Abstract
Real-time biofeedback is a promising post-stroke gait rehabilitation strategy that can target specific gait deficits preferentially in the paretic leg. Our previous work demonstrated that the use of an audiovisual biofeedback interface designed to increase paretic leg propulsion, measured via anterior ground reaction force (AGRF) generation during late stance phase of gait, can induce improvements in peak AGRF production of the targeted and paretic limb of able-bodied and post-stroke individuals, respectively. However, whether different modes of biofeedback, such as visual, auditory, or a combination of both, have differential effects on AGRF generation is unknown. The present study investigated the effects of audio only, visual only, or audiovisual AGRF biofeedback in able-bodied and post-stroke individuals. Seven able-bodied (6 females, 27±2 years) and nine post-stroke individuals (6 females, 54±12 years, 42±26 months post-stroke) completed four 30-second walking trials on a treadmill under 4 conditions: no biofeedback, audio biofeedback, visual biofeedback, or audiovisual biofeedback. Compared to walking without biofeedback, all three biofeedback modes significantly increased peak AGRF in the targeted and paretic leg. There was no significant difference in peak AGRF between the three biofeedback modes. Able-bodied individuals demonstrated greater feedback-induced increase in stride-to-stride variation of AGRF generation during audio biofeedback compared to visual biofeedback; however, similar results were not observed in the post-stroke group. The present findings may inform future development of real-time gait biofeedback interfaces for use in clinical or community environments.
Keywords: biofeedback, gait biomechanics, post-stroke hemiparesis, gait training, paretic propulsion
Introduction
Hemiparesis following stroke results in biomechanical impairments to the paretic leg that disrupt walking ability, mobility, and community participation. Generation of propulsion is an important component of the normal gait cycle. During the double support phase of gait, propulsion generated by the trailing limb facilitates stance-to-swing and enables forward progression of the body’s center of mass. Reduced propulsive force generation by the paretic leg, measured as anteriorly-directed ground reaction forces (AGRF) from a force platform, is a common post-stroke deficit that is associated with reduced inter-limb symmetry, hemiparetic severity, and decreased walking speed (Balasubramanian et al. 2007; Bowden et al. 2006; Chen et al. 2005a; Mulroy et al.; Nadeau et al. 1999; Neptune et al. 2001). Underscoring the importance of paretic leg propulsion, rehabilitative efforts designed to improve paretic AGRF in post-stroke individuals have resulted in improvements to walking speed, long-distance walking function, and energy expenditure (Awad et al. 2015; Awad et al. 2014; Awad et al. 2016; Bowden et al. 2013; Hsiao et al. 2016).
Biofeedback can provide real-time information regarding physiological processes that may not be consciously perceptible to users, enabling awareness and self-correction of incorrect gait patterns (Stanton et al. 2017). Considerable progress has been made from the original use of EMG biofeedback of individual muscles to improve post stroke paretic gait (Binder et al. 1981; Wolf and Binder-MacLeod 1983). Recently, real-time biofeedback representative of limb positioning or force-generation during gait has shown promise as a post-stroke rehabilitation strategy that may preferentially target paretic leg deficits to improve gait function (Drużbicki et al. 2015; Drużbicki et al. 2016; Genthe et al. 2018; Ki et al. 2015). Following two weeks of treadmill training with visual biofeedback displaying step length, post-stroke individuals demonstrated improvements in step length symmetry post-training and at 6-month follow-up (Drużbicki et al. 2015; Drużbicki et al. 2016). Ki et al. demonstrated significant improvements in paretic leg single limb stance duration following weight-bearing biofeedback during gait in post-stroke individuals (Ki et al. 2015). Recently, our study on post-stroke individuals demonstrated that the use of audiovisual biofeedback displaying real-time paretic AGRF generation can induce significant improvements to paretic leg peak AGRF without changes to non-paretic leg AGRFs (Genthe et al. 2018).
While the use of real-time biofeedback may improve specific paretic leg gait deficits in individuals post-stroke, several questions remain unanswered regarding the most effective biofeedback modes, as well as strategies to enhance gait performance and learning. Biofeedback can be delivered through various senses, such as visual, auditory, tactile, or a combination of different modes. Different modes of biofeedback may differentially affect performance and learning of a motor skill (Molier et al. 2010; Sigrist et al. 2013). For example, Hasegawa et al. demonstrated that able-bodied individuals decreased spatial and temporal variability during a dynamic postural control task following auditory but not visual biofeedback (Hasegawa et al. 2017). Thus, there is a need to compare the effects of different modes or interfaces for providing biofeedback regarding targeted gait parameters during post-stroke gait training.
The present study assessed the immediate effects of real-time audio only, visual only, and audiovisual AGRF biofeedback on propulsive force generation in able-bodied and post-stroke individuals. We hypothesized that walking with any biofeedback mode would increase peak AGRF in the targeted and paretic leg, but that the greatest feedback-induced increases in gait performance would be observed during the audiovisual biofeedback trial rather than with either visual or auditory biofeedback. Moreover, we hypothesized that compared to the visual and combined audiovisual biofeedback trials, participants would increase stride-to-stride variability of peak AGRF during the audio biofeedback trial when a visual display to guide propulsive force generation is absent.
Methods
Seven neurologically unimpaired (6 females, 27±2 years) and nine post-stroke (6 females, 54±12 years, 42±26 months post-stroke) individuals participated in one session of treadmill walking. All participants provided informed consent and the study was approved by the Institutional Human Individuals Review Board. Inclusion criteria for post-stroke individuals included: >6 months post-stroke, ability to walk on a treadmill continuously for 6-minutes, and ability to communicate with investigators. Exclusion criteria included neurologic diagnosis other than stroke, hemineglect, orthopedic conditions limiting walking, and cerebellar dysfunction.
Determination of self-selected gait speed
Self-selected gait speeds were determined individually for each participant by increasing the treadmill speed in 0.1 m/s increments until participants reported that the treadmill speed matched their comfortable walking speed (Fig. 1). All subsequent gait trials were performed at this self-selected speed. Participants walked on a dual belt treadmill with force platforms embedded under each belt (Bertec Corporation, Ohio, USA), with one foot on each belt to allow for collection of ground reaction force (GRF) data from each leg. The force platforms measured 3 components of GRF – vertical, medio-lateral, and antero-posterior; of these, the antero-posterior component was used to derive AGRF, the primary variable in our current study. All participants used a handrail for safety during all gait trials and were instructed to maintain consistent handrail grip throughout all trials. We visually monitored handrail grip and excessive trunk lean, and stopped and redid a gait trial if we observed excessive trunk lean or reliance on the handrail.
Fig. 1. Experimental protocol.
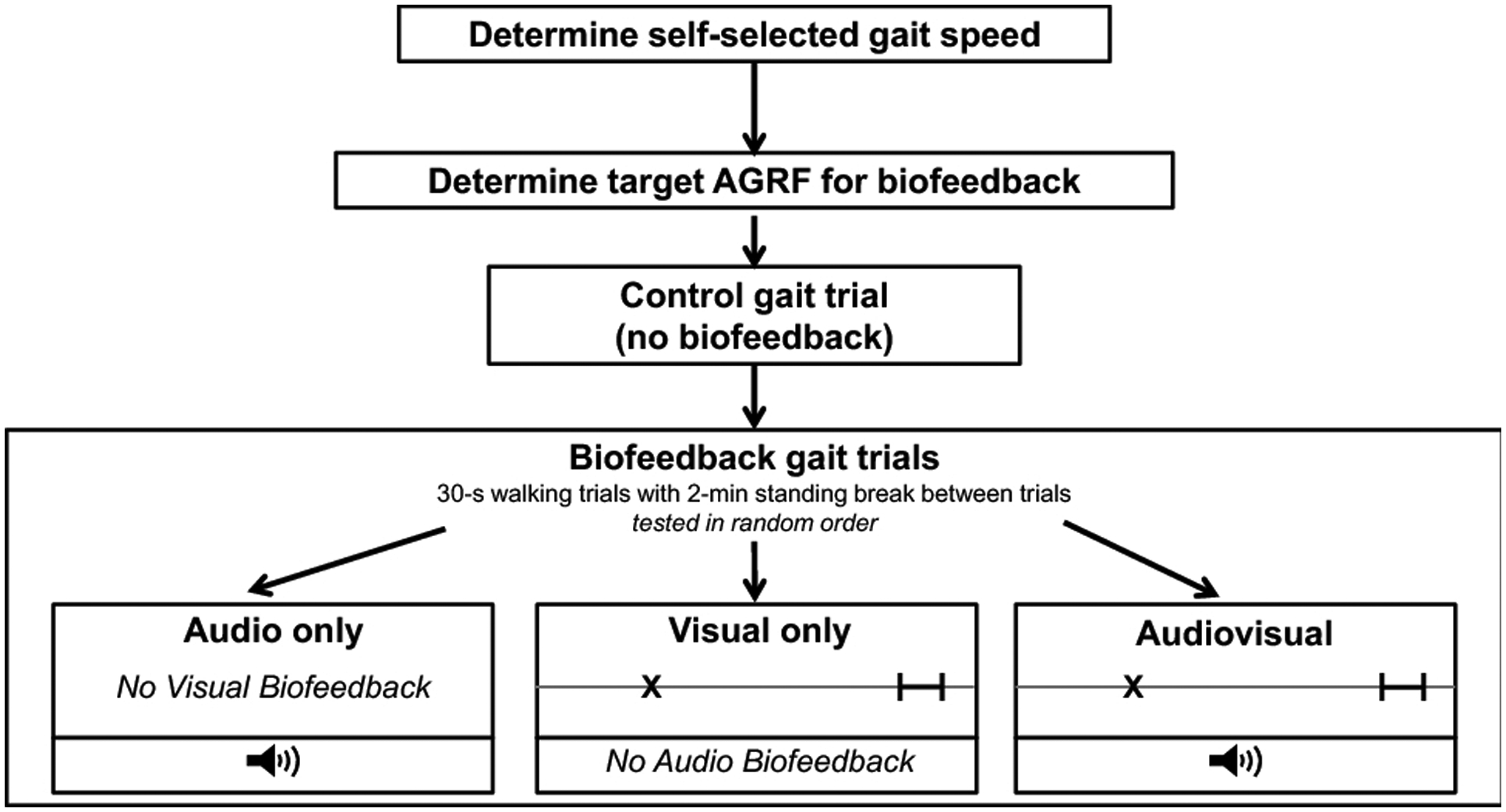
Verbal instructions regarding the mode of biofeedback and the goal for the subsequent trial were provided before each trial. For the audio biofeedback trial, the speaker symbol denotes that participants heard an audible beep when their current AGRF reached or exceeded their target AGRF. For the visual and audiovisual biofeedback trials, the visual display shown here was projected and displayed on a screen in front of the participant. The X cursor denotes the current measured AGRF during the stride cycle, and the black bar indicates the target AGRF
Determination of target AGRF for biofeedback trials
For able-bodied individuals, a 15-s walking trial was used to calculate the average peak AGRF produced by the right (targeted) leg. This peak AGRF baseline was increased by 20% to calculate the target AGRF (Schenck and Kesar 2017).
For post-stroke individuals, the target AGRF calculation employed methods similar to those in our recent study (Genthe et al. 2018). Using paretic and non-paretic leg peak AGRFs measured during a 30-s walking trial, five AGRF targets were calculated (see equation below) to determine a challenging individualized AGRF target for each post-stroke participant that was a greater than baseline peak AGRF by a specific magnitude or percentage (n=0.2, 0.4, etc.).
After calculating the five target AGRFs, stroke participants completed five 30-s trials with audiovisual AGRF biofeedback at each target value in a sequential order from lowest to highest. The AGRF target selected for all subsequent biofeedback trials corresponded to the gait trial with the largest AGRF target during which the participant achieved the target for ≥50% gait cycles.
Methodology for AGRF gait biofeedback
Participants were provided with three modes or types of biofeedback during the session: audio, visual, and audiovisual. Audio feedback consisted of an audible cue or beep that emitted a sound when the participant’s current AGRF was within a 6-Newton range of the target AGRF (Fig. 1). The audible beep indicated that the participant had successfully reached their target AGRF for the preceding gait cycle. The visual feedback display consisted of a horizontal line graph with a moveable cursor that represented the current measured AGRF of the targeted leg (MotionMonitor, Illinois, USA). Audiovisual biofeedback comprised both the visual display (similar to the visual biofeedback condition) and audio beep (similar to the audio biofeedback condition) (Fig. 1). A speaker and projector screen connected to the MotionMonitor biofeedback interface was placed in front of the treadmill to provide audio and visual feedback, respectively (Fig. 1).
Control and biofeedback gait trials
After determining the self-selected walking speed and target AGRF for biofeedback trials, participants completed a 30-s control trial without biofeedback, during which participants were instructed to walk normally and AGRF data were collected (Fig. 1). Next, three 30-s walking trials were collected in an order randomized across participants: audio, visual, and audiovisual biofeedback. Before each trial, participants received verbal instruction regarding the type of biofeedback provided and their goals during the subsequent trial. A 2-minute standing break was provided between gait trials.
Control trial:
No biofeedback was provided to participants during the control trial.
Audio biofeedback:
Participants were instructed to “push back into the ground harder until a beep sounded”, signaling that their current AGRF had reached their target AGRF. No visual biofeedback was provided during this trial.
Visual biofeedback:
Participants were shown a cursor that represented the current measured AGRF from the targeted (able-bodied) or paretic (stroke) leg. Participants were instructed to “push back into the ground harder until their cursor reached the target AGRF.” No audio biofeedback was provided during this trial.
Audiovisual biofeedback:
Participants were provided with both audio and visual biofeedback during this trial (Fig. 1).
Dependent Variables & Data Analysis
The primary dependent variables were peak AGRF of the targeted (right) leg and the paretic leg for able-bodied and post-stroke individuals, respectively. Secondary variables included the stride-to-stride coefficient of variance (CV) of peak AGRF for the targeted/paretic leg, peak AGRF for the non-targeted/non-paretic leg, and absolute percent error in peak AGRF production in the targeted/paretic leg with respect to the target AGRF. Additionally, a change score was calculated to evaluate the feedback-induced change in peak AGRF and CV of peak AGRF for the targeted (able-bodied) and paretic (post-stroke) leg.
The peak AGRF was calculated as the maximal value of anteriorly directed GRFs during the terminal double support phase of the leg, averaged across all gait cycles during the 30-s gait trials (Fig. 2c). The CV of peak AGRF was calculated by dividing the standard deviation of peak AGRF across all gait cycles by the average peak AGRF during a 30-s trial, providing a measure of stride-to-stride variability or consistency of AGRF generation. The absolute percent error was calculated as the absolute percent difference of the average generated peak AGRF minus the AGRF target. The change scores of peak AGRF and CV of peak AGRF were calculated as the difference between the peak AGRF during each of the three biofeedback trials with respect to the control trial without biofeedback.
Fig. 2. Peak AGRF of the (a) targeted (right) and paretic leg and (b) non-targeted and non-paretic leg for able-bodied (N=7, filled symbols) and post-stroke (N=9, unfilled symbols) individuals during the control and biofeedback (audio, visual, audiovisual) gait trials.
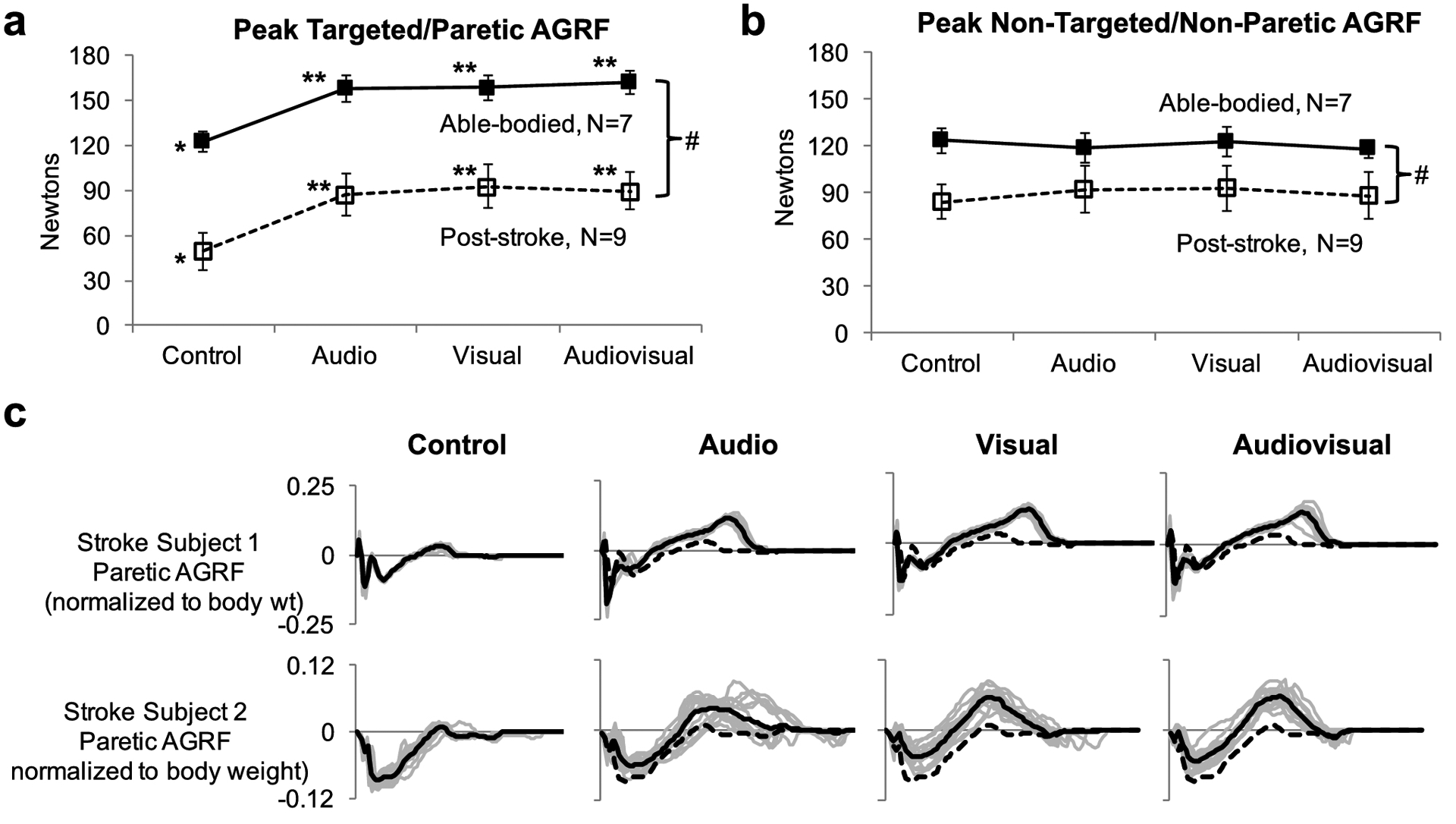
The 2-way repeated measures ANOVA detected a significant main effect of group (p<0.001, # symbol). The symbol * indicates a significant main effect (p<0.05) of biofeedback mode detected by the 1-way repeated measures ANOVA. The symbol ** indicates a significant difference (p<0.05) compared to the control trial, detected by Bonferroni-corrected post-hoc paired comparisons. Error bars represent standard error. (c) Raw antero-posterior GRF data during the control and biofeedback trials are shown for two representative stroke individuals. Solid black lines indicate the GRF profiles throughout the gait cycle (x-axis is normalized to the gait cycle) averaged over multiple strides during the trial. Light grey lines indicate GRFs for individual gait cycles. The dotted black line in the biofeedback trials (audio, visual, audiovisual) displays the average AGRF during the control trial (for comparison). Note that both representative stroke individuals significantly increased peak AGRF during each of the three biofeedback gait trials compared to the control trial
Statistical analyses
A 2-way repeated measures ANOVA was conducted to evaluate the effect of group (stroke, able-bodied) and biofeedback mode (control, audio, visual, and audiovisual) on each dependent variable. If a main effect of feedback type was found, a 1-way repeated measures ANOVA was conducted for each group. Additionally, three Bonferroni-corrected planned post-hoc paired comparisons were conducted to evaluate differences between control and audio biofeedback, control and visual biofeedback, and control and audiovisual biofeedback trials. To evaluate differences in feedback-induced change scores for AGRF and CV for the targeted and paretic leg between the three biofeedback modes, three paired t-tests were conducted to compare audio and visual, audio and audiovisual, and visual and audiovisual biofeedback change scores. Significance level was set at α≤0.05 for all tests. Additionally, effect sizes (Cohen’s d) were calculated for selected pairwise comparisons.
Results
Peak AGRF of the targeted and paretic leg
The 2-way repeated-measures ANOVA evaluating the effect of group (able-bodied, stroke) and biofeedback mode (audio, visual, audiovisual) on targeted peak AGRF showed a significant main effect of group (p<0.001) and biofeedback mode (p=0.003), and no significant interaction effect (p=0.990) (Fig. 2a).
The 1-way repeated measures ANOVA showed a significant main effect of biofeedback mode on peak AGRF of the targeted (right) leg for able-bodied participants (p=0.000, F=52.392) (Fig. 2a). Bonferroni-corrected post hoc paired comparisons showed a significantly greater targeted peak AGRF for audio (p<0.001, Cohen’s d= 1.71), visual (p=0.001, Cohen’s d= 1.78), audiovisual (p<0.001, Cohen’s d= 2.05) compared to that of the control.
The 1-way repeated measures ANOVA showed a significant main effect of biofeedback mode on peak AGRF of the paretic leg for stroke participants (p=<0.001, F=59.631) (Fig. 2a). Bonferroni-corrected post hoc paired comparisons revealed a significantly greater paretic peak AGRF for audio (p<0.001, Cohen’s d=0.962), visual (p<0.001, Cohen’s d=1.07), audiovisual (p<0.001, Cohen’s d=1.06) compared to that of the control.
Peak AGRF of the non-targeted and non-paretic leg
The 2-way repeated measures ANOVA evaluating the effect of group (able-bodied, stroke) and biofeedback mode (audio, visual, audiovisual) on non-targeted or non-paretic peak AGRF showed a significant main effect of group (p=0.001), no significant main effect on biofeedback mode (p=0.984), and no significant interaction effect (p=0.961) (Fig. 2b).
Feedback-induced change in peak AGRF for the targeted/paretic leg
In able-bodied individuals, the paired t-test evaluating feedback-induced change of peak AGRF in the targeted leg revealed no significant difference between audio and visual (p=0.906, Cohen’s d=0.067), audio and audiovisual (p=0.246, Cohen’s d= 0.516), and visual and audiovisual (p=0.210, Cohen’s d=0.381) biofeedback (Fig. 3a).
Fig. 3. The feedback-induced change in peak AGRF for the (a) targeted leg in able-bodied individuals, and (b) the paretic leg in post-stroke individuals during the audio, visual, and audiovisual biofeedback trials.
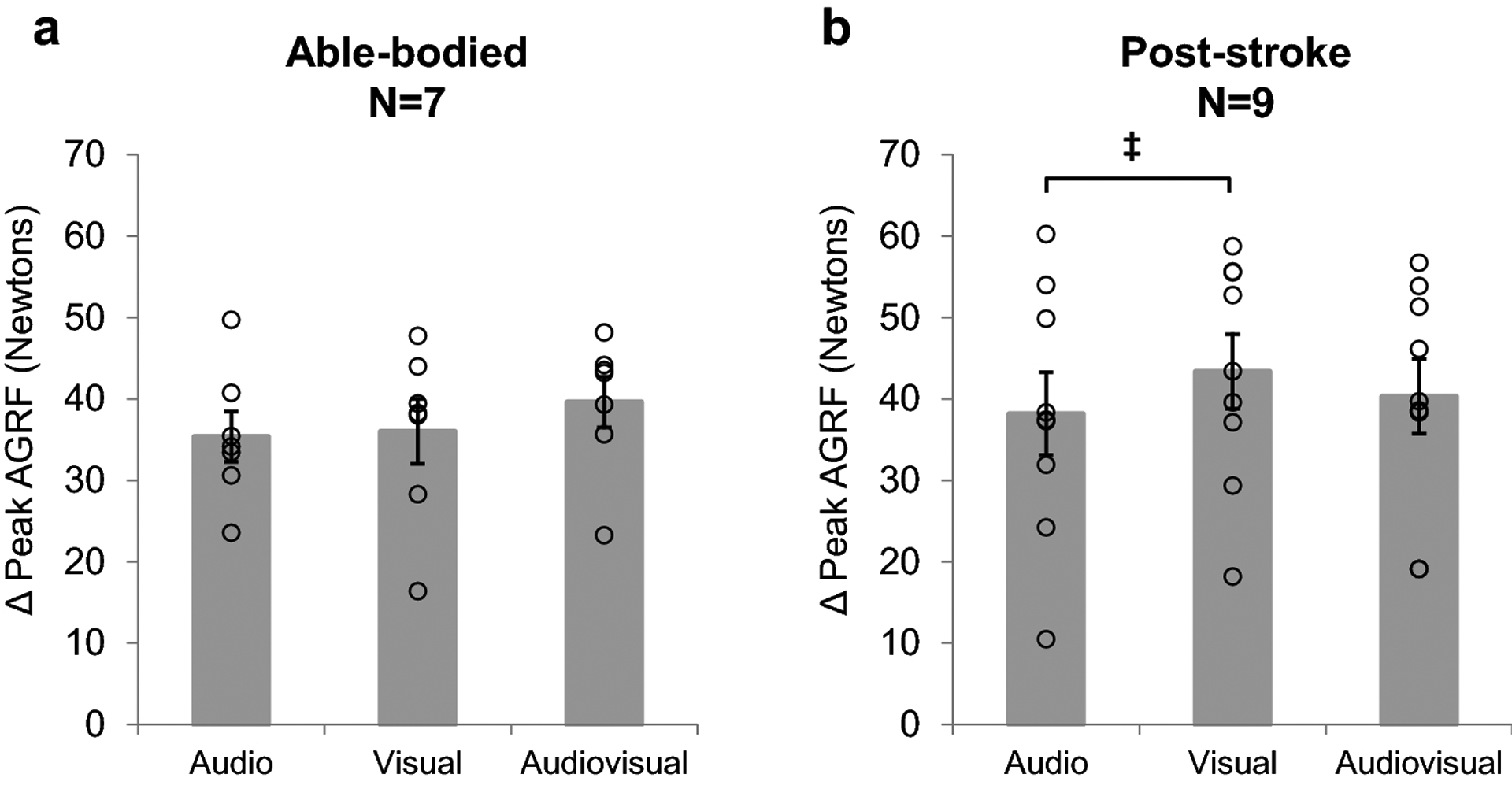
Change was calculated as the difference in peak AGRF between each biofeedback mode (audio, visual, audiovisual) and control. Open circles represent individual subject data. No significant differences in feedback-induced change of peak AGRF were observed in the targeted leg of able-bodied individuals. Paired t-test revealed a trend (p<0.1, ‡ symbol) towards a greater feedback-induced change during visual compared to audio biofeedback in post-stroke individuals. Error bars indicate standard error
In post-stroke individuals, the paired t-test evaluating feedback-induced change of paretic leg peak AGRF revealed a trend of a larger change score during visual compared to audio biofeedback (p=0.0832, Cohen’s d=0.356) (Fig. 3b), but no significant differences in paretic leg peak AGRF change score between audio and audiovisual biofeedback (p=0.329, Cohen’s d=0.146) and visual and audiovisual biofeedback (p=0.208, Cohen’s d=0.222).
Stride-to-stride CV of peak AGRF
The 2-way repeated measures ANOVA evaluating the effect of group (able-bodied, stroke) and biofeedback mode (audio, visual, audiovisual) on stride-to-stride coefficient of variation (CV) of peak AGRF showed a significant main effect of group (p<0.001), but no significant main effect on biofeedback mode (p=0.770), and no significant interaction effect (p=0.309) (Fig. 4a).
Fig. 4. (a) Stride-to-stride coefficient of variation (CV) of peak targeted AGRF in able-bodied (N=7, filled symbols) and paretic legs of post-stroke (N=9, open symbols) participants, respectively.
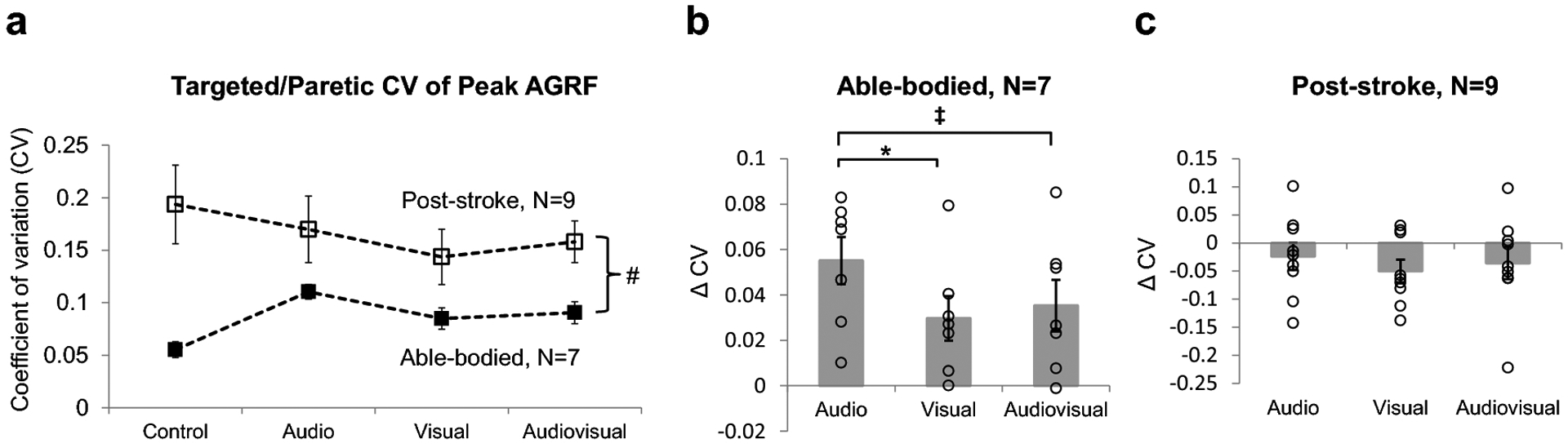
The 2-way repeated measures ANOVA found a significant main effect of group (p<0.001, # symbol), but no significant main effect of biofeedback mode. (b, c) The feedback-induced change in targeted or paretic leg CV of peak AGRF (with respect to the control trial without biofeedback) during the audio, visual, and audiovisual biofeedback trials for (b) able-bodied individuals and (c) post-stroke individuals. Feedback-induced changes were calculated as the difference in CV of peak AGRF between the biofeedback (audio, visual, audiovisual) and control trials. Open circles represent individual subject data. Able-bodied individuals demonstrated a significantly larger feedback-induced change in targeted CV of peak AGRF during the audio compared to visual biofeedback trial (p=0.021, * symbol), as well as a trend towards a larger feedback-induced change during the audio compared to audiovisual biofeedback trial (p=0.067, ‡ symbol). Post-stroke individuals did not demonstrate a significant difference in feedback-induced change in paretic leg CV of peak AGRF among the three biofeedback trials. Error bars indicate standard error
Feedback-induced change in CV of peak AGRF for the targeted/paretic leg
In able-bodied individuals, the paired t-test evaluating feedback-induced change in targeted leg CV of peak AGRF showed a significantly larger change score during audio compared to visual biofeedback (p=0.021, Cohen’s d=0.951) (Fig. 4b). A statistical trend for an increase in CV of peak AGRF was found during audio biofeedback compared to audiovisual biofeedback (p=0.067, Cohen’s d=0.688). No significant difference in CV of peak AGRF change score was found between the visual and audiovisual biofeedback conditions (p=0.569, Cohen’s d=0.2).
In post-stroke individuals, the paired t-tests evaluating feedback-induced change in paretic leg CV of peak AGRF found no significant differences between audio and visual (p=0.138, Cohen’s d= 0.388), audio and audiovisual (p=0.676, Cohen’s d= 0.148), and visual and audiovisual (p=0.480, Cohen’s d= 0.191) biofeedback (Fig. 4c).
Absolute percent error in peak AGRF of targeted or paretic leg with respect to target AGRF
The 2-way repeated measures ANOVA evaluating the effect of group (able-bodied, stroke) and biofeedback mode (audio, visual, audiovisual) on absolute percent error in peak AGRF of the targeted/paretic leg with respect to the target AGRF showed a significant main effect of group (p<0.001), no significant effect of biofeedback mode (p=0.765), and no significant interaction effect (p=0.791) (Fig. 5).
Fig. 5. Average absolute percent error of the targeted (able-bodied) and paretic (stroke) peak AGRF with respect to the target AGRF during biofeedback.
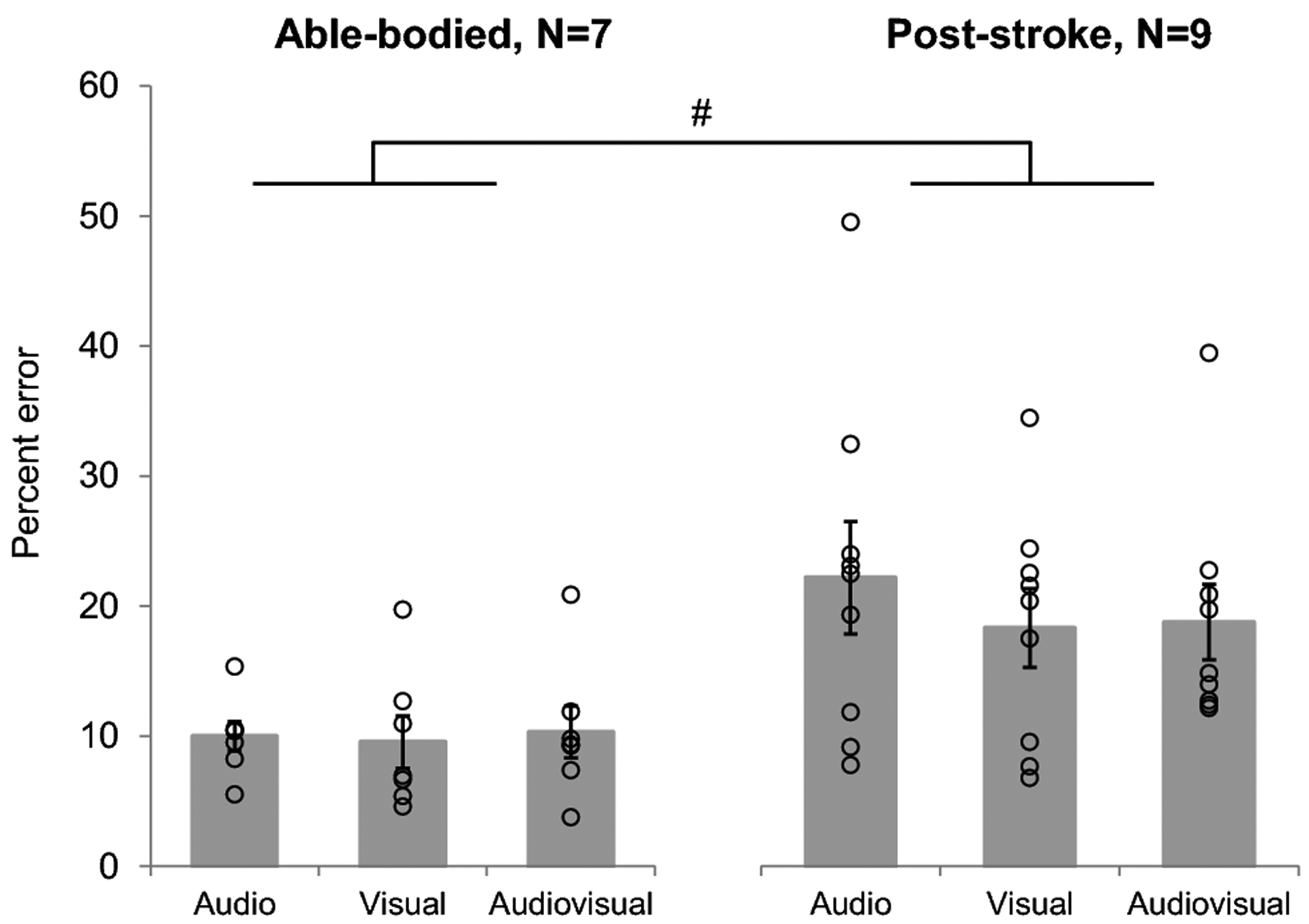
Compared to able-bodied individuals, post-stroke individuals demonstrated significantly greater absolute percent error in paretic peak AGRF with respect to the AGRF target, detected by the 2-way repeated measures ANOVA (p<0.001, # symbol). Absolute percent error did not significantly change between the biofeedback conditions in either able-bodied or post-stroke individuals. Open circles represent individual subject data. Errors bars indicate standard error
DISCUSSION
The present study investigated the effects of three different modes of real-time AGRF biofeedback (audio, visual, audiovisual) on propulsive force generation during the terminal stance phase of gait in able-bodied and post-stroke individuals. During short-duration gait trials comprising exposure to audio, visual, or audiovisual AGRF biofeedback, both able-bodied and post-stroke individuals demonstrated a significant increase (with large effect size magnitudes, Cohen’s d > 0.9) in the targeted/paretic limb peak AGRF compared to control gait trials without biofeedback (Fig. 2a). Interestingly, we failed to find a significant difference in the feedback-induced change in targeted and paretic leg peak AGRF among the three modes of biofeedback. However, post-stroke participants demonstrated a trend towards a larger feedback-induced change score, with a small magnitude of effect (Cohen’s d=0.36) for paretic leg peak AGRF during the visual compared to audio biofeedback trial (Fig. 3b). In able-bodied subjects, while the peak AGRF change scores were not different between the audio and visual biofeedback trials, there was a significantly larger change score for CV of peak AGRF, with a large effect size (Cohen’s d>0.9), during the audio compared to visual biofeedback trial. Moreover, able-bodied participants demonstrated a statistical trend (with a medium effect size) towards a larger change score for CV of peak AGRF during the audio compared to audiovisual biofeedback trial (Fig. 4b). In other words, when visual information is removed, able-bodied individuals showed significantly greater stride-to-stride variability of peak AGRF generation, compared to when visual information was present. Post-stroke individuals did not demonstrate any significant differences in CV of peak AGRF change score between the three biofeedback modes, suggesting that regardless of mode of biofeedback provided, post-stroke participants did not significantly alter the stride-to-stride variability of peak AGRF generation (Fig. 4c). Absolute percent error of peak AGRF with respect to the AGRF target was not significantly different among the three biofeedback modes (Fig. 5). For both able-bodied and post-stroke individuals, no significant differences in non-targeted/non-paretic leg peak AGRF were observed between the control trial and the three biofeedback trials (Fig 2b). The lack of feedback-induced change in the non-targeted leg further confirms our recent findings (Genthe et al. 2018; Schenck and Kesar 2017), and showcases the advantage of biofeedback for specifically and preferentially targeting the paretic leg gait parameters in people with unilateral gait deficits such as post-stroke hemiparesis.
Our present findings align with results from previous studies that have demonstrated increased targeted leg AGRF in able-bodied individuals or paretic leg AGRF in post-stroke individuals in response to real-time audiovisual AGRF gait biofeedback (Genthe et al. 2018; Schenck and Kesar 2017). Surprisingly, the mode of biofeedback provided to participants, whether audio only, visual only, or both audio and visual, did not differentially affect feedback-induced changes in peak AGRF of the targeted and paretic leg (all pairwise comparison effect sizes < 0.5) (Fig. 2a and 2b). Our results may have important clinical implications given the varied feasibility of introducing different modes of biofeedback into clinical or community environments. Given that audio feedback requires only speakers or earphones and no visual display interface, audio biofeedback may be more feasible for overground gait training, and more readily applicable in clinical or community environments. In contrast, visual and audiovisual feedback require a visual display interface (screen or head-mounted headsets), which may limit their application during overground gait training. The use of visual biofeedback during overground gait training may also be challenging because overground walking presents more visual distractions and obstacles for participants. Our results suggest that for both able-bodied and post-stroke individuals, audio biofeedback may hold promise for use in clinical environments without sacrificing the beneficial effects of biofeedback on gait propulsion. Future studies, however, are needed to investigate the effects of audio, visual, and audiovisual AGRF biofeedback in a clinical or overground setting.
Our study did reveal a trend, with a small-to-medium effect size, towards a larger feedback-induced improvement in peak AGRF during the visual compared to audio biofeedback trial in post-stroke individuals (Fig 2b). While audio or visual biofeedback alone may be sufficient for inducing a significant increase in paretic leg peak AGRF, for post stroke individuals, visual biofeedback may be better than audio biofeedback to induce improvements in peak AGRF. Sigrist et al. suggested that visual biofeedback may be more effective at increasing task performance and retention when utilized during complex compared to simpler tasks (Sigrist et al. 2013).Walking is generally considered a simple and automated task for able-bodied individuals, but may be considerably more challenging for some individuals with post-stroke hemiparesis. Our results indicate that post-stroke individuals may benefit more from visual compared to audio biofeedback during a propulsive force generation walking task. Eventually, however, the goal of post-stroke gait retraining is for walking to become more automated (Canning et al. 2006); a visual cue may potentially disrupt this automaticity of gait. Future work can investigate how incorporating different modes of biofeedback during different stages of learning can best enhance gait propulsion. For example, subsequent studies could explore biofeedback protocols that transition post-stroke individuals from audiovisual to audio to no AGRF biofeedback over the course of multiple training sessions.
In the able-bodied individuals, our results demonstrated that the feedback-induced change in stride-to-stride CV of peak AGRF was significantly larger, with a large magnitude of effect (Cohen’s d>0.9) during the audio compared to visual biofeedback trial (Fig. 3b). The CV (coefficient of variation) of AGRF, similar to the CV or variance of other gait performance variables such as step length or kinematics, provides a measure of the variability of gait the parameter from one step to another. During walking, a higher magnitude of CV may show up as greater variability or lack of consistency in an individual’s walking patterns. Our current findings suggest that depriving able-bodied subjects of visual information regarding their targeted AGRF with respect to their ongoing force generation may lead to increased stride-to-stride variability. A visual target may increase the likelihood of aiming for the target AGRF more consistently with every gait cycle, thus reducing the CV of peak AGRF. Our results also reveal that, as expected, individuals post-stroke walk with significantly larger stride-to-stride CV of peak AGRF compared to able-bodied young adults. This finding is consistent with other studies that have reported increased variability in spatiotemporal variables such as step length, stride length, and swing time in post-stroke compared to able-bodied individuals (Balasubramanian et al. 2009). Surprisingly, post-stroke individuals did not demonstrate a significant difference in the feedback-induced change in paretic leg CV of peak AGRF between the three biofeedback modes (Fig. 3c). Our results failed to confirm our hypothesis that CV of peak AGRF would decrease with the addition of biofeedback, and that the feedback-induced change would be the greatest during the visual and audiovisual biofeedback conditions. One potential explanation may be that the biofeedback task in the current study comprised a short duration of walking and/or did not demand accuracy. The criteria for success during gait biofeedback trials did not require participants to bring their AGRF within a certain range of peak AGRF, but rather to simply reach or exceed the target AGRF; that is, classical “shaping” was not emphasized in this training paradigm. Thus, participants may have used the strategy of overshooting with respect to the target AGRF range rather than consistently meeting the exact target AGRF. Because the biofeedback task was setup to reward generation of AGRF at or above target as “success” without the need to accomplish “accuracy” of AGRF generation through gradations within a specified range, the visual and audiovisual biofeedback conditions may have failed to improve gait variability more than audio biofeedback alone.
We did not observe a significant main effect of biofeedback mode on absolute percent error of peak AGRF with respect to the AGRF target (Fig. 5). Our results were contrary to our hypothesis that individuals would demonstrate a greater percent error of peak AGRF during the audio condition compared to the visual and audiovisual condition. We had predicted that biofeedback trials with visual information, (i.e. audiovisual and visual alone trials) would show improved percent error or accuracy compared to biofeedback trials without visual information (audio only). However, as noted previously, the lack of significant difference between the audio and visual modes may be explained by the fact that the participants adopted the strategy of overshooting the target AGRF to accomplish success. For our biofeedback system, the criterion for success was generation of AGRF equal to or exceeding the target. A pertinent question related to the future development of gait biofeedback training paradigms is whether gait tasks, such as increasing paretic push-off force generation during terminal stance require accuracy. We posit that push-off generation during gait is not a skilled, accuracy-based task, and that the goal of improving gait for individuals post-stroke is to simply increase the push-off force generation in the paretic leg, without precision requirements with regards to remaining within a prescribed or constrained AGRF target range. However, the temporal aspects of propulsive force generation during late stance phase may be a biofeedback variable that requires accuracy during gait (Turns et al. 2007), and warrant future investigation.
Compared to the control gait trial without biofeedback, there was no significant main effect of biofeedback type on peak AGRF in the non-targeted or non-paretic leg in either able-bodied or post-stroke individuals (Fig. 2b). Our findings regarding lack of change in the non-targeted leg during biofeedback support our previous work that used audiovisual gait AGRF biofeedback (Genthe et al. 2018; Schenck and Kesar 2017) and also suggest that peak AGRF in the non-targeted/non-paretic leg does not change during walking with other modes of biofeedback, such as audio alone or visual alone. These findings support the notion that real-time gait biofeedback, regardless of the mode of delivery, provides a promising gait training technique to unilaterally target propulsive force deficits in the paretic limb of post-stroke individuals, without inducing concomitant compensatory changes in gait parameters in the non-paretic limb.
In addition to differences between biofeedback modes within the able-bodied and post-stroke groups, our results also show significant differences in propulsion between able-bodied and post-stroke participants. As expected, compared to the targeted leg of able-bodied individuals, post-stroke individuals demonstrate significantly reduced peak AGRF in the paretic leg (Fig. 2a). The statistical tests also found a significant main effect of group for non-paretic and non-targeted leg peak AGRF, revealing that post-stroke participants even demonstrate significantly less AGRF in their non-affected limb compared to the non-targeted limb of young, able-bodied individuals (Fig. 2b). The difference in non-paretic and non-targeted leg peak AGRF is likely because our able-bodied group consisted of younger individuals compared to the older individuals in the post-stroke group. Our findings are in agreement with previous studies showing that older individuals walk with less propulsion compared to healthy younger adults (Franz 2016; Kerrigan et al. 1998). Our results also revealed that individuals post-stroke walk with larger stride-to-stride CV of peak AGRF compared to able-bodied young adults, which is consistent with previous reports of increased variability in post-stroke gait parameters (Balasubramanian et al. 2009). Our analyses also revealed greater absolute percent error with respect to the biofeedback target AGRF in post-stroke compared to able-bodied participants, which may reflect post-stroke motor control deficits.
There are several limitations in our present study. First, the duration of gait trials with biofeedback was short (30-second), and potentially, differences between the 3 modes of biofeedback may emerge during longer-duration walking bouts (e.g. 6 minutes) or a complete training session (e.g. a 30-minute session comprising six 5-minute bouts). Second, although the order of biofeedback trials was randomized across all participants, all post-stroke participants were initially exposed to audiovisual biofeedback during the AGRF target determination trials. This methodological choice was related to ensuring stroke participant safety. We could not feasibly and safely find an appropriate AGRF target for post-stroke individuals without first exposing individuals to a range of AGRF targets in a sequential manner. The initial exposure to audiovisual biofeedback (during target AGRF determination) before all other gait biofeedback trials may contribute to the lack of significant differences between the three biofeedback modes in post-stroke individuals. Third, both able-bodied and post-stroke participants were allowed to use a light-touch on the handrail during all gait trials, which may have resulted in reliance on the handrail as a compensatory mechanism to increase AGRF production. However, to prevent the effects of variable handrail grip from influencing study results, participants were reminded throughout the study to maintain a consistent magnitude of handrail grip force and a light fingertip touch on the handrail during walking.
In summary, we found that audio, visual, and audiovisual modes of AGRF biofeedback significantly increased peak AGRF in the targeted and paretic legs of able-bodied and post-stroke individuals, respectively. However, no significant differences in targeted and paretic leg peak AGRF were observed among the three biofeedback modes. Our findings may hold implications for the use of different gait biofeedback modes in the clinical and community environments, given the feasibility of audio biofeedback in non-laboratory settings. Future studies are needed to compare the longer-term effects and carry-over to overground for audio, visual, and audiovisual AGRF gait biofeedback. Our study results provide insights that can inform the future development of effective biofeedback rehabilitative strategies to improve post-stroke gait function.
Acknowledgements:
We would like to thank Steven Eicholtz and Christopher Schenck for assisting with data-collection and data-processing.
Funding Sources: TMK is supported by the National Institute of Child Health and Human Development grant number K01 HD079584. SLW is supported by grants U10NS086607 and U01 NS091951.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare that they have no conflict of interest
References
- Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS (2015) Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke Neurorehabil Neural Repair 29:499–508 doi: 10.1177/1545968314554625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA (2014) Targeting paretic propulsion to improve poststroke walking function: a preliminary study Arch Phys Med Rehabil 95:840–848 doi: 10.1016/j.apmr.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA (2016) Reducing The Cost of Transport and Increasing Walking Distance After Stroke: A Randomized Controlled Trial on Fast Locomotor Training Combined With Functional Electrical Stimulation Neurorehabil Neural Repair 30:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA (2007) Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis Arch Phys Med Rehabil 88:43–49 [DOI] [PubMed] [Google Scholar]
- Balasubramanian CK, Neptune RR, Kautz SA (2009) Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke Gait Posture 29:408–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder SA, Moll CB, Wolf SL (1981) Evaluation of electromyographic biofeedback as an adjunct to therapeutic exercise in treating the lower extremities of hemiplegic patients Physical therapy 61:886–893 [DOI] [PubMed] [Google Scholar]
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA (2006) Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking Stroke 37:872–876 doi: 10.1161/01.STR.0000204063.75779.8d [DOI] [PubMed] [Google Scholar]
- Bowden MG, Behrman AL, Neptune RR, Gregory CM, Kautz SA (2013) Locomotor rehabilitation of individuals with chronic stroke: difference between responders and nonresponders Archives of physical medicine and rehabilitation 94:856–862 [DOI] [PubMed] [Google Scholar]
- Canning CG, Ada L, Paul SS (2006) Is automaticity of walking regained after stroke? Disability and Rehabilitation 28:97–102 [DOI] [PubMed] [Google Scholar]
- Chen G, Patten C, Kothari DH, Zajac FE (2005a) Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds Gait Posture 22:51–56 [DOI] [PubMed] [Google Scholar]
- Drużbicki M, Guzik A, Przysada G, Kwolek A, Brzozowska-Magoń A (2015) Efficacy of gait training using a treadmill with and without visual biofeedback in patients after stroke: A randomized study Journal of rehabilitation medicine 47:419–425 [DOI] [PubMed] [Google Scholar]
- Drużbicki M, Guzik A, Przysada G, Kwolek A, Brzozowska-Magoń A, Sobolewski M (2016) Changes in Gait Symmetry After Training on a Treadmill with Biofeedback in Chronic Stroke Patients: A 6-Month Follow-Up from a Randomized Controlled Trial Med Sci Monit 22:4859–4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR (2016) The age-associated reduction in propulsive power generation in walking Exercise and sport sciences reviews 44:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthe K, Schenck C, Eicholtz S, Zajac-Cox L, Wolf S, Kesar TM (2018) Effects of real-time gait biofeedback on paretic propulsion and gait biomechanics in individuals post-stroke Topics in stroke rehabilitation 25:186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa N, Takeda K, Sakuma M, Mani H, Maejima H, Asaka T (2017) Learning effects of dynamic postural control by auditory biofeedback versus visual biofeedback training Gait & posture 58:188–193 [DOI] [PubMed] [Google Scholar]
- Hsiao HY, Awad LN, Palmer JA, Higginson JS, Binder-Macleod SA (2016) Contribution of paretic and non-paretic limb peak propulsive forces to changes in walking speed in individuals poststroke Neurorehabil Neural Repair 30:743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ (1998) Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments Archives of physical medicine and rehabilitation 79:317–322 [DOI] [PubMed] [Google Scholar]
- Ki KI, Kim MS, Moon Y, Choi JD (2015) Effects of auditory feedback during gait training on hemiplegic patients’ J Phys Ther Sci 27:1267–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molier BI, Van Asseldonk EH, Hermens HJ, Jannink MJ (2010) Nature, timing, frequency and type of augmented feedback; does it influence motor relearning of the hemiparetic arm after stroke? A systematic review Disability and rehabilitation 32:1799–1809 [DOI] [PubMed] [Google Scholar]
- Mulroy SJ, Klassen T, Gronley JK, Eberly VJ, Brown DA, Sullivan KJ Gait parameters associated with responsiveness to treadmill training with body-weight support after stroke: an exploratory study Phys Ther 90:209–223 [DOI] [PubMed] [Google Scholar]
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D (1999) Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors Clin Biomech (Bristol, Avon) 14:125–135 [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE (2001) Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking J Biomech 34:1387–1398 [DOI] [PubMed] [Google Scholar]
- Schenck C, Kesar TM (2017) Effects of unilateral real-time biofeedback on propulsive forces during gait Journal of neuroengineering and rehabilitation 14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist R, Rauter G, Riener R, Wolf P (2013) Augmented visual, auditory, haptic, and multimodal feedback in motor learning: a review Psychonomic bulletin & review 20:21–53 [DOI] [PubMed] [Google Scholar]
- Stanton R, Ada L, Dean CM, Preston E (2017) Biofeedback improves performance in lower limb activities more than usual therapy in people following stroke: a systematic review Journal of physiotherapy 63:11–16 [DOI] [PubMed] [Google Scholar]
- Turns LJ, Neptune RR, Kautz SA (2007) Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking Archives of physical medicine and rehabilitation 88:1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Binder-MacLeod SA (1983) Electromyographic biofeedback applications to the hemiplegic patient. Changes in upper extremity neuromuscular and functional status Phys Ther 63:1393–1403 [DOI] [PubMed] [Google Scholar]


