Abstract
The action of FTO on myoblasts proliferation and differentiation and molecular mechanism underlying it were investigated by transfecting with FTO lentiviral overexpression vector and gene expression profile sequencing. Compared with the control group, myoblasts with FTO transfection was significantly enhanced proliferation; the expression of MYOG and MYOD mRNA was significantly increased. In cells transfected with FTO, 129 differentially expressed genes were determined compared with control group, with 104 up-regulated and 25 down-regulated genes. Twelve pathways (Phagosome, Focal adhesion, Adrenergic signaling in cardiomyocytes, Endocytosis, Cardiac muscle contraction, Toll-like receptor, Ribosome, Tight junction, Regulation of actin cytoskeleton, Cytokine–cytokine receptor interaction, Adrenergic signaling in cardiomyocytes and MAPK) were significantly enriched. Eight genes known to be directly or indirectly related to skeletal muscle development (LAMA5, SPP1, CAV3, RASGRF1, FAK, PDGFB, PDGFRα, and RAC2) were enriched in the focal adhesion and expressed differentially. Altogether, these data suggested that FTO stimulated differentiation of myoblasts through regulation of eight genes enriched in the focal adhesion.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02386-z) contains supplementary material, which is available to authorized users.
Keywords: FTO, Chicken, Myoblast proliferation, Myoblast differentiation, Focal adhesion
Introduction
Fat mass- and obesity-associated (FTO) gene is highly conserved and widely exits in vertebrate. However, it did not attract much attention until in 2007 when a cluster of SNPs (single nucleotide polymorphisms) located in the first intron of FTO was found to be closely associated with obesity-related traits in human (Dina et al. 2007; Frayling et al. 2007; Scuteri et al. 2007). In addition, studies had indicated that FTO played an essential role in body development, metabolic homeostasis, and hypothalamus-governed food intake in mice (Caruso et al. 2011; Church et al. 2010). The mice lacking Fto completely, compared to control mice, showed immediate postnatal growth retardation with shorter body length, lower body weight, and lower bone mineral density (Gao et al. 2010). More importantly, skeletal muscle development was impaired in FTO-deficient mice (Wang et al. 2017). These findings suggested that FTO not only had been found to be important role in adipose deposition but also in skeletal muscle development in mammals.
In birds, FTO-regulated gluconeogenesis in DF-1 cells (Guo et al. 2015). The expression of FTO mRNA was identified in muscle, liver, visceral fat, hypothalamus, cerebellum and telencephalon tissues, and there was significant difference in liver and visceral fat in broilers and layer chickens (Wang et al. 2012). However, studies of our research team have showed that FTO mRNA was expressed in breast and leg muscle tissues, and exhibited tissue- and breed-specific patterns in the Recessive White Plymouth Rock and the Qingyuan partridge chicken from embryo to postnatal period (Song et al. 2015). These findings indicated that FTO was likely to play an essential role in skeletal muscle development in chickens.
Skeletal muscle development is closely associated with myogenesis but the physiological significance of its connection with FTO needs to be clarified. Thus, our present study was undertaken to examine the effect of FTO and its underlying molecular mechanisms on the differentiation of chicken myoblasts, which could lay a foundation on further elucidating the molecular mechanism of skeletal muscle development in chicken.
Material and methods
Ethical approval of the study protocol
The study project was conducted in accordance with the Guidelines for Experimental Animals formulated by the Ministry of Science and Technology (Beijing, China). All experimental programs were approved by the Science Research Department (in charge of animal welfare) of the Institute of Animal Sciences, CAAS (Beijing, China).
Culture of myoblasts
The hatching eggs of S3 strain, which has characteristic by yellow feather, good flavor and excellent growth performance, were used in this study. More than three female embryo were selected for breast muscle separation at 13 embryo age (Luo et al. 2014). Cells were seeded in 6- and 96-well culture dishes (culture medium: DMEM/F12 (HyClone, USA) + 20% FBS (Gibco, USA) + 1% penicillin/streptomycin ( HyClone, USA) at 37 ℃ in a humidified atmosphere of 5% CO2 in air. When cells reached 70 ~ 80% confluence, myoblast differentiation was induced with 2% horse serum (BI, Israel).
Construction and transfection of FTO lentiviral vectors
The vectors containing cDNA of FTO (HM050377.1) was synthesized by GenePharma (GenePharma, Shanghai, China). Primers including NotI and NsiI sites were used to generate PCR fragments that were subcloned into LV5(EF-1α/GFP/Puro) lentiviral expression vector. Positive clones were identified by sequencing. Virus drops is 10^8 TU/mL.
Transfection
Transfections were undertaken at 40–50% confluence using polybrene (5 μg/mL). Myoblasts cultured in six-well plates were transfected with FTO lentiviral vector and negative vector to detect gene expression and cell differentiation using qPCR in three replicate wells, respectively. Cells of the same group in 96-well plates were transfected with FTO lentiviral vector to monitored cell proliferation using Cell Counting Kit-8 (CCK-8; Dojindo, Fukuoka, Japan) and Edu staining (RiboBio, Guangdong, China) in eight replicate wells.
RNA extraction
Total RNA was isolated from myoblasts using the Total RNA kit (TIANGEN, Beijing, China) following the manufacturer’s instructions. An ND-1000 spectrophotometer (Agilent Technologies, Palo Alto, CA, USA) was used to measure the concentration and purity of RNA (A260/280 ≥ 1.8 and ≤ 2.0). RNA integrity (RIN ≥ 7) was evaluated by a 2100 Bioanalyzer Lab-on-chip system (Agilent Technologies). RNA samples were stored at − 80 ℃ until use.
Gene expression profile
The gene expression profile was undertaken by LC (Hangzhou, China) using total RNA from three replicate differentiated myoblasts treated with FTO lentiviral and negative control vector for 9 days, respectively. Raw data were determined by FASTQC (v0.11.2), and Clean data were mapped to the reference genome and genes of chickens (Gallus_gallus-5.0) using Tophat2 (v2.0.12) (Huang et al. 2015). Fragments per kilobase of exon per million mapped reads (FPKM) was employed to quantifying gene expression. P values ≤ 0.05 and fold changes ≥ 1.5 were assumed to indicate differentially expressed genes (DEGs). Pathways were analyzed by Kobas v3.0 (kobas.cbi.pku.edu.cn/help.do) (Mao et al. 2005; Wu et al. 2006). Pathways with < 3 known chicken genes were discarded.
qPCR of genes
Abundance of MYOD and MYOG and differentially expressed genes from three replicate cells treated with FTO lentiviral and negative control vector for 9 days were quantified by qPCR (Qiagen, Düsseldorf, Germany). The final concentration of each primer was 10 μmol/μL. The primers are detailed in Table 1.
Table 1.
qPCR primers
| Gene name | Sequence (5′ to 3′) | Fragments/bp | Nnnealing temperature/℃ |
|---|---|---|---|
| SPP1 | S: 5′ CAAAGCTGCCAGGAAGCTCAT 3' | 171 | 60 |
| A: 5′ TCCACCTCAGGGCTGTCAAA 3' | |||
| CAV3 | S:5′ CTTCCTCTTCGCCCTCATCT 3' | 182 | 60 |
| A:5′ CCTTCCGCAGCATAACCCT 3' | |||
| PTK2 | S: 5′ GCAGTCTGAGGAGGTTCACT 3' | 123 | 60 |
| A: 5′ TTGGGCAGGTACCGAATTCT 3' | |||
| PDGFB | S: 5′ TGGACAGCACCAATGCCAA 3' | 192 | 60 |
| A: 5′ GGTCCTCCAAAGGCACGAT 3' | |||
| PDGFRα | S: 5′ CTTGGCAGCTCGTAATGTCC 3' | 125 | 60 |
| A: 5′ TTACTGGGAGGAAGGTGCTG 3' | |||
| RAC2 | S: 5′ TGCTGGACAAGAGGATTACGA 3' | 165 | 60 |
| A: 5′ ACAAGGATGATGGGAGTGCTG 3' | |||
| MYOD | S: 5′GCTACTACACGGAATCACCAAAT 3' | 21 | 60 |
| A: 5′ CTGGGCTCCACTGTCACTCA 3' | |||
| MYOG | S: 5′ CGGAGGCTGAAGAAGGTGAA 3' | 314 | 60 |
| A: 5′CGGTCCTCTGCCTGGTCAT 3' | |||
| RASGRF1 | S: 5′ TTTGACATCCTGCTGCCCAT 3' | 110 | 60 |
| A: 5′ AGCTTGTCGAAGTCACGGTT 3' | |||
| ß-actin | S: 5 'GAGAAATTGTGCCTGACATCA 3' | 152 | 60 |
| A: 5 'CCTGAACCTCTCATTGCCA 3' |
Statistical analyses
Statistical differences between treatment groups were determined by t tests using Excel software. The data are the mean ± standard deviation (SD), and P < 0.05 was considered significant.
Results
FTO promoted the proliferation of myoblast
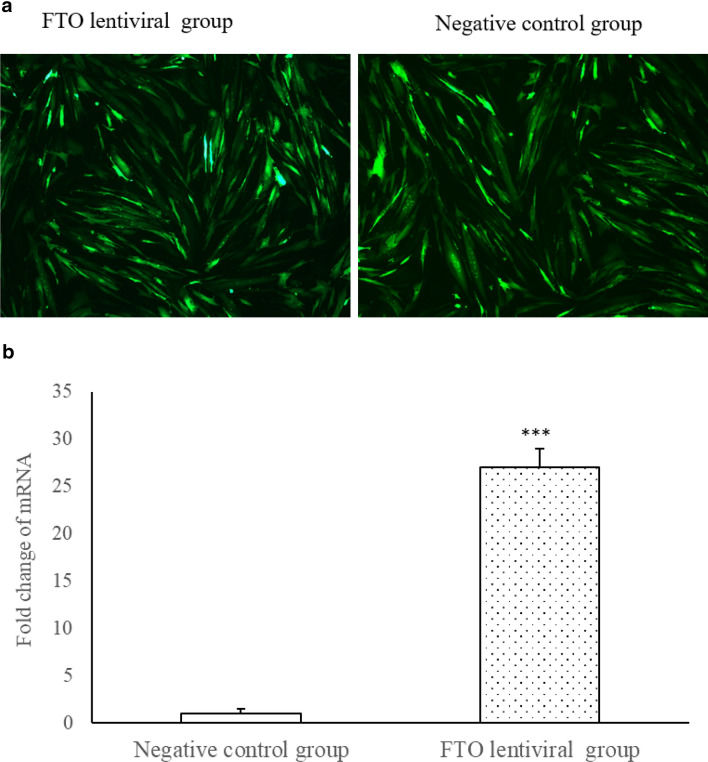
FTO lentiviral and negative control vector were successfully transfected with myoblast (Fig. 1a), respectively. The expression of FTO mRNA, compared with the control group, was increased significantly 72 h after transfection with FTO (Fig. 1b, P < 0.001).
Fig. 1.
a Transfection of FTO lentiviral overexpression and negative control vectors (inverted microscope, × 100 magnification, n = 3); Green fluorescence denotes successful transfection. b The expression of FTO mRNA was increased significantly 72 h after transfection with FTO lentiviral overexpression vectors, ***P < 0.001
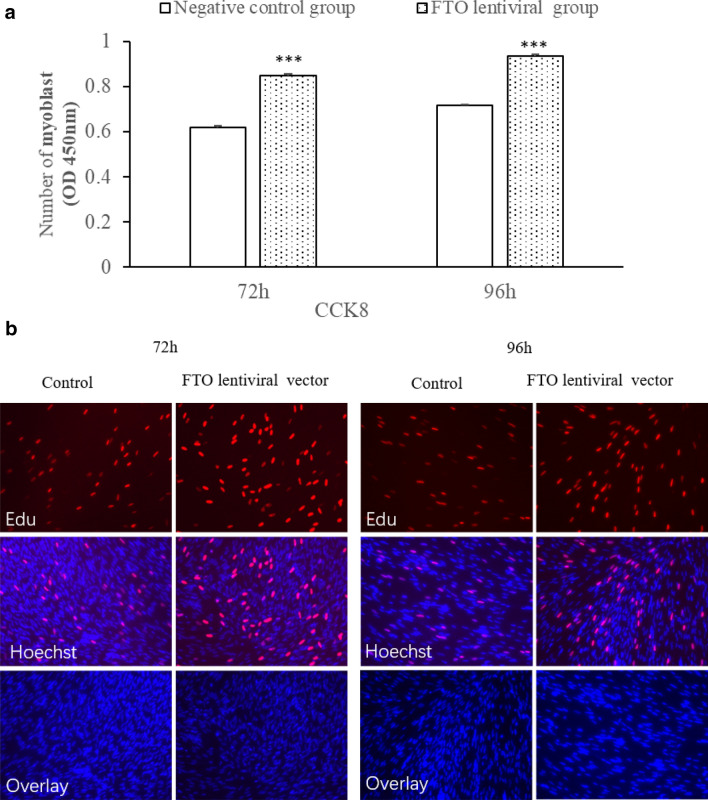
The effect of FTO on the proliferation of myoblastwas showed in Fig. 2a, b, respectively. Cell numbers were enhanced significantly at 72 and 96 h after transfection with FTO (Fig. 2a, P < 0.05). EdU staining also indicated that the proliferation rate of FTO lentiviral cells was increased compared to control cells. Altogether, the data suggested that FTO increased myoblast proliferation.
Fig. 2.
Effect of FTO on proliferation of cells. a The number of myoblasts was significantly enhanced after transfection with FTO lentiviral overexpression vectors (n = 8, ***P < 0.001 vs. control). b The number of EdU-stained cells was significantly enhanced after transfection with FTO lentiviral overexpression vectors (n = 3, × 200 magnification)
FTO-enhanced myoblast differentiation
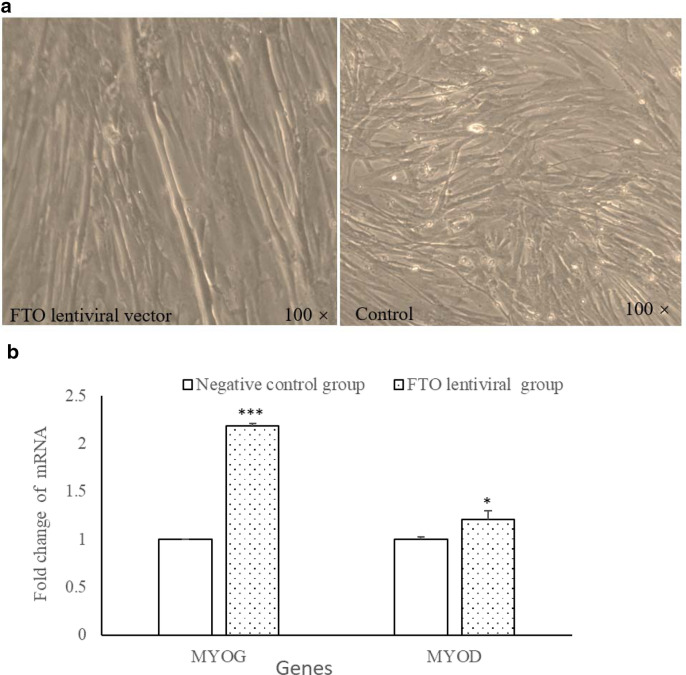
The myotubes were longer and more complete compared with those of control myoblasts after transfection FTO (Fig. 3a). Two major myoblast differentiation marker genes (MYOG and MYOD) were detected at 9d after transfection with FTO lentiviral using qPCR. The results showed that MYOG and MYOD expression transfection FTO were significant higher that of control group (Fig. 3b).
Fig. 3.
Effect of FTO on myoblast differentiation. a Microscopic images of myoblasts at 144 h (× 100 magnification). The myotubes were observed to be longer and more complete in the FTO lentiviral overexpression vectors than that of control group. b MYOG and MYOD mRNA were significantly enhanced at 9d after transfection with FTO lentiviral overexpression vectors (n = 3, *P < 0.05, ***P < 0.001 vs. control)
FTO-regulated myoblast differentiation through a focal adhesion pathway
The gene expression profile was used to explore the key genes and pathways by which FTO influenced myoblast differentiation. In the present study, three cDNA libraries were constructed using total RNA from transfection FTO lentiviral vector and negative control vector, respectively (for details of quality control, see table S1). A total of 129 DEGs (P < 0.05, log1.5 > 0.67 or < − 0.67) were identified, with 104 up-regulated and 25 down-regulated genes (Table S2).
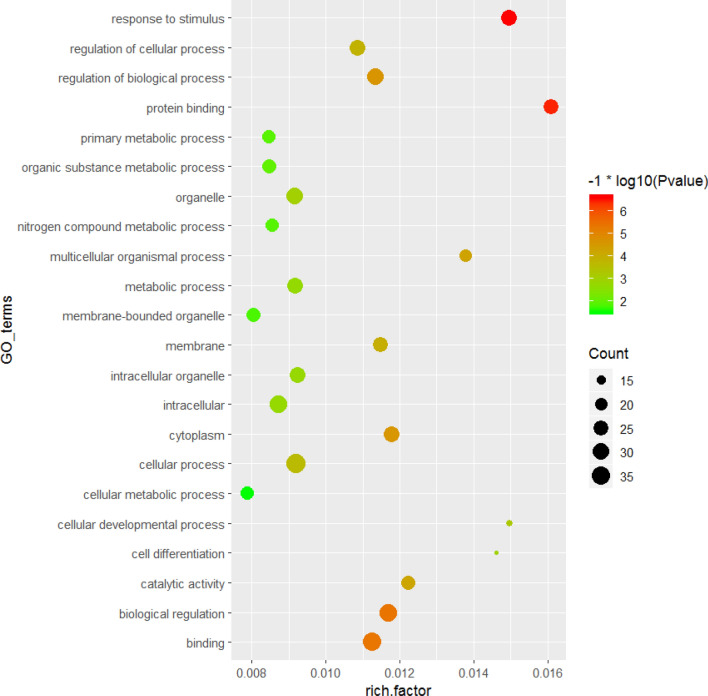
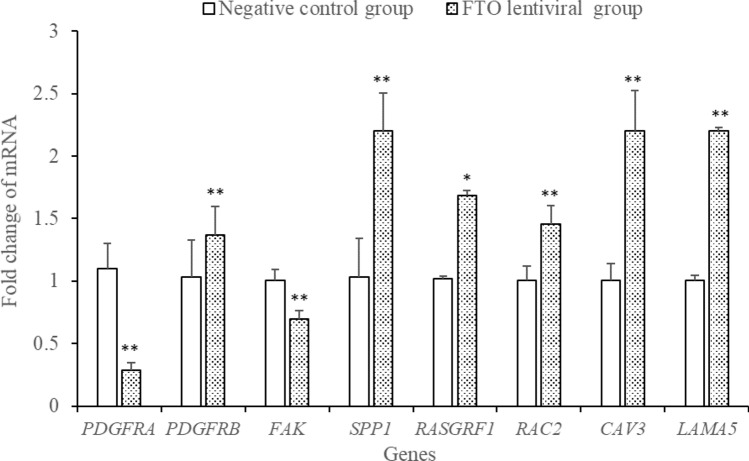
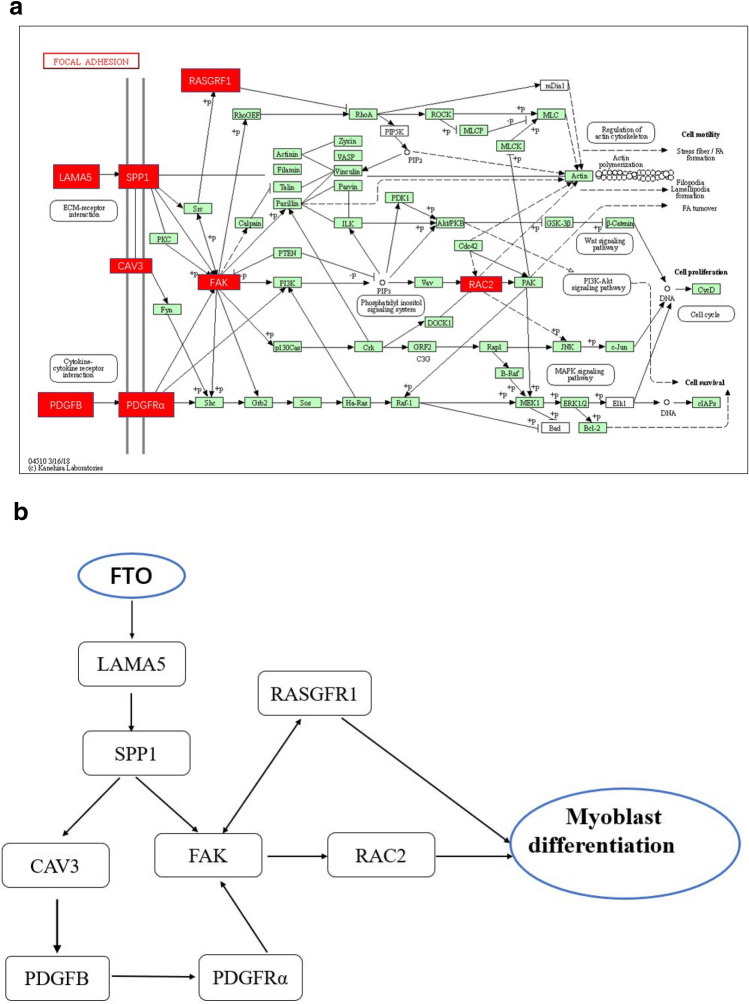
94 GO terms (Gene number ≥ 10, P < 0.05) were significantly enriched based on 129 DEGs, including regulation of cellular process, cytoplasm, regulation of biological process, biological regulation, cellular metabolic process, cellular developmental process, cell differentiation, developmental process, etc. As shown in Fig. 4, the top of twenty GO terms related to cell differentiation were showed (Fig. 4). Using Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway to analyze the DEGs, twelve pathways were significantly enriched: Phagosome, Focal adhesion, Adrenergic signaling in cardiomyocytes, Endocytosis, Cardiac muscle contraction, Toll-like receptor signaling pathway, Ribosome, Tight junction, Regulation of actin cytoskeleton, Cytokine–cytokine receptor interaction, Adrenergic signaling in cardiomyocytes and MAPK (Table 2, P ≤ 0.05). Most noticeably of all, six genes (LAMA2, RASGRF1, RAC2, SPP1, CAV3, and PDGFB) enriched in focal adhesion pathway were reported to be closely related to the morphology, metabolism, insulin sensitivity and myoblast differentiation in skeletal muscle. The six genes showed consistency between the qPCR assays and the deep sequencing analysis in terms of the direction of regulation and statistical significance (Fig. 5). The two genes, PDGFRα and FAK located in focal adhesion pathway and related to skeletal muscle development, were also detected using Q-PCR and differentially expression (Fig. 5). As shown in Fig. 6, FAK may play an important pivotal role in the response of the FTO regulation of myoblast differentiation. Therefore, it was hypothesized that FTO could contribute to myoblast differentiation through regulation of the expression of genes (e.g., LAMA5, SPP1, CAV3, RASGRF1, FAK, PDGFB, PDGFRα, and RAC2) enriched in focal adhesion (Fig. 6).
Fig. 4.
GO analysis of the differentially expressed genes between FTO lentiviral overexpression and negative vectors groups. Significantly enriched GO terms of biological process in the top 20 were used (P ≤ 0.05)
Table 2.
Significantly enriched pathways for differentially expressed gene
| Pathway | P value | Genes |
|---|---|---|
| Phagosome | 7.75E-05 | CYBB, MARCO, TLR4, CTSS, NCF1, ITGB2 |
| Focal adhesion | 5 E-05 | RASFRF, PDGFB, RAC2, SPP1, LAMA5,CAV3 |
| Regulation of actin cytoskeleton | 0.0003 | CXCR4, LPAR5, RAC2, ITGB2, PDGFB |
| Cytokine-cytokine receptor interaction | 0.0042 | CXCR4, INHA, IL2RG, CCL4, CSF1R |
| Adrenergic signaling in cardiomyocytes | 0.0047 | ACTC1, TNNC1, TNNT2, PIK3R5L |
| Calcium signaling pathway | 0.0147 | CXCR4, TNNC2, TNNC1, PTK2B |
| Endocytosis | 0.0312 | CXCR4, DNAJC6, IL2RG, CAV3 |
| MAPK signaling pathway | 0.0470 | RAC2, RASGRF1, CSF1R, PDGFB |
| Cardiac muscle contraction | 0.0053 | ACTC1, TNNC1, TNNT2 |
| Toll-like receptor signaling pathway | 0.0100 | TLR4, SPP1, CCL4 |
| Ribosome | 0.0252 | RPS28, RPS29, RPL3L |
| Tight junction | 0.0480 | CD1B, MYH1C, HCLS1 |
Fig. 5.
Validation of differentially expressed genes by qPCR (n = 3). *p < 0.05, **P < 0.01 vs. control
Fig. 6.
Exploration of pathways by which FTO regulates chicken myoblast differentiation using analyses of gene expression profile. a Focal adhesion pathway from the KEGG database. Genes marked in red show differential expression between the FTO lentiviral overexpression vectors and control group. b FTO promotes the proliferation, differentiation of myoblast through regulation of gene expression (LAMA2, SPP1, CAV3, RASGRF1, PTK2, PDGFB, PDGFRα, and RAC2) enriched in focal adhesion pathway
Discussion
Skeletal muscle constitutes the largest proportion and most valuable component of meat mass in chicken, and its development is a complex physiological process (Bloise et al. 2018). Myoblast differentiation plays a decisive role in number of the muscle fibers after birth in animals. Hence, it is essential to clarify the molecular mechanisms of myoblast differentiation. Many genes and pathways were involved in the process of skeletal muscle development. Focal adhesion is one of the important molecular signaling pathways and its play pivotal role in cell proliferation, differentiation, motility, migration, and survival (Block et al. 2009; Cebrian-Serrano et al. 2014; Saeed-Zidane et al. 2019; Wu 2007). Of the special interest, eight genes (LAMA5, SPP1, CAV3, RASGRF, FAK, PDGFB, PDGFRα, and RAC2) known to be directly or indirectly related to skeletal muscle, were enriched in the focal adhesion pathway and showed differential expression. Based on the previous studies, these genes have been found to be important role in focal adhesion pathway. Mutation of LAMA2 was associated with merosin-deficient congenital muscular dystrophy (Ge et al. 2018; Hashemi-Gorji et al. 2018); SPP1 may contribute to regulating the myogenic in the early stages of muscle regeneration (Uaesoontrachoon et al. 2008) and interact with AKT1/MSTN/FoxO1 to modify normal and dystrophic muscle (Nghiem et al. 2017); Cav3 is the main structural component of caveolae in skeletal muscle (Galbiati et al. 2001) and its deficiency resulted in disturbances of the mitochondrial respiration and energy status (Shah et al. 2020); FAK, a mechanosensitive/exercise responsive protein, regulated myoblast development and myofibrogenesis, skeletal muscle morphology, and insulin sensitivity (Bisht et al. 2008; Graham et al. 2015); palmitate oxidation and reduced glycogen synthesis enhanced after silencing FAK in primary human skeletal muscle cells (Lassiter et al. 2018). The function of PDGF member is mediated by two receptors (PDGFRα and PDGFRβ). PDGFRα signaling was associated with muscle pathology (Ito et al. 2013; Olson and Soriano 2009; Tidball et al. 1992; Zhao et al. 2003), and its promoted muscle development in growing embryos and angiogenesis in regenerating adult muscle (Orr-Urtreger et al. 1992; Soriano 1997). Focal adhesion mainly included ECM–receptor intersection and cytokine–cytokine receptor intersection signaling. As shown in Fig. 6a, eight genes mentioned above were located in the important position in each branches of the focal adhesion pathway. Spp1 is located at the beginning of ECM–receptor intersection, it was hypothesized that SPP1 firstly respond to FTO regulation, then CAV3, FAK simultaneously receive the signaling of SPP1, and inspire of the PDGFB, PDGFRα located in the beginning of cytokine–cytokine receptor intersection; FAK receives the feedback from PDGFB, PDGFRα, and deliver the signaling to RAC2, ultimately resulting in altered myoblast proliferation and differentiation. FAK may be an important node factor and is likely to play a key role in myoblast differentiation respond to FTO regulation.
In summary, our data showed that FTO promotes the differentiation of myoblast through regulation of gene expression (LAMA5, SPP1, CAV3, RASGRF1, FAK, PDGFB, PDGFRα and RAC2) enriched in focal adhesion pathway.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank International Science Editing (https://www.international science editing. com) for editing this manuscript.
Author contributions
Conceptualization: HH and SL; validation: CL and LL; data curation: ZL; formal analysis: QW and ZH; writing the original draft: HH; supervision: SL.
Funding
The research was supported by the Natural Science Fund project in Jiangsu (BK20191217), China Meat-type Chicken Research System (CARS-42-Z06), Key Laboratory of Poultry Genetics and Breeding in Jiangsu (JQLAB-ZZ-201705), and the Special Funds for Transformation of Scientific and Technological Projects in Jiangsu Province (BA2019049).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study project was conducted in accordance with the Guidelines for Experimental Animals formulated by the Ministry of Science and Technology (Beijing, China). All experimental programs were approved by the Science Research Department (in charge of animal welfare) of the Institute of Animal Sciences, CAAS (Beijing, China).
Footnotes
Huayun Huang, Longzhou Liu and Chunmiao Li contributed equally to this work.
Contributor Information
Huayun Huang, Email: huanghuayun520@163.com.
Longzhou Liu, Email: 826062982@qq.com.
Chunmiao Li, Email: cherrylcm@163.com.
Zhong Liang, Email: lzxzsd163@163.com.
Zhenyang Huang, Email: zyhuang@qq.com.
Qianbao Wang, Email: wqb15855142436@163.com.
Shoufeng Li, Email: yzlsf3333@126.com.
Zhenhua Zhao, Email: zzh0514@163.com.
References
- Bisht B, Srinivasan K, Dey CS. vivo inhibition of focal adhesion kinase causes insulin resistance. J Physiol. 2008;586:3825–3837. doi: 10.1113/jphysiol.2008.157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J, Bonilla L, Hansen PJ. Effect of addition of hyaluronan to embryo culture medium on survival of bovine embryos in vitro following vitrification and establishment of pregnancy after transfer to recipients. Theriogenology. 2009;71:1063–1071. doi: 10.1016/j.theriogenology.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Bloise FF, Cordeiro A, Ortiga-Carvalho TM. Role of thyroid hormone in skeletal muscle physiology. J Endocrinol. 2018;236:R57–r68. doi: 10.1530/joe-16-0611. [DOI] [PubMed] [Google Scholar]
- Caruso V, Chen H, Morris MJ. Early hypothalamic FTO overexpression in response to maternal obesity–potential contribution to postweaning hyperphagia. PLoS ONE. 2011;6:e25261. doi: 10.1371/journal.pone.0025261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrian-Serrano A, Salvador I, Silvestre MA. Beneficial effect of two culture systems with small groups of embryos on the development and quality of in vitro-produced bovine embryos. Anat Histol Embryol. 2014;43:22–30. doi: 10.1111/ahe.12043. [DOI] [PubMed] [Google Scholar]
- Church C, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42:1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina C, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science (New York, NY) 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Gao X, Shin YH, Li M, Wang F, Tong Q, Zhang P. The fat mass and obesity associated gene FTO functions in the brain to regulate postnatal growth in mice. PLoS ONE. 2010;5:e14005. doi: 10.1371/journal.pone.0014005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, et al. Deletion of exon 4 in LAMA2 is the most frequent mutation in Chinese patients with laminin alpha2-related muscular dystrophy. Sci Rep. 2018;8:14989. doi: 10.1038/s41598-018-33098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ZA, Gallagher PM, Cardozo CP. Focal adhesion kinase and its role in skeletal muscle. J Muscle Res Cell Motil. 2015;36:305–315. doi: 10.1007/s10974-015-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Zhang Y, Zhang C, Wang S, Ni Y, Zhao R. Fatmass and obesity associated (FTO) gene regulates gluconeogenesis in chicken embryo fibroblast cells. Comp Biochem Physiol A Mol Integr Physiol. 2015;179:149–156. doi: 10.1016/j.cbpa.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Hashemi-Gorji F, Yassaee VR, Dashti P, Miryounesi M. Novel LAMA2 gene mutations associated with merosin-deficient congenital muscular dystrophy. Iran Biomed J. 2018;22:408–414. doi: 10.29252/.22.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, et al. Brain natriuretic peptide stimulates lipid metabolism through its receptor NPR1 and the glycerolipid metabolism pathway in chicken adipocytes. Biochemistry. 2015;54:6622–6630. doi: 10.1021/acs.biochem.5b00714. [DOI] [PubMed] [Google Scholar]
- Ito T, et al. Imatinib attenuates severe mouse dystrophy and inhibits proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord NMD. 2013;23:349–356. doi: 10.1016/j.nmd.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Lassiter DG, et al. FAK tyrosine phosphorylation is regulated by AMPK and controls metabolism in human skeletal muscle. Diabetologia. 2018;61:424–432. doi: 10.1007/s00125-017-4451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, et al. The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation. Cell Death Dis. 2014;5:e1347. doi: 10.1038/cddis.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics (Oxford, England) 2005;21:3787–3793. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- Nghiem PP, et al. Osteopontin is linked with AKT, FoxO1, and myostatin in skeletal muscle cells. Muscle Nerve. 2017;56:1119–1127. doi: 10.1002/mus.25752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A, Bedford MT, Do MS, Eisenbach L, Lonai P. Developmental expression of the alpha receptor for platelet-derived growth factor, which is deleted in the embryonic lethal Patch mutation. Development (Cambridge, England) 1992;115:289–303. doi: 10.1242/dev.115.1.289. [DOI] [PubMed] [Google Scholar]
- Saeed-Zidane M, et al. Hyaluronic acid and epidermal growth factor improved the bovine embryo quality by regulating the DNA methylation and expression patterns of the focal adhesion pathway. PLoS ONE. 2019;14:e0223753. doi: 10.1371/journal.pone.0223753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri A, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DS, Nisr RB, Stretton C, Krasteva-Christ G, Hundal HS. Caveolin-3 deficiency associated with the dystrophy P104L mutation impairs skeletal muscle mitochondrial form and function. J Cachexia Sarcopenia Muscle. 2020 doi: 10.1002/jcsm.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Song WT, Shu JT, Tao ZY, Zhu WQ, Di C, Li HF. Tissue- and breed-specific expression of the chicken fat mass- and obesity-associated gene (FTO) Genet Mol Res GMR. 2015;14:10500–10506. doi: 10.4238/2015.September.8.11. [DOI] [PubMed] [Google Scholar]
- Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development (Cambridge, England) 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Spencer MJ, St Pierre BA. PDGF-receptor concentration is elevated in regenerative muscle fibers in dystrophin-deficient muscle. Exp Cell Res. 1992;203:141–149. doi: 10.1016/0014-4827(92)90049-e. [DOI] [PubMed] [Google Scholar]
- Uaesoontrachoon K, Yoo HJ, Tudor EM, Pike RN, Mackie EJ, Pagel CN. Osteopontin and skeletal muscle myoblasts: association with muscle regeneration and regulation of myoblast function in vitro. Int J Biochem Cell Biol. 2008;40:2303–2314. doi: 10.1016/j.biocel.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. FTO is required for myogenesis by positively regulating mTOR-PGC-1alpha pathway-mediated mitochondria biogenesis. Cell Death Dis. 2017;8:e2702. doi: 10.1038/cddis.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rao K, Yuan L, Everaert N, Buyse J, Grossmann R, Zhao R. Chicken FTO gene: tissue-specific expression, brain distribution, breed difference and effect of fasting. Comp Biochem Physiol A Mol Integr Physiol. 2012;163:246–252. doi: 10.1016/j.cbpa.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Wu C. Focal adhesion: a focal point in current cell biology and molecular medicine. Cell Adhes Migr. 2007;1:13–18. doi: 10.4161/cam.1.1.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mao X, Cai T, Luo J, Wei L. KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic Acids Res. 2006;34:W720–724. doi: 10.1093/nar/gkl167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Haginoya K, Sun G, Dai H, Onuma A, Iinuma K. Platelet-derived growth factor and its receptors are related to the progression of human muscular dystrophy: an immunohistochemical study. J Pathol. 2003;201:149–159. doi: 10.1002/path.1414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.