Abstract
Background
miRNAs regulate a multitude of cellular processes and their aberrant regulation is linked to human cancer. However, the role of miR-425-5p in lung cancer (LCa) is still largely unclear. Here, we explored the role of miR-425-5p during LCa tumorigenesis.
Methods
Cell proliferation was evaluated by cell counting Kit-8 and colony formation assay. Western blot and real-time PCR were accordingly used to detect the relevant proteins, miRNA and gene expression. Luciferase reporter assays were used to illustrate the interaction between miR-425-5p and PTEN.
Results
We demonstrate that miR-425-5p is overexpressed in LCa tissue and enhances the proliferative and colony formation capacity of the LCa cell lines A549 and NCI-H1299. Through predictive binding assays, PTEN was identified as a direct gene target and its exogenous expression inhibited the pro-cancer effects of miR-425-5p. Through its ability to down-regulate PTEN, miR-425-5p activated the PI3K/AKT axis.
Conclusion
We conclude that miR-425-5p promotes LCa tumorigenesis through PTEN/PI3K/AKT signaling.
Keywords: miR-425-5p, Lung cancer, PTEN, PI3K/AKT signaling pathways
Background
Lung cancer (LCa) is a leading cause of cancer related mortality across the globe. LCa is prevalent in males [1] and asymptomatic during early disease stages. As many as 2 in every 3 cases are at an advanced stage (III or IV) when diagnosed and the 5-year survival rates remain low, particularly for those with metastatic LCa [2]. Improved LCa therapeutics is thus urgently required.
MicroRNAs (miRNAs) regulate many cell processes including differentiation, metabolism and tumorigenesis [3–6]. Emerging evidence suggests that miRNAs are key players during LCa tumorigenesis [7–10]. The aberrant expression of miR-425-5p is linked to hepatocellular carcinoma (HCC), gastric cancer (GCa) and colorectal cancer (CRC) [11–13]. Here, we report the upregulation of miR-425-5p in LCa and highlight its contribution to LCa development. We further identify PTEN as a novel miR-425-5p target that is inhibited in LCa to promote PTEN/PI3K/AKT signaling.
Methods
Patient specimens
LCa samples (n = 25) and adjacent healthy tissue (at least 2 cm from the resection margin) were collected from the Fourth Affiliated Hospital, Zhejiang University School of Medicine. The study was fully supported by the Institutional Review Board of the Fourth Affiliated Hospital, Zhejiang University School of Medicine (No.2015-0-09). All participants provided consent for sample analysis and anything about their identities will not be included in the data.
LCa cell lines, cell culture and cell transfection
Human lung cancer cell lines A549, NCI-H1299, NCI-H460, HCC827 and Normal Human Lung Epithelial cell line (BEAS-2B) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). A549 & HCC827 cells were cultured in DMEM plus 10% FBS and pen/strep. NCI-H1299 & NCI-H460 cells were grown in RPMI-1640 plus 10% FBS at 37 °C in 5% CO2. BEAS-2Bs were cultured in Clonetics™ media.
The miR-425-5p mimics and negative control miRNA (NC) were chemically synthesized by Shanghai GenePharma Co., Ltd. (Songjiang, Shanghai, China). Lipofectamine 2000 (Invitrogen, Eugene, OR, USA) was used for transfection according to the manufacturer’s protocol. PI3K activity was assayed as previously described [6]. PI3K inhibitor LY294002 was obtained from Abcam. To analyze the effects of miR-425-5p on PI3K/AKT, indicated Lca cells were cultured in the presence or absence of the drugs for 24 h at 37 °C, the working concentrations of LY294002 were 30 μM. For experiments with LY294002 treatments, indicated Lca cells were pre-treated with LY294002 for 1 h prior to exposure to proteasome inhibitors.
Cell growth assays
LCa viability was assessed using Cell Counting Kit-8 from Shanghai Haling Biotechnology, Co., Ltd. (Shanghai, China) as per the manufacturer’s protocols. Briefly, after incubating the transfected cells for one full day, they were collected after trypsinization and seeded (~ 5000 cells/well) into 96-well plates. Ten microliters of CCK-8 solution were added per well and kept for 2 h at 37 °C. The absorbance of the mixture was estimated in a microplate reader from Bio-Rad Laboratories, Inc. (Hercules, USA) at 450 nm.
Colony formation assay
The colony formation assays were performed as previous [6]. Each group of treated cells (1 × 103 per well) was seeded into 10 cm culture dish, and cultured for 2 weeks. Finally, colonies were stained using 1% crystal violet and the number of cell colonies was counted.
qRT-PCR analysis
Total RNA was isolated by TRIzol® reagent from Invitrogen (Thermo Fisher Scientific, Inc.), and a NanoDrop (NanoDrop Technologies; Thermo Fisher Scientific, Inc.) was used to estimate its quality and concentration. The expression of miR-425-5p was done by reverse transcription using the Mir-X™ miRNA First-Strand Synthesis Kit from TaKaRa Biotechnology, Co., Ltd. (Dalian, China), and quantitative evaluation of the synthesized cDNA was done by quantitative PCR (RT-qPCR) using the Mir-X™ miRNA qRT-PCR TB Green® Kit from TaKaRa Biotechnology. As an endogenous control, the small nuclear RNA U6 normalized the expression of miR-425-5p. The 2−ΔΔCq system was used to evaluate all genes expressions and the primer sequences were shown as follows: miR-425-5p (forward: 5′- GGGGAGTTAGGATTAGGTC-3′, reverse: 5′- TGCGTGTCGTGGAGTC-3′), U6 (forward: 5′-CTCGCT TCGGCAGCACA-3′, reverse: 5′-AAC GCT TCA CGA ATT TGC GT-3′), PTEN (forward: 5′- TGGATTCGACTTAGACTTGACC − 3′, reverse: 5′- AGGATATTGTGCAACTCTGCAA -3′), GAPDH (forward: 5′-CATCACCATCTTCCAGGAGCG-3′, reverse: 5’TGACCTTGCCCACAGCCTT-3′).
Dual luciferase assays
The design and synthesis of PTEN fragments containing binding sites for WT (wild-type) and MUT (mutant) on miR-425-5p was done by Shanghai GenePharma. These were cloned into the Target Expression Vector pmirGLO Dual-luciferase from Promega Corporation (WI, USA) to get the reporter plasmids WT-PTEN and MUT-PTEN. One night prior to transfection, seeding of cells (60–70% confluence) was done in plates with 24-wells. Transfection of these cells was done with reporter plasmids harboring WT or MUT in the presence of miR-NC or miR-425-5p mimic. Post 48 h of transfection, luciferase activity of the cells was estimated as per instructions using the Dual-Luciferase Reporter Assay System from Promega Corporation (Promega, Fitchburg, WI, USA). The data normalization was done by the activity of Renilla luciferase.
WB
Cell lysates (RIPA lysates) were resolved on SDS page and transferred to PVDF membranes. Membranes were blocked for 1 h in 5% milk plus TBST, incubated with primary antibodies against PTEN (dilution, 1:5000; cat. no. ab170941; Abcam), PI3K (dilution, 1:8000; cat. no. ab32089; Abcam), p-AKT (dilution, 1:8000; cat. no. ab81283; Abcam), AKT (dilution, 1:8000; cat. no. ab32505; Abcam), p-AKT (dilution, 1:8000; cat. no. ab81283; Abcam), ACTIN (dilution, 1:8000; cat. no. ab8226; Abcam) at 4 °C overnights, and labeled with HRP-conjugated secondary’s for 2 h at room temperature. Bands were visualized using chemiluminescent HRP Substrate and analyzed using Image Lab TM Software.
Statistical analyses
Data analysis was performed using SPSS 19.0. Treatment groups were compared using a one-way ANOVA. P-values < 0.05 were taken as significant. Experiments were performed on at least three occasions. Data represent the mean ± SD.
Results
miR-425-5p is upregulated in LCa
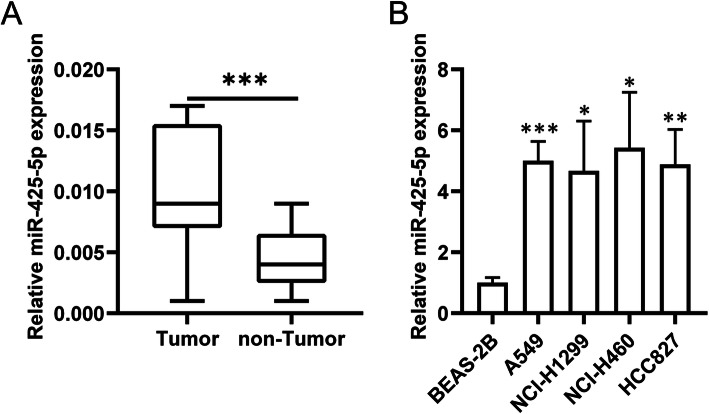
We compared miR-425-5p levels in 25 paired LCa and normal lung tissue samples by qRT-PCR analysis. miR-425-5p was upregulated in LCa specimens (Fig. 1a) and expressed to high levels in A549, NCI-H1299, NCI-H460, and HCC827 cells compared to normal human lung epithelial cell line (BEAS-2B) (Fig. 1b) consistent with previous findings in other cancer types. Previous results indicated that miR-425-5p is up-regulated in LCa.
Fig. 1.
MiR-425-5p in highly expressed in LCa (a). qRT-PCR of miR-425-5p. b. qRT-PCR of A549, NCI-H1299, NCI-H460, HCC827 and BEAS-2B cells. Data: mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001
miR-425-5p enhances proliferation and inhibits apoptosis in LCa cells
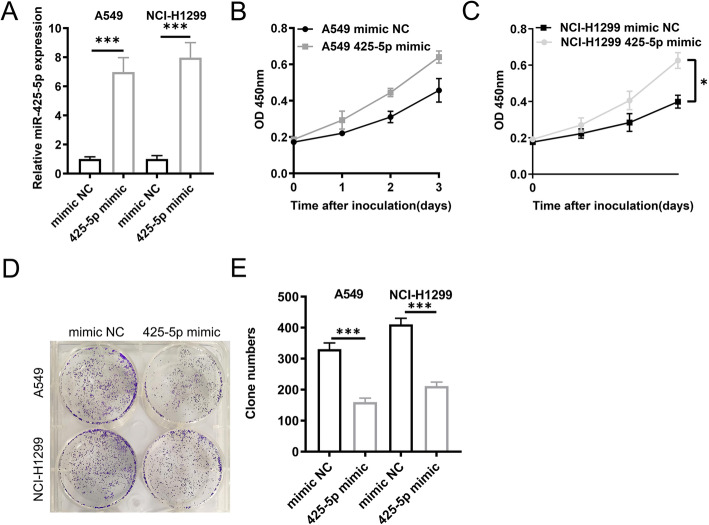
To dissect the role of miR-425-5p in LCa, its expression was manipulated using miR-425-5p mimics. Figure 2a-c shows that miR-425-5p upregulation enhanced cell survival, meanwhile enhanced cell colony formation ability (Fig. 2d and e). Taken together, above results indicated miR-425-5p is thus an oncogene in LCa cells.
Fig. 2.
MiR-425-5p promotes LCa. (a). miR-425-5p expression in A549 and NCI-H1299 cells. b & c. CCK-8 assays in miR-425-5p mimic transfected cells. d & e. Colony-forming assay in miR-425-5p mimic transfected cells. The raw data of all colony formation experiments was listed in supplemental Table 1. Data: mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001
miR-425-5p targets PTEN
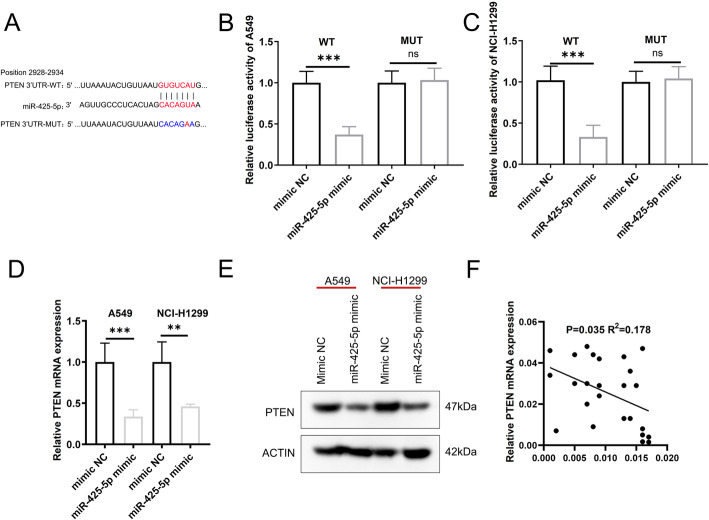
From TargetScan 7, PTEN was identified as a predicted miR-425-5p target (Fig. 3a). In PTEN 3′-UTR reporter assays, miR-425-5p suppressed WT PTEN expression (Fig. 3b-c) but had little effect on mutated PTEN 3′-UTR fragments (Fig. 3b-c). The levels of PTEN were lower in cells transfected with miR-425-5p mimics (Fig. 3d-e). MiR-425-5p also negatively related PTEN mRNA levels in LCa tissue (P = 0.035, R2 = 0.178, Fig. 3f). These data implicate PTEN as a cellular target of miR-425-5p.
Fig. 3.
MiR-425-5p targets PTEN in LCa. a. Predicted binding of miR-425-5p and PTEN. b & c. Relative luciferase activity of PTEN-WT, PTEN-MUT in LCa cells expressing miR-425-5p mimic. d. PTEN mRNA levels are significantly lower after transfection with miR-425-5p mimic. e. PTEN expression assessed by WB. Full-length blots/gels are presented in Supplementary Figure 1. f. miR-425-5p and PTEN negatively correlate in LCa tissue. Data: mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001
miR-425-5p promotes LCa via PTEN
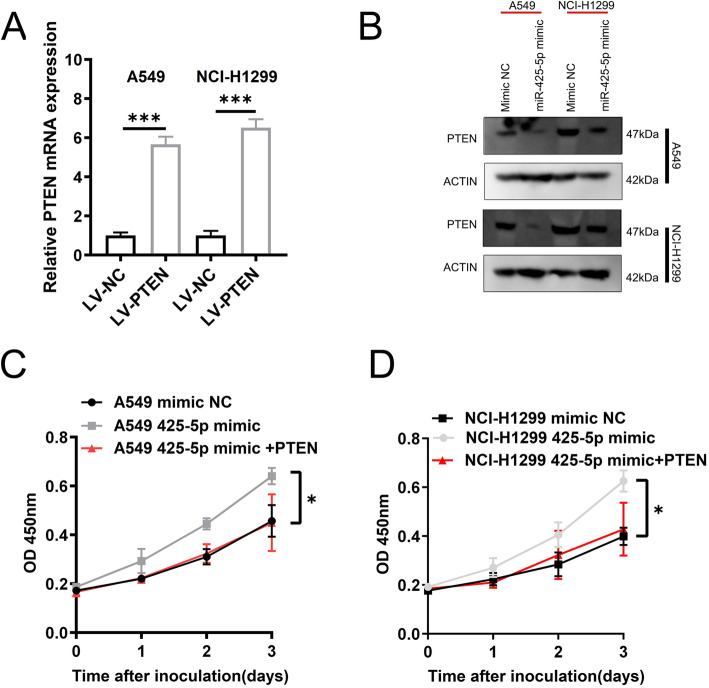
To define whether miR-425-5p regulate PTEN in Lca, we firstly overexpression PTEN in LCa (Fig. 4a). WB analysis demonstrated that miR-425-5p reduces PTEN levels which could be recovered by exogenous expression (Fig. 4b). Cell viability assays showed that miR-425-5p enhances LCa proliferation which could be reversed by PTEN transfection (Fig. 4c-d), suggesting miR-425-5p mediates its activities via PTEN. This indicates that miR-425-5p targets PTEN to mediate its pro-tumor effects.
Fig. 4.
MiR-425-5p promotes LCa growth by targeting PTEN. a. qRT-PCR of PTEN in indicated Lca cell lines. b. WB analysis of PTEN expression. Full-length blots/gels are presented in Supplementary Figure 2. c & d. CCK-8 analysis of cell viability in the indicated cell lines with empty vector, miR-425-5p mimic, and/or PTEN in A549/NCI-H1299 cells. Data: mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001
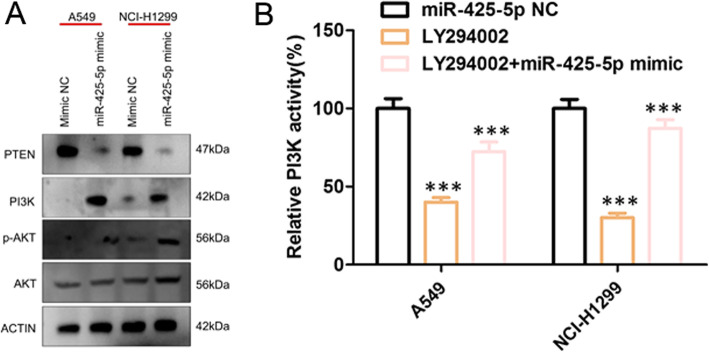
miR-425-5p activates PTEN/PI3K/AKT signaling
It has been reported PTEN/PI3K/AKT signaling was closely related to cell proliferation and apoptosis [14–18]. Next, we explored the effect of miRNA-425-5p on PTEN/PI3K/AKT signaling. As shown in Fig. 5a, in comparison to NC groups, PTEN was down regulated in response to miR-425-5p mimics, whilst PI3K and p-AKT levels increased. In addition, NSCLC cells were treated with the PI3K/Akt inhibitor LY294002 or LY294002 + miR-425-5p mimics mimic (Fig. 5b), Kinase activity assays further showed that PI3K activity in NSCLC transfected with LY294002 was significantly lower than that transfected with mimic control (P < 0.01), and PI3K activity in NSCLC cells transfected with both LY294002 and miR-425-5p mimic was significantly higher than that transfected with LY294002 (P < 0.01). Take together; this implicates PTEN/PI3K/AKT signaling in the pro-tumorigenic effects of miR-425-5p.
Fig. 5.
Effects of miR-425-5p on PTEN/PI3K/AKT. a. WB analysis of PTEN, PI3K, p-AKT, and AKT in LCa cells. Full-length blots/gels are presented in Supplementary Figure 3. b The PI3K kinase activity was determined in NSCLC cells transfected with LY294002 was significantly lower than that transfected with mimic control, and the PI3K kinase activity in NSCLC transfected with both LY294002 and miR-425-5p mimic was significantly higher than that transfected with LY294002. Data: mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001
Discussion
The poor prognosis of LCa highlights the need for urgent therapeutic strategies. miRNAs are novel targets for cancer therapies and their dysregulation occurs in LCa tissue [19–22]. Duan et al. showed that miR-203 binds to ZEB2 to suppress EMT [23], Yuan et al. showed that miR-30a inhibits EYA2 migration and invasion [24], and Li et al. showed that miR-1304 inhibits LCa cell division through heme oxygenase-1 [25]. The cellular roles of miR-425-5p in LCa are poorly understood. In the present work, we further explore the underlying mechanisms of miR-425-5p-induced LCa cell progression.
In the present study, we confirmed that miR-425-5p is overexpressed in LCa cell lines and tissues implicating a role in LCa tumorigenesis. Upregulating miR-425-5p levels enhanced the cell survival and colony formation ability of LCa cells in vitro, implicating it as a novel LCa oncogene. In the mechanism, using the algorithms TargetScan website tools, we identified PTEN as the potential target of miR-425-5p. Furthermore, we performed Luciferase reporter assays and the results showed that miR-425-5p may directly target PTEN-3’UTR. The result of qRT-PCR and western blot also confirmed that over-expression of miR-425-5p could suppress the expression level of PTEN. All the above suggested that PTEN was a potential functional target of miR-425-5p. Moreover, MiR-425-5p also negatively related PTEN mRNA levels in LCa tissue. Rescue experiment indicated that exogenous PTEN expression inhibited the pro-cancer effects of miR-425-5p. PTEN was downregulated in LCa tissue. PTEN is a tumor suppressor with well-characterized phosphatase activity [26]. PI3K/AKT promotes cell cycle progression, inhibits apoptosis, and is known to be overactive in a multitude of human cancers [27, 28]. PTEN can suppress PI3K/AKT signaling and thus displays anti-cancer effects [29]. Upon assessment of the molecular mechanisms of miR-425-5p in LCa cells, its pro-cancer effects were found to be mediated through manipulation of the PTEN/PI3K/AKT signaling axis.
Conclusion
In conclusion, we highlight miR-425-5p as an oncogene in LCa that promotes an oncogene phenotype by inhibiting PTEN. These findings enhance our knowledge of the role of miR-425-5p and reveal new therapeutic strategies for the diagnosis and treatment of LCa. Angiogenesis and metastasis biological experiments will clarify the functions and roles of miR-425-5p in LCa.
Supplementary information
Additional file 1: Supplement Figure S1. Uncropped images of blots and gels related to Fig. 3. Supplement Figure S2. Uncropped images of blots and gels related to Fig. 4. Supplement Figure S3. Uncropped images of blots and gels related to Fig. 5. Table S1. The raw data of all colony formation experiments.
Acknowledgements
Not applicable.
Abbreviations
- Lca
Lung cancer
- PTEN
Gene of phosphate and tension homology deleted on chromsome ten
- PI3K
Phosphatidylinositol 3-kinase
- NSCLC
Non-small cell lung cancer
- GCa
Gastric cancer
- CRC
Colorectal cancer
- HCC
Hepatocellular carcinoma
- EMT
Epithelial-Mesenchymal Transition
Authors’ contributions
JSZ, ZSY, SYC, and QF did patient recruitment and assessment, and performed the experiments. JHY and CJH were responsible for statistical analysis. JSZ, ZSY, and SYC interpreted the data and wrote the manuscript. All authors contributed to the subsequent drafts and approved the final version.
Funding
This research was funded by Zhejiang Provincial Natural Science Foundation of China/Exploration Program (LQ19H020010); Core talent program of Department of Health of Zhejiang Province (2013RCA018); Zhejiang Provincial Natural Science Foundation of China/General Program (LY15HO2004). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethics approval for this study was obtained from the Fourth Affiliated Hospital, Zhejiang University School of Medicine (No.2015-0-09). All patients gave written informed consent for participation in the study.
Consent for publication
Not applicable.
Competing interests
All authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12890-020-01261-0.
References
- 1.Chen W, Zheng R, Zuo T, Zeng H, Zhang S, He J. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28(1):1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni JS, Zheng H, Huang ZP, et al. MicroRNA-197-3p acts as a prognostic marker and inhibits cell invasion in hepatocellular carcinoma. Oncol Lett. 2019;17(2):2317–2327. doi: 10.3892/ol.2018.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang TJ, Wang YX, Yang DQ, et al. Down-regulation of miR-186 correlates with poor survival in de novo acute myeloid leukemia. Clin Lab. 2016;62(1–2):113–120. doi: 10.7754/clin.lab.2015.150606. [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Teng Y, Liu Q. MicroRNA-153 regulates NRF2 expression and is associated with breast carcinogenesis. Clin Lab. 2016;62(1–2):39–47. doi: 10.7754/clin.lab.2015.150518. [DOI] [PubMed] [Google Scholar]
- 6.Lin H, Huang ZP, Liu J, et al. MiR-494-3p promotes PI3K/AKT pathway hyperactivation and human hepatocellular carcinoma progression by targeting PTEN. Sci Rep. 2018;8(1):10461. doi: 10.1038/s41598-018-28519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Li J, Le Y, Zhou C, Zhang S, Gong Z. PFKL/miR-128 axis regulates glycolysis by inhibiting AKT phosphorylation and predicts poor survival in lung cancer. Am J Cancer Res. 2016;6(2):473–485. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R, Chen X, Xu T, et al. MiR-326 regulates cell proliferation and migration in lung cancer by targeting phox2a and is regulated by HOTAIR. Am J Cancer Res. 2016;6(2):173–186. [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Q, Wei F, Zhang J, Wang X, Li B. miR-134 inhibits non-small cell lung cancer growth by targeting the epidermal growth factor receptor. J Cell Mol Med. 2016;20(10):1974–1983. doi: 10.1111/jcmm.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu H, Xu Z, Li C, et al. MiR-145 and miR-203 represses TGF-beta-induced epithelial-mesenchymal transition and invasion by inhibiting SMAD3 in non-small cell lung cancer cells. Lung Cancer. 2016;97:87–94. doi: 10.1016/j.lungcan.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Fang F, Song T, Zhang T, Cui Y, Zhang G, Xiong Q. MiR-425-5p promotes invasion and metastasis of hepatocellular carcinoma cells through SCAI-mediated dysregulation of multiple signaling pathways. Oncotarget. 2017;8(19):31745–31757. doi: 10.18632/oncotarget.15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cristobal I, Madoz-Gurpide J, Rojo F, Garcia-Foncillas J. Potential therapeutic value of miR-425-5p in metastatic colorectal cancer. J Cell Mol Med. 2016;20(11):2213–2214. doi: 10.1111/jcmm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Wen M, Guo J, et al. Clinical value of miR-425-5p detection and its association with cell proliferation and apoptosis of gastric cancer. Pathol Res Pract. 2017;213(8):929–937. doi: 10.1016/j.prp.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Xing S, Liu M, Deng W, Wang Y, Huang Z, Huang Y, Huang X, Wu C, Guo X, Pan X, Jiang J, Feng F, Li T. miR-26a-5p enhances cells proliferation, invasion and apoptosis resistance of fibroblast-like synoviocytes in rheumatoid arthritis by regulating PTEN/PI3K/AKT pathway. Biosci Rep. 2019;39:7. [DOI] [PMC free article] [PubMed]
- 15.Meng J, Liu GJ, Song JY, Chen L, Wang AH, Gao XX, Wang ZJ. Preliminary results indicate resveratrol affects proliferation and apoptosis of leukemia cells by regulating PTEN/PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23(10):4285–4292. doi: 10.26355/eurrev_201905_17933. [DOI] [PubMed] [Google Scholar]
- 16.Liu HY, Zhang YY, Zhu BL, Feng FZ, Yan H, Zhang HY, Zhou B. miR-21 regulates the proliferation and apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur Rev Med Pharmacol Sci. 2019;23(10):4149–4155. doi: 10.26355/eurrev_201905_17917. [DOI] [PubMed] [Google Scholar]
- 17.Yu G, Wang C, Song X, Liu S, Zhang Y, Fan L, Yang Y, Huang Y, Song J. Formaldehyde induces the apoptosis of BMCs of BALB/c mice via the PTEN/PI3K/Akt signal transduction pathway. Mol Med Rep. 2019;20(1):341–349. doi: 10.3892/mmr.2019.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun XH, Wang X, Zhang Y, Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23–32. doi: 10.1016/j.thromres.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Mou X, Liu S. MiR-485 inhibits metastasis and EMT of lung adenocarcinoma by targeting Flot2. Biochem Biophys Res Commun. 2016;477(4):521–526. doi: 10.1016/j.bbrc.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 20.Su TJ, Ku WH, Chen HY, et al. Oncogenic miR-137 contributes to cisplatin resistance via repressing CASP3 in lung adenocarcinoma. Am J Cancer Res. 2016;6(6):1317–1330. [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Wang F, Xu P. miR-590 accelerates lung adenocarcinoma migration and invasion through directly suppressing functional target OLFM4. Biomed Pharmacother. 2017;86:466–474. doi: 10.1016/j.biopha.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Luo P, Song Q, Fei X. DNMT1/miR-200a/GOLM1 signaling pathway regulates lung adenocarcinoma cells proliferation. Biomed Pharmacother. 2018;99:839–847. doi: 10.1016/j.biopha.2018.01.161. [DOI] [PubMed] [Google Scholar]
- 23.Duan X, Fu Z, Gao L, et al. Direct interaction between miR-203 and ZEB2 suppresses epithelial-mesenchymal transition signaling and reduces lung adenocarcinoma chemoresistance. Acta Biochim Biophys Sin Shanghai. 2016;48(11):1042–1049. doi: 10.1093/abbs/gmw099. [DOI] [PubMed] [Google Scholar]
- 24.Yuan Y, Zheng S, Li Q, et al. Overexpression of miR-30a in lung adenocarcinoma A549 cell line inhibits migration and invasion via targeting EYA2. Acta Biochim Biophys Sin Shanghai. 2016;48(3):220–228. doi: 10.1093/abbs/gmv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li CG, Pu MF, Li CZ, et al. MicroRNA-1304 suppresses human non-small cell lung cancer cell growth in vitro by targeting heme oxygenase-1. Acta Pharmacol Sin. 2017;38(1):110–119. doi: 10.1038/aps.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li DM, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci U S A. 1998;95(26):15406–15411. doi: 10.1073/pnas.95.26.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellacosa A, Chan TO. Ahmed NN, et al. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17(3):313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 28.Li JW, Wang XY, Zhang X, Gao L, Wang LF, Yin XH. Epicatechin protects against myocardial ischemiainduced cardiac injury via activation of the PTEN/PI3K/AKT pathway. Mol Med Rep. 2018;17(6):8300–8308. doi: 10.3892/mmr.2018.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin Y, Huo Z, Song X, Chen X, Tian X, Wang X. Mir-106a regulates cell proliferation and apoptosis of colon cancer cells through targeting the PTEN/PI3K/AKT signaling pathway. Oncol Lett. 2018;15(3):3197–3201. doi: 10.3892/ol.2017.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplement Figure S1. Uncropped images of blots and gels related to Fig. 3. Supplement Figure S2. Uncropped images of blots and gels related to Fig. 4. Supplement Figure S3. Uncropped images of blots and gels related to Fig. 5. Table S1. The raw data of all colony formation experiments.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.