual limb, Medicare functional classification level (K-level) 2 or higher, daily use (6 h or more per day) of a prosthesis with a definitive socket, and ability to walk on a treadmill for at least 90 s at a comfortable speed. Participants also needed to have a residual limb at least 9.0 cm in length to accommodate placement and spacing of bioimpedance electrodes. Exclusion criteria included skin breakdown (or other conditions that would preclude prosthetic ambulation) or presence of metal implants that would adversely affect the quality of bioimpedance data.
Instrumentation
A custom, semi-portable bioimpedance analyzer was used to measure participants’ residual limb fluid volume. Details of the bioimpedance system are described elsewhere.6 Briefly, the instrument injected short packets of alternating current (~300 μA peak-to-peak, 5 kHz to 1 MHz) to electrodes positioned on the participant’s proximal thigh and distal inferior residual limb. Voltage was sensed by electrodes positioned on the anterior and posterior aspects at the level of the patellar tendon, mid-limb, and distal tibia, producing four regions of measurement: anterior-distal; anterior; posterior-distal; and posterior. We measured from anterior and posterior regions separately because the interosseous membrane between the tibia and fibula is a natural conduction barrier thus helps to isolate measurements from the two regions. A prior study suggested clinically meaningful differences between proximal and distal regions.7 For short residual limbs (<12 cm) only the patellar-tendon-level and distal-tibia-level voltage-sensing electrodes were used, thus there were only two regions of measurement: anterior and posterior. The electrodes and wires connecting the electrodes to the analyzer were less than 1.0 mm diameter, and did not inhibit normal use of the prosthesis or movements of the limb. The electrodes were fabricated using conductive tape (ARCare8881, Adhesives Research Inc., Glen Rock, PA), multi-stranded silver-plated copper wire with an aramid core and poly vinyl chloride insulation (New England Wire, Lisbon, NH), a flattened metal crimp to strain relieve wires as they exited the insulation within the electrode, and a thin layer of hydrogel (KM10B, Katecho, Des Moines, IA) between the electrode and skin. This electrode configuration was used successfully in previous studies5,8,9,20. Sensed voltages were transmitted via a 3-meter cable back to a personal computer (PC) for subsequent analysis (Figure 1a,b). Evaluation tests conducted on the custom bioimpedance analysis system demonstrated minimal signal drift (0.02%/h), root-mean-square (RMS) noise (0.026%/h), and measurement error across the sensing range of the instrument (−0.4%).6
Figure 1a,b.
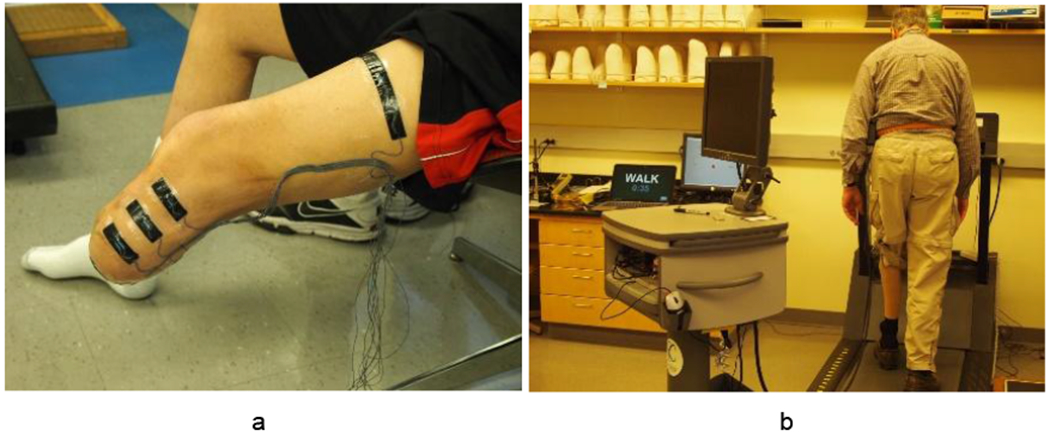
a: Residual limb instrumented with electrodes (distal current-injection electrode not visible). b: Limb fluid volume monitoring test session.
Self-report measures
A self-report survey was used to solicit information about participants’ prosthesis-related function, health, and quality of life over the past 2 weeks. The survey included several standardized measures, including the Socket Comfort Score (SCS)10 and the Ambulation, Residual Limb Health, Utility, and Well-Being subscales from the Prosthesis Evaluation Questionnaire (PEQ). One additional question from the PEQ that is not included in the PEQ subscales (i.e., “Over the past two weeks, rate how happy you have been with your current prosthesis”) was included to assess participants’ overall satisfaction with the prosthesis.11 The SCS and PEQ were developed to evaluate prosthesis-related health outcomes in people with lower limb amputation, and both instruments have been reported to be valid and reliable when used for this purpose.10,11 Measures were presented to participants on paper using the developers’ recommended instructions and response options.10,11
Procedures
Participants first attended an in-person screening session to verify eligibility criteria and determine their comfortable walking speed. Before leaving the laboratory, participants were provided a two-week sock log12 to record their normal, daily use of prosthetic socks. A sock log was used to characterize participants’ sock use, because self-report (i.e., asking participants, “Do you change socks throughout the day?”) was previously found to be an unreliable indicator of accommodation in people with trans-tibial amputation.12 Participants were also instructed to eat a normal breakfast and avoid caffeine or alcohol (diuretics) during the morning before their next visit. Participants returned to the laboratory after two weeks for an assessment that included a morning (AM) test session, a between-sessions activity monitoring period, and an afternoon (PM) test session.
Upon arrival at the AM test session, participants met with the study prosthetist to have their residual limb inspected and to discuss recent changes in their health, prosthetic fit, or activity that might affect limb fluid volume, limb fluid volume changes, or other studied outcomes. Participants were then administered the self-report survey. Upon finishing the survey, participants sat for 10 min with both feet on the floor to establish a stable limb fluid volume baseline. After resting, participants doffed their prosthesis and were instrumented for limb fluid volume monitoring with the biomimpedance analyzer.6 Participants’ prostheses were also equipped with an activity monitor (Actigraph GT3X+, Pensacola, FL). The GT3X+ is a small (4.6x3.3x1.5 cm), lightweight (19 g), accelerometer-based sensor (±6 g dynamic range, 0.0023 g resolution) that has been used previously to monitor prosthesis users’ activity.13,14,15,16
Participants then performed a standardized, ‘sit-stand-walk’ protocol intended to reflect typical daily activities. Participants first sat in a stationary chair for 90 s with their prosthesis donned. Then they rose and stood for 90 s. Weight-bearing through the prosthetic leg was monitored with a digital scale (349KLX Health-O-Meter, Pelstar, McCook, IL) and participants were prompted to adjust their weight allocation as needed to achieve equal weight-bearing. Participants next stepped onto the treadmill and walked at their previously determined preferred walking speed for 90 s. Lastly, participants stepped off the treadmill and stood on the scale with equal weight-bearing for 10 s. This sit-stand-walk protocol was repeated four more times. Upon completion of the last cycle, participants remained sitting for 10 min. The system was then turned off and the electrode wires were disconnected from the analyzer. The wires were coiled neatly and taped to the prosthesis so that the participant could move without restriction.
Participants were instructed to leave the laboratory for at least 3 h and go about their normal activities to the extent possible. Participants were informed that they could doff their prosthesis or change socks to accommodate volume changes as desired, but were asked not to doff their liner in order to avoid damaging the electrodes.
Participants returned to the laboratory for the PM test session and were re-administered the limb fluid volume test using the same sit-stand-walk protocol described for the AM test session. Upon conclusion of the test, participants doffed the prosthesis and the instrumentation was removed.
Analysis
Sock logs and self-report measures were scored by research staff according to developers’ instructions.10,11,12 Participants were classified as “accommodators” if they reported adding or removing socks during any day in the 2-week monitoring period. They were classified as “non-accommodators” if no daily sock changes were made over the monitoring period.
Collected bioimpedance data were post-processed to determine extracellular fluid impedance using de Lorenzo’s form of the Cole model.17 To convert extracellular fluid impedances to extracellular fluid volumes, a limb segment model was used.18,19 In this study, we investigated only extracellular fluid volume changes because extracellular changes were expected to be most relevant to the relatively short-term limb fluid volume fluctuations of interest in this study.
The rate of limb fluid volume change between AM and PM sessions expressed in %/h was calculated for each of the four channels. Residual limb fluid volumes measured during the brief 5-10 s stand after the 5th walk cycle during the AM and PM test sessions were used. Residual limb fluid volume change was expressed as a percentage difference:
Differences in distributions between accommodators and non-accommodators were assessed with the Fisher’s Exact test (for sex and activity level) and Mann-Whitney U test (for age, time since amputation and body mass index [BMI]). Pearson correlation was used to assess the strength of linear association between outcomes and residual limb fluid volume change. Non-parametric test of medians (percent time doffed, siting, standing, walking, and weight bearing; SCS; PEQ Satisfaction, Ambulation, Residual Limb Health, Utility, and Weight Bearing) was used for comparisons between “accommodators” and “non-accommodators.”
Results
Participant characteristics
Twenty-nine people with trans-tibial amputation participated in the study. On average, participants were male (82.8%), middle-age (mean=56.7 [SD=14.8] years old), and established amputees (mean=15.3 [14.4] years post-amputation). Most participants (93.1%) were unlimited or active community ambulators. About half of the participants (48.3%) used socks to manage daily limb volume changes, and were classified as “accommodators.” Four accommodators started days with different sock ply. All of the non-accommodators started their days with the same sock ply. There were no significant differences between accommodators and non-accommodators according to sex (p=0.65), age (p=0.95), BMI (p=0.56) or activity level (K-2 vs. higher level, p=1.0). However, non-accommodators had their amputation significantly longer than accommodators (median 12.9 years vs. 5.5 years, respectively, p=0.01). Individual participant characteristics are listed in Appendix 1.
Morning-to-afternoon fluid volume changes
Of the 29 participants, 21(72.4%) lost fluid volume between sessions in most regions of the residual limb (3 of 4, or 2 of 2 regions), 5(17.2%) gained fluid volume in most regions, and 3(10.3%) gained in half of the regions and lost in the other half (Appendix 2).
Participants spent most of their time between sessions sitting with their prosthesis donned (mean=62.7% [18.0%], median=65.4%), and spent less time walking (mean=19.0% [13.0%], median=13.0%) or standing (mean=11.6% [7.0%], median=10.4%). Participants doffed their prosthesis a mean of 6.5% [12.9%] and median of 3.4% of the time between sessions.
Morning-to-afternoon percent fluid volume change per hour generally increased with decreased percent time walking, standing, and weight-bearing (weight-bearing was the sum of standing and walking). However, the correlations were weak (Table 1, Appendix 3, Figures 5 to 7). Correlation coefficients for all channels ranged from −0.34 to 0.11 and p-values ranged from 0.01 to 0.64. The high correlations for percent fluid volume change per hour in the anterior and anterior distal regions with percent time doffed were due largely to two individuals with long percent doff times (37.7% and 62.9%). The remaining participants had percent doff durations ranging from 0.0% to 11.1%. Similarly, for the posterior-distal region and weight-bearing, two individuals had high percent fluid volume changes per hour (5.3%/h and 10.8%/h), while the remaining ranged from −2.4%/h to 2.4%/h.
Table 1.
Pearson correlations between activities and percent residual limb fluid volume change per hour by region.
| Activity | Anterior Distal | Anterior | Posterior Distal | Posterior | |
|---|---|---|---|---|---|
| % Doffed | Corr. | .41 | .56 | .08 | .11 |
| P-Value | .04 | .002 | .68 | .56 | |
| % Sit | Corr. | −.13 | −.21 | .23 | .17 |
| P-Value | .53 | .28 | .25 | .37 | |
| % Stand | Corr. | .11 | −.15 | −.10 | −.09 |
| P-Value | .60 | .43 | .62 | .64 | |
| %Walk | Corr. | −.29 | −.18 | −.34 | −.30 |
| P-Value | .14 | .35 | .08 | .11 | |
| % Wt-Bearing | Corr. | −.20 | −.21 | −.32 | −.28 |
| P-Value | .33 | .27 | .01 | .13 |
Morning-to-afternoon percent fluid volume change per hour was not strongly correlated with reported socket comfort, satisfaction, ambulation, residual limb health, utility, or well-being (Table 2). Correlation coefficients ranged from −0.20 to 0.24 and p-values ranged from 0.22 to 0.94.
Table 2.
Pearson correlations between self-reported health outcomes and percent residual limb fluid volume change per hour by region.
| Health Outcome | Anterior Distal | Anterior | Posterior Distal | Posterior | |
|---|---|---|---|---|---|
| SCS | Corr. | .02 | .04 | .02 | −.02 |
| P-Value | .92 | .83 | .94 | .92 | |
| Satisfaction | Corr. | −.03 | −.03 | .04 | −.04 |
| P-Value | .90 | .88 | .86 | .85 | |
| Ambulation | Corr. | .24 | .11 | −.09 | −.12 |
| P-Value | .22 | .56 | .67 | .54 | |
| Residual Limb Health | Corr. | .12 | .16 | −.16 | −.15 |
| P-Value | .55 | .41 | .44 | .44 | |
| Utility | Corr. | .04 | .07 | −.11 | −.12 |
| P-Value | .86 | .71 | .58 | .52 | |
| Well-Being | Corr. | −.20 | −.15 | .09 | .12 |
| P-Value | .33 | .44 | .67 | .52 |
Accommodators vs. non-accommodators
Participants who accommodated to daily limb volume changes spent more time weight-bearing than those who did not accommodate, but the difference in medians was not statistically significant (p=0.47) (Figure 2). Interestingly, participants who did not accommodate had a greater median percent time with their prosthesis doffed than accommodators, though the medians were not statistically different (p=0.72). The distribution of percent time doffed for accommodators and non-accommodators showed that about a third of the participants were at the extremes: 5 accommodators and 2 non-accommodators spent 0.0-0.9% percent time doffed, and 3 non-accommodators spent >10.0% time doffed.
Figure 2. Activities between sessions.

Accommodators (Acc) and non-accommodators (Non). Black horizontal lines are medians.
Median percent fluid volume changes per hour were not statistically different for accommodators vs. non-accommodators (Table 3) with p-values ranging from 0.45 to 1.0. Across all self-report measures, persons who accommodated to daily fluid volume had lower median self-report scores than those who did not accommodate (Figure 3). Differences in medians for all PEQ subscales ranged from 5 to 11 (100 point scale) and was 1 for SCS (10 point scale). However, none of the differences were statistically significant. Correlations between self-report measures and percent limb fluid volume change per hour were weak (Figures 6 and 7 in Appendix 3). P-values ranged from 0.27 to 0.72.
Table 3.
Morning-to-Afternoon Percent fluid volume change per hour. Accommodators and non-accommodators.
| Region | Accommodators mean (SD) median (min, max) | Non-Accommodators mean (SD) median (min, max) | P-Value* |
|---|---|---|---|
| Anterior-distal | −0.9 (1.1) −0.9 (−3.1, 0.6) |
−0.7 (1.0) −0.5 (−2.5, 1.1) |
0.45 |
| Anterior | −0.8 (0.7) −0.9 (−2.2, 0.2) |
−0.7(1.1) −0.7 (−2.5, 2.0) |
0.46 |
| Posterior-distal | 1.0 (3.5) −0.2 (−2.5, 10.8) |
−0.1 (1.1) −0.5 (−1.5, 2.4) |
0.71 |
| Posterior | 0.3 (3.8) −0.9 (−2.5, 12.0) |
−0.5 (0.8) −0.8 (−1.5, 1.5) |
1.0 |
P-value from non-parametric test of medians; SD: standard deviation
Figure 3. Self-report results.

Accommodators and non-accommodators. Left: SCS. Right: PEQ subscales (SAT = Satisfaction, AM = Ambulation, RLH = Residual Limb Health, Satisfaction, UT = Utility, WB = Well-Being). Black horizontal lines are medians.
Discussion
Results from the present study do not support the hypothesis that prosthesis users who spend much time weight-bearing, i.e. standing and walking, experience greater percent limb fluid volume losses over the day compared with people who spend little time weight-bearing. Part of the reason this expectation is not supported may be because of the influence of doffing. Doffing the prosthesis periodically during the day, even for short periods, may help the user to counter fluid volume losses experienced during weight-bearing.20 Also, previous research showed that walking did not necessarily cause a fluid volume loss, particularly if preceded by standing. In a prior investigation,21 fluid volume gains were measured in 16 of 24 (66.7%) participants during walking in a PM in-laboratory test sessions. In the present investigation we calculated fluid volume changes within PM test sessions and found that during walking, 21 of 29 (72.4%) participants gained limb fluid volume in the majority of monitored limb regions (3 of 4, 4 of 4, or 2 of 2) (Appendix 4). Fluid volume gains from both periodic doffing and from walking may have contributed to the relatively low correlation between percent time weight-bearing and percent limb fluid volume loss per hour in the present study.
Results do not support the hypothesis that prosthesis users who report low healthy outcomes experience greater rates of percent limb fluid volume loss from the morning to afternoon compared with people who report high health outcomes. This result may have been influenced by the short testing interval (half a day), the atypical environment for the participants (laboratory and nearby locations accessible during the between-session period), and the design of the self-report measures (they were not specific to the test day but instead reflected 2-week periods immediately prior to the test day).
Since they had more mature residual limbs, non-accommodators in the present study may have been less prone to daily limb fluid volume changes than accommodators. However, the result that, on average, non-accommodators spent more time doffed than accommodators suggests that non-accommodators may have regularly used temporary doffing as an accommodation method. Thus they may have been “accommodating,” just not by adding socks. Temporary doffing facilitates limb fluid volume recovery and retention, enlarging the residual limb and helping to keep it within an acceptable volume range so as to maintain fit (Figure 4a).20
Figure 4. Accommodation strategies.

Temporary doffing (a) compared with sock addition (b).
Sock accommodation affects limb fluid volume differently than temporary doffing. Prior research has shown that a prosthesis user’s limb fluid volume decreases when a sock is added.22 By adding socks, accommodators shift the location of their acceptable volume window to lower volumes (Figure 4b). It is unknown whether using periodic doffing instead of sock addition as an accommodation method reduces long-term residual limb volume loss (limb atrophy). If it did then regular periodic doffing would lengthen the duration a socket remained comfortable to the user. Interestingly, non-accommodators showed a tendency to report higher health outcome scores than accommodators (Figure 3). Maintaining limb fluid volume by temporary doffing rather than by accentuating limb volume loss with sock addition may be more satisfactory to prosthesis users. Future research efforts are needed to compare effectiveness of sock addition vs. temporary doffing, and to determine if individual prosthesis users benefit using one method over the other. It would also benefit the field to know what characteristics of prosthesis users cause one method to provide more favorable user outcomes.
Research studies designed to assess other factors that may influence residual limb fluid volume loss such as diet, hydration, and medications need to be conducted and their impact compared with activity. By providing the clinical community with insight into the relative influence of these and other factors, researchers will help practitioners treat patients who experience poor socket fit.
Conclusions
Factors other than time weight bearing (standing and walking) contribute to the rate of morning-to-afternoon limb fluid volume change on trans-tibial prosthesis users. Further investigation is needed to determine if temporary doffing is a more effective and satisfying accommodation method than sock addition.
Supplementary Material
Clinical Relevance.
Practitioners should be mindful that daily limb fluid volume change and prosthesis satisfaction are not dictated exclusively by activity. Temporarily doffing the prosthesis may slow daily limb fluid volume loss, and should be investigated as an alternative strategy to sock addition.
Acknowledgments
Funding/Support
Research reported in this publication was supported by the National Institute of Child Health and Human Development under award number R01HD060585. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations:
- cm
centimeter
- d
day
- h
hour
- min
minute
- mo
month
- PEQ
Prosthesis Evaluation Questionnaire
- s
second
- SCS
Socket Comfort Score
- SD
standard deviation
- U.S.
United States
- y
year
Footnotes
Disclosure
The authors declare no conflict of interest.
Contributor Information
Joan E Sanders, Department of Bioengineering, University of Washington, Seattle Washington USA..
Robert T Youngblood, Department of Bioengineering, University of Washington, Seattle Washington USA..
Brian J Hafner, Department of Rehabilitation Medicine, University of Washington, Seattle Washington USA..
Marcia A Ciol, Department of Rehabilitation Medicine, University of Washington, Seattle Washington USA..
Katheryn J Allyn, Department of Bioengineering, University of Washington, Seattle Washington USA..
David Gardner, Department of Bioengineering, University of Washington, Seattle Washington USA..
John C Cagle, Department of Bioengineering, University of Washington, Seattle Washington USA..
Christian B Redd, Department of Bioengineering, University of Washington, Seattle Washington USA..
Colin R Dietrich, Department of Bioengineering, University of Washington, Seattle Washington USA..
References
- 1.Schaffalitzky E, Gallagher P, Maclachlan M and Wegener ST. Developing consensus on important factors associated with lower limb prosthetic prescription and use. Disabil Rehabil. 2012; 34: 2085–94. [DOI] [PubMed] [Google Scholar]
- 2.Marks LJ and Michael JW. Artificial limbs. BMJ. 2001; 323: 732–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legro MW, Reiber G, del Aguila M, Ajax MJ, Boone DA, Larsen JA, Smith DG and Sangeorzan B. Issues of importance reported by persons with lower limb amputations and prostheses. J Rehabil Res Dev. 1999; 36: 155–63. [PubMed] [Google Scholar]
- 4.Sanders JE, Severance MR and Allyn KJ. Computer-socket manufacturing error: how much before it is clinically apparent? J Rehabil Res Dev. 2012; 49: 567–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders JE, Allyn KJ, Harrison DS, Myers TR, Ciol MA and Tsai EC. Preliminary investigation of residual limb fluid volume changes within one day. J Rehabil Res Dev. 2012; 49(10):1467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders J, Moehring M, Rothlisberger T, Phillips RH, Hartley T, Dietrich CR, Redd CB, Gardner DW and Cagle JC. A versatile segmental bioimpedance analysis platform for use on people with limb amputation. IEEE Trans Biomed Eng. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders JE, Youngblood RT, Hafner BJ, Cagle JC, McLean JB, Redd CB, Dietrich CR, Ciol MA, Allyn KJ. Effect of socket size on metrics of socket fit in trans-tibial prosthesis users. Med Engi Phys. 2017; 44: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders JE, Cagle JC, Harrison DS, Myers TR, Allyn KJ. How does adding and removing liquid from socket bladders affect residual-limb fluid volume? J Rehabil Res Dev. 2013; 50: 845–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders JE , Redd CB , Cagle JC , Hafner BJ , Gardner DL , Allyn KJ , Harrison DS, Ciol MA. Preliminary evaluation of a novel bladder-liner for facilitating residual limb fluid vol-ume recovery without doffing. J Rehabil Res Dev. 2016; 53: 1107–20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanspal RS, Fisher K and Nieveen R. Prosthetic socket fit comfort score. Disabil Rehabil. 2003; 25: 1278–80. [DOI] [PubMed] [Google Scholar]
- 11.Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J and Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998; 79: 931–8. [DOI] [PubMed] [Google Scholar]
- 12.D’Silva K, Hafner BJ, Allyn KJ and Sanders JE. Self-reported prosthetic sock use among persons with transtibial amputation. Prosthet Orthot Int. 2014; 38: 321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redfield MT, Cagle JC, Hafner BJ and Sanders JE. Classifying prosthetic use via accelerometry in persons with transtibial amputations. J Rehabil Res Dev. 2013; 50: 1201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent JA, Stergiou N and Wurdeman SR. Step activity and stride-to-stride fluctuations are negatively correlated in individuals with transtibial amputation. Clin Biomech (Bristol, Avon). 2015; 30: 1225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuelsen BT, Andrews KL, Houdek MT, Terry M, Shives TC and Sim FH. The impact of the immediate postoperative prosthesis on patient mobility and quality of life after transtibial amputation. Am J Phys Med Rehabil. 2017; 96: 116–9. [DOI] [PubMed] [Google Scholar]
- 16.Gardner D, Redd C, Cagle J, Hafner BJ and Sanders JE. Monitoring Prosthesis User Activity and Doffing Using an Activity Monitor and Proximity Sensors. J Prosthet Orthot. 2016; 28: 68–77. [Google Scholar]
- 17.De Lorenzo A, Andreoli A, Matthie J and Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. Journal of applied physiology (Bethesda, Md : 1985). 1997; 82: 1542–58. [DOI] [PubMed] [Google Scholar]
- 18.Fenech M and Jaffrin MY. Extracellular and intracellular volume variations during postural change measured by segmental and wrist-ankle bioimpedance spectroscopy. IEEE Trans Biomed Eng. 2004; 51: 166–75. [DOI] [PubMed] [Google Scholar]
- 19.Hanai T Electrical properties of emulsions, in Emulsion Science. London, England: Academic Press, 1968, pp. 354–477. [Google Scholar]
- 20.Sanders JE, Hartley TL, Phillips RH, Ciol MA, Hafner BJ, Allyn KJ and Harrison DS. Does temporary socket removal affect residual limb fluid volume of trans-tibial amputees? Prosthet Orthot Int. 2016; 40: 320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders JE, Cagle JC, Allyn KJ, Harrison DS and Ciol MA. How do walking, standing, and resting influence transtibial amputee residual limb fluid volume? J Rehabil Res Dev. 2014; 51: 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders JE, Harrison DS, Allyn KJ, Myers TR, Ciol MA and Tsai EC. How do sock ply changes affect residual-limb fluid volume in people with transtibial amputation? J Rehab Res Dev. 2012; 49: 241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


