Abstract
Functional properties of proteins, such as emulsification, foam formation and antioxidant activity, can be improved by conjugating the proteins with phenolic substances. We reported here changes in the structural, physicochemical and functional properties of Soy Protein Isolate (SPI) after conjugation with phenolic substances in the green tea and black tea extracts. Conjugation of SPI with tea extracts were conducted using alkaline treatment (pH 9.0) followed by air exposure. The results showed that conjugation of SPI increased the protein molecular size and decreased the protein hydrophobicity. The hydrophobic decreasing effect by the treatment was larger with the black tea extract than by green tea extract. SPI-tea polyphenol conjugates significantly (p < 0.05) increased the emulsifying ability of SPI up to 43% and the emulsifying stability of SPI up to 59%. SPI-tea polyphenol conjugates which was prepared using 0.75% (w/w SPI) green tea polyphenol extract showed the best emulsifying properties with strong repulsion forces between the droplets, smaller emulsion droplet size and lower polydispersity index of droplets size distribution. Although the conjugation product is still inferior to egg lecithin in emulsion stability, antioxidant activity of SPI was significantly (p < 0.05) improved in a concentration dependant manner. SPI-black tea polyphenol conjugates showed greater antioxidant activity than SPI-green tea polyphenol conjugate. The present study shows the feasibility and benefits of the use of SPI-tea polyphenol conjugates as a food emulsifier.
Keywords: Soy protein isolate, Tea polyphenol, Protein–polyphenol conjugate, Emulsifying properties, Antioxidant activity
Introduction
The tendency to use vegan product as protein sources such as soy protein is increasing due to healthy reason, dietary preferences and food sensitivities. Soy bean is a multifaceted legume with enormous economic value; this protein source has been consumed for over 2000 years especially by the Asian, and nowadays are getting more popular among the Westerners as well. Therefore application of these protein in food industries tent to increase in the last decade.
Soy protein isolate (SPI) contains approximately 90% of protein which has high digestibility (Babji et al. 2010). SPI is used as ingredient in various food products such as meat products, beverages, infant formulas, and food supplements (Singh et al. 2008). SPI has various important functional properties for industrial applications, i.e. as emulsifier, foaming agent, gelation agent, and as antioxidant (Abu-salem et al. 2013; Kempka et al. 2014). Surface activity and emulsifying properties of soy protein is considered still limited due to its molecular structure. Modification of soy protein is directed to achieve better desirable structure, improved physicochemical and functional properties.
Previous studies reported that interactions of protein with polyphenol improved the functional properties of protein by modifying its structural characteristics (Liu et al. 2015; Rohn 2014). This resulted in improving features such as emulsifying ability, foaming ability, gelation properties and antioxidant activity (Liu et al. 2016; Wang et al. 2014a). Protein–polyphenols interaction consists of two types of interactions: non-covalent linkage (hydrogen bonding, Van der Waals interaction, hydrophobic effect and ion pairing) and covalent linkage. Non-covalent linkage forms a reversible interaction (Le Bourvellec and Renard 2012; Ozdal et al. 2013), while covalent linkage formed irreversible interaction (Oliveira et al. 2016; Rohn 2014). For food product development, covalent complex (protein–polyphenol conjugate) is more desirable than non-covalent complex since it is more stable (Curcio et al. 2012).
Tea is well known as an excellent polyphenol sources (Perez-Jimenez et al. 2010). Polyphenol compounds in tea consist of flavonoid and phenolic acid groups (Wang et al. 2000). Three basic flavonoid groups in tea are catechins, theaflavins and thearubigins. Both green tea and black tea are the most popular tea products and contain a similar quantity of flavonoids, but differ in their chemical structures. Green tea has higher content of simple flavonoids, catechins, while black tea contains many complex polymers of catechins, theaflavins and thearubigins, which are produced during fermentation of the black tea (Yashin et al. 2015). Recent studies have been reported that tea polyphenol is one of the most commonly used antioxidants to bind with protein and plays role in enhancing antioxidant activity of the protein (Prodpran et al. 2012; Aewsiri et al. 2013). The use of tea polyphenols, such as catechins, EGCG and tannic acid in protein–polyphenol cross-linking systems also helps to improve functional properties of protein, such as thermal stability (Kanakis et al. 2011), emulsifying properties (Aewsiri et al. 2013) and protein based-films mechanical properties (Prodpran et al. 2012). However, most studies have been limited to the use of purified tea polyphenol or a narrow range of tea polyphenols.
Considering the availability of tea, that is about three billions kilograms of tea being produced and consumed yearly across the world, which makes the global tea extracts market grow at 6.8% during 2019–2027, to account for US$ 4500.7 millions by 2027 (Market Research 2020), we chose tea extract as the phenolic source to modify the soy protein. The aim of this study was to create favourable condition to utilize the natural tea extract in the making of complex soy protein–polyphenol conjugate with good functional characteristics. This favourable condition involved alkaline treatment which was expected to promote formation of better interaction between the soy protein and the phenolic compounds of the tea extracts. Our hypothesis is that under alkaline treatment, addition the various phenolic substances in the tea extract would react extensively with the functional group in the soy protein and thus, improve the functional characteristic of soy protein and at the same time add another health feature of the modified protein, that is antioxidant activity, useful for many food applications.
Our main goal is proposing new food emulsifier with high antioxidant activity useful for applications in the food industries.
Materials and methods
Materials
Commercial soy protein isolate (protein 90%), green tea (GT) and black tea (BT) (Camellia sinensis) were from local markets in Bogor, Indonesia. Dialysis bag (MWCO 8 kDa), ethanol, methanol and NaOH were from Sigma-Aldrich Company Ltd. (Gillingham, UK). 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and the Pre-stained Protein Markers (low range) for SDS-PAGE were from Nacalai Tesque, Inc. (Kyoto, Japan). Lecithin from egg yolk was from Wako Pure Chemical Industries (Tokyo, Japan). All other chemicals used were analytical grade, unless otherwise stated.
Methods
Extraction of polyphenols
Green tea and black tea polyphenols were extracted according to the method defined by International Organization for Standardization (2005) with slight modifications. Briefly, 200 g of tea leaves (green or black tea) were grinded for 1 min using a blender. Then 20 g of the ground tea powder was dissolved in 500 mL of distilled water (70 °C) in an extraction tube. The extraction tube was heated at 70 °C for 10 min in a shaker waterbath at 150 rpm, cooled at room temperature and then centrifuged at 1760 × g for 10 min. The supernatant was carefully collected and put into a graduated tube. This extraction step was repeated twice. Both supernatant was combined and the total volume was made up to 1.00 L with water. The extract was freeze-dried and stored at − 20 °C until used.
Total polyphenols were measured by spectrophotometric analysis using Folin–Ciocalteu’s reagent according to the method defined by the International Organization for Standardization (2005). Briefly, 0.5 mL of the extract solution was mixed with 2.5 mL of Folin–Ciocalteu reagent (10 N), and then the solution was vortexed for 1 min. Within 8 min, 2 mL of Na2CO3 (7.5% w/v) was added to the mixture, and then the tube was allowed to stand for 60 min in the dark room. The absorbance of the solution was measured at 765 nm. The blank was prepared using distilled water instead of extract solution. Total polyphenol content (TPC) was expressed as gallic acid equivalents (GAE) by using the calibration curves of gallic acid standard. TPC value was 18.21 ± 0.71 mg GAE/g for green tea extract powder and 12.23 ± 0.86 mg GAE/g for black tea extract powder.
Preparation of SPI-tea polyphenol conjugates
SPI-tea polyphenol conjugates was prepared according to Liu et al. (2016) with slight modifications. Briefly, 1.0 g of SPI was dissolved in 100 mL of deionized water in an erlenmeyer flask, and then shaked at 150 rpm for 30 min to facilitate protein solubilization. Then, the solution was mixed with tea polyphenol extracts at several concentrations [0 (underivatized SPI), 0.10, 0.25, 0.50, 0.75 and 1.00% w/w]. The pH of solution was adjusted to 9.0 with 0.1 M NaOH. After incubation at ambient temperature for 24 h under continuous stirring at 150 rpm, the mixture solution was dialyzed for 48 h against water in a dialysis tubing (8 kDa MWCO) to remove free polyphenols and their derivatives. After that, the resulting solution was dried using a freeze dryer to obtain porous solid. The control was prepared under the same condition but in the absence of tea polyphenol extract.
Structural and physicochemical characterizations of SPI-tea polyphenol conjugates
SDS-PAGE under reducing condition was performed according to the method of Fling and Gregerson (1986). The stacking and resolving gel were prepared with 4% and 12.5% (w/v) acrylamide, respectively. The protein–tea polyphenol conjugates sample was diluted with SDS sample buffer containing 2% β-mercaptoethanol, and then heated at 100 °C for 2 min. After running the electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250.
Molar mass distribution of SPI-tea polyphenol conjugates solution was carried out by Size Exclusion Chromatography (SEC) using Sephacryl S400-HR open column. Briefly, sample solution containing 1% (w/v) protein was dissolved in 6 M Urea and 0.4% β-mercaptoethanol, followed by centrifugation at 14,355 × g for 10 min. 5 mL of the supernatant was loaded to a Sephacryl S400-HR (bed volume, 200 mL) column and eluted with 0.5 M NaCl solution as the eluent at a nominal flow rate of 0.5 mL/min. The elution curve of protein was monitored by absorbance at 280 nm.
Surface hydrophobicity was determined using 1-anilino-8-naphthalene-sulfonate (ANS) as a fluorescence probe according to Wang et al. (2014b) with some modifications. SPI-tea polyphenol conjugates (4 mg/mL) was suspended in 0.1 M phosphate buffer pH 7.0 at room temperature. The sample was stirred for 30 min, and then centrifuged at 14,355 × g for 30 min. The protein content of supernatant was determined by Lowry method using BSA as a standard. Each sample was diluted in 0.1 M phosphate buffer pH 7.0 at several concentration (0.0025–4 mg/mL). After that, 4 mL of each diluted sample was mixed with 40 μL of 8 mM ANS solution in 0.1 M phosphate buffer pH 7.0. Fluorescence intensity (FI) of the mixed solution was measured at 365 nm (excitation) and 484 nm (emission) using a spectrofluorometer. The initial slope in FI-protein concentration plot was determine as an index of protein surface hydrophobicity (S0).
Functional properties evaluation of SPI-tea polyphenol conjugates
Emulsifying Ability Index (EAI) and Emulsifying Stability Index (ESI) of SPI-tea polyphenol conjugates were measured according to Liu et al. (2015) with some modifications. The sample was dispersed in deionized water to give the protein concentration of 0.3% (w/v). Then, 15 mL of protein solution was mixed with 5 mL of soybean oil, and followed by stirring at 18,000 rpm for 2 min with a Polytron Brinkmann PT 10/35 homogenizer (Kinematica, Switzerland). An aliquot of the emulsion (0.1 mL) was pipetted from the container’s bottom and 200-fold diluted with 0.1% SDS solution. The emulsion solution after 30 min incubation was also 200-fold diluted with 0.1% SDS solution. Each of the diluted solutions was blended thoroughly for 10 s with a vortex mixer. Then, the absorbance was measured at 500 nm. 1% (w/v) lecithin was used as a positive control.
EAI was calculated by the following formula:
where A the absorbance at 500 nm of sample solution without incubation, l path length of cuvette (0.01 m), DF dilution factor (200), Φ oil volume fraction, and C protein concentration in solution.
ESI was calculated by the following formula:
where A0 the absorbance at 500 nm of sample solution without incubation, Δt the time difference (30 min), and ΔA the absorbance difference at 500 nm between sample solution without incubation and sample solution after 30 min incubation.
Zeta potential, which refers to the droplets size and polydispersity index (PdI) of freshly made emulsion sample were measured using a zeta potential and particle size analyzer ELSZ-2000 G (Photal Otsuka Electronics Co., Ltd, Osaka, Japan). Each fresh emulsion sample was diluted with deionized water at a ratio of 1:25 prior to analysis. Cells used for the measurement of zeta potential and droplet size were a rectangular quartz standard cell and a plastic cell, respectively. Zeta potential, mean droplets size and polydispersity index of the diluents were calculated by using the software (version 3.60/2.30) provided from the manufacturer. The data were recorded at 25 °C.
Antioxidant activity of SPI-tea polyphenol conjugates was measured by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity method described in Zhu et al. (2014). Briefly, 1.5 mL of 0.2 mM DPPH in ethanol was mixed with 0.75 mL of standard Trolox solutions with several concentrations (50–250 µM) or SPI-tea polyphenol conjugates solution in each test tube. Then the test tube was allowed to stand at room temperature for 45 min in the dark. The absorbance was measured at 517 nm. The DPPH radical scavenging activity was expressed as Trolox equivalent antioxidant activity (TEAC) which was calculated using the calibration curves of Trolox standard. Each sample was prepared in triplicate.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Statistical analysis was performed using the SPSS (version 16.0.0) software (univariate analysis). One-way variance analysis was conducted using HSD’s post hoc test at significance level of 5%.
Result and discussion
Molecular mass distribution of SPI-tea polyphenol conjugates
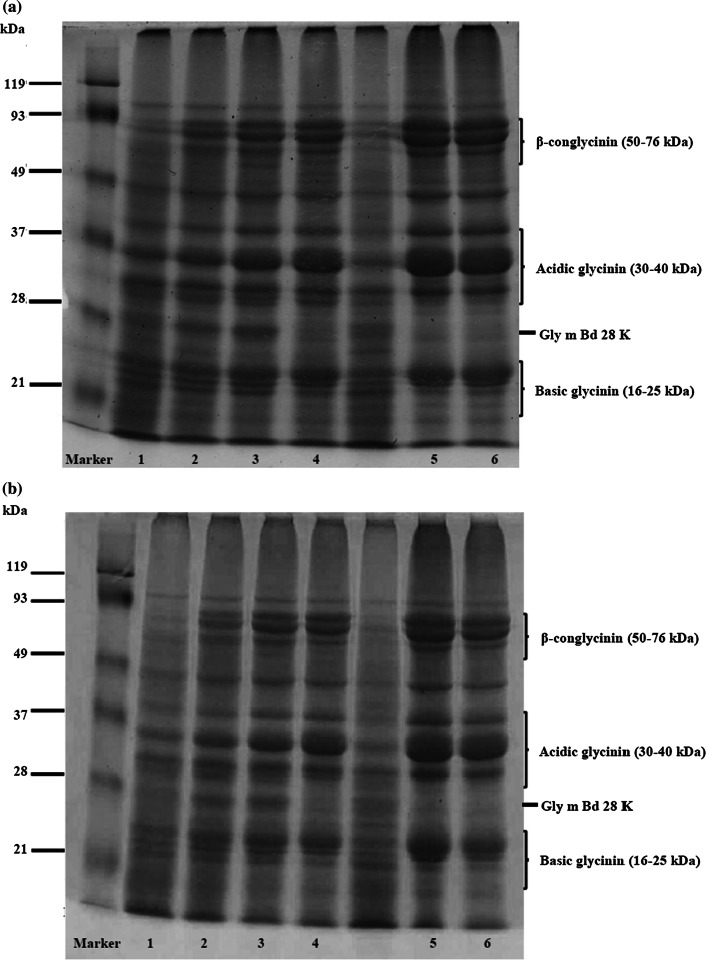
SDS-PAGE pattern of SPI treated with tea polyphenol extract is shown in Fig. 1. Underivatized SPI (control) had dozens of protein bands. Their major bands are attributed to basic glycinin (16–22 kDa), acidic glycinin (30–40 kDa) and β-conglycinin (50–76 kDa) subunits. Protein band of SPI treated with green tea polyphenol extract, was different from that of the underivatized SPI. Band intensity of 28 kDa protein, known as Gly m Bd 28 K (glycoprotein allergen) (Tsuji et al. 1997), was increased at first and then decreased later as polyphenol concentration was increased. In contrast, band intensity of 22 kDa protein (basic glycinin subunit), 35 kDa protein (acidic glycinin subunit) and 76 kDa protein (β-conglycinin subunit) were increased with increasing polyphenol concentration.
Fig. 1.
SDS-PAGE of a SPI treated with green tea polyphenols and b SPI treated with black tea polyphenols in alkaline treatment: Lane 1-underivatized SPI; Lane 2-SPI-tea polyphenol 0.10% w/w; Lane 3-SPI-tea polyphenol 0.25% w/w; Lane 4-SPI-tea polyphenol 0.50% w/w; Lane 5-SPI-tea polyphenol 0.75% w/w; Lane 6-SPI-tea polyphenol 1.00% w/w
Changes in the band pattern and those in band intensity are clearly indicative of interaction between soy protein and tea polyphenol. The protein band intensity at the origin of the SDS-PAGE separation gel, which is attributed to high molecular weight protein complexes, was increased as well. The complex has non-disulfide type covalent bonds, because the electrophoresis was conducted under reducing condition using β-mercaptoethanol. This result suggests that the formation of high molecular weight SPI complex was facilitated by addition of green tea polyphenol extract and this treatment induces polymerization of SPI subunit proteins.
Similar result was found in SPI treated with black tea polyphenol extract (Fig. 1b). Band intensity of the 28 kDa allergen protein was decreased, whereas the band intensity of basic glycinin subunit (22 kDa), acidic glycinin subunit (35 kDa) and β-conglycinin subunit (76 kDa) were increased with increasing polyphenol concentration. These results suggest that both green tea polyphenol and black tea polyphenol induce molecular mass shift of the SPI proteins.
The SDS-PAGE results demonstrated that treatment of tea polyphenol increased the band intensity of 22 kDa, 37 kDa or higher molecular weight (50–76 kDa) proteins and decreased the band intensity of 28 kDa or lower molecular weight proteins (16–21 kDa). There was a molecular mass shift of the soy proteins after treated with the tea extract under alkaline condition. Tea leaf, especially black tea leaf, contain large amount of tannins with molecular mass between 500 and 3000 dalton (Bate-Smith and Swain 1962). Attachment of gallic acid, flavonoids, and tannins to a single protein molecule does not cause such large molecular mass shift. Thus, we think that those phenolic compounds act as a bridge between proteins and produce high molecular protein complexes. Previous studies reported that polyphenol is oxidized under alkaline condition, and the resulting quinone reacts with nucleophilic side chains of proteins (Rohn 2014), and further oxidation process induces intermolecular cross-linking between the proteins (Rawel et al. 2002; Liu et al. 2015). The conjugation reactions between protein and polyphenol lead to change of the protein molecular weight.
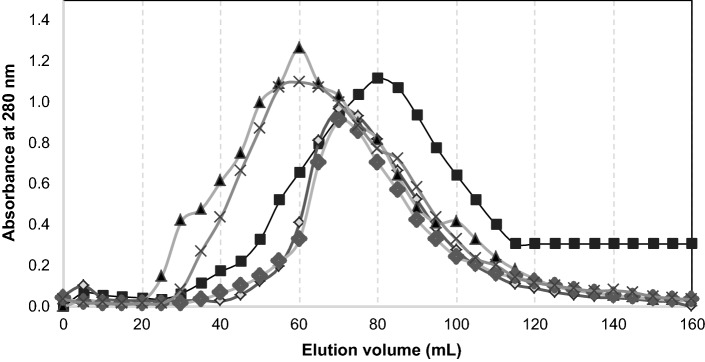
The size exclusion chromatography (SEC) profiles of SPI-tea polyphenol conjugates was different from that of the underivatized SPI (Fig. 2). SPI-green tea polyphenol conjugates showed similar SEC profile to SPI-black tea polyphenol conjugates at concentration levels of 0.10% and 1.00%. The peaks of SPI-tea polyphenol conjugates were shifted to smaller elution volume with increasing tea extract concentration, indicating that larger molecular size protein complex is formed as polyphenol concentration is increased. The data confirmed that the conjugation of SPI with polyphenols in the tea extract alters the molecule size and molar mass distribution of the proteins.
Fig. 2.
Size exclusion chromatography profile of underivatized SPI (filled square), SPI-GT 0.10% w/w (unfilled diamond), SPI-GT 1.00% w/w (filled triangle), SPI-BT 0.10% w/w (filled diamond), and SPI-BT 1.00% w/w (×)
Surface hydrophobicity of SPI-tea polyphenol conjugates
Surface hydrophobicity of SPI-tea polyphenol conjugates is shown in Fig. 3. Increase of polyphenol concentration decreased the surface hydrophobicity. In green tea extract treatments, the surface hydrophobicity index was decreased with increasing tea extracts, indicating that increasing in polyphenol concentration made SPI more hydrophilic. The decreased surface hydrophobicity of soy protein is due to attachment of oxidized polyphenol groups on the protein surface. As reported earlier, in aqueous alkaline, catechin is oxidized and then polymerized to form flavonoid oligomers, which have multiple hydroxyl groups (Liang et al. 2016). These multiple hydroxyl groups assist the soy protein becoming more hydrophilic, and the aromatic rings of the oligomeric polyphenol may well block some hydrophobic residues like tryptophan through hydrophobic interactions. Treatment with black tea polyphenol extract also induced concentration-dependent decrease in the surface hydrophobicity. The hydrophobic decreasing effect by polyphenol treatment was larger in the black tea extract than in the green tea extract. Attachment of the black tea polyphenol, which contain higher number of hydroxyl groups than green tea polyphenol (Leung et al. 2001), attract the protein into more hydrophilic environment, resulting in significant decrease in the protein surface hydrophobicity. It can be concluded that the chemical state and amount of tea polyphenol molecules which bind to the protein affected the surface property of the soy protein molecule.
Fig. 3.
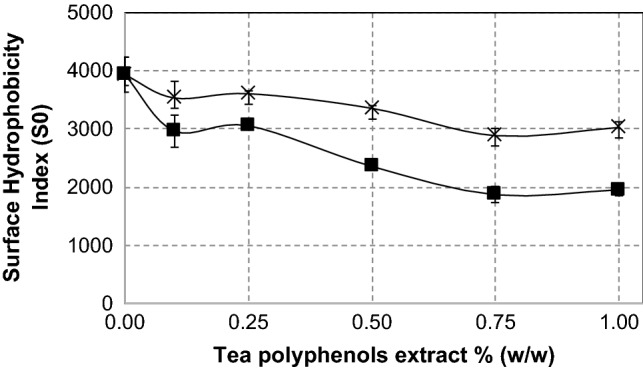
Surface hydrophobicity behavior of SPI-green tea polyphenols conjugates (×) and SPI-black tea polyphenols conjugates (filled square) at several levels of tea polyphenols extract concentration. Values are the means of two replicates
Emulsifying properties of SPI-tea polyphenols conjugates
Protein possesses moderate emulsifying ability since it is an amphiphilic macromolecule. Structural modifications of some food proteins can improve their emulsifying ability. Liu et al. (2015) reported that lactoferrin conjugated with epigallocatechin gallate (EGCG), chlorogenic acid (CA) and gallic acid (GA) showed positive impact on the food functionalities: emulsifying ability and foaming ability.
In this study, Emulsifying Ability Index (EAI) of SPI-tea polyphenol conjugates are shown in Fig. 4a. EAI of SPI-black tea polyphenol conjugates underwent 21% increase from the treatment of 0.1 to 0.25% black tea extract, and remained constant up to 0.75% black tea extract and then went to higher value when the black tea extract concentration was 1.00%. This result implys that attachment of black tea polyphenols to protein improves the emulsifying ability of the protein. The highest EAI value (61.73 ± 2.83 m2/g) was achieved by treatment of SPI-green tea polyphenol 0.75% (w/w) which was 43% higher than that of the underivatized SPI and 38% higher than that of lecithin (44.76 ± 4.04 m2/g), a commercial food emulsifier. On the other hand, the lowest EAI value obtained at treatment of 1.00% green tea extract, indicate that excessive concentration of green tea extract inhibit the protein to form emulsion.
Fig. 4.
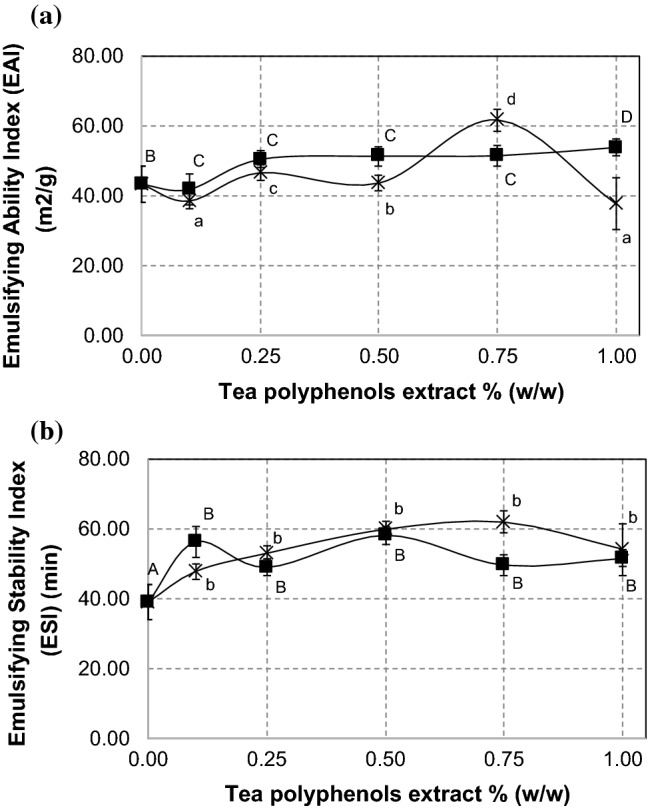
Emulsifying Ability Index (EAI) (a) and Emulsifying Stability Index (ESI) (b) of SPI-green tea polyphenols conjugates (×) and SPI-black tea polyphenols conjugates (filled square) at several levels of tea polyphenols extract concentration. Different superscript letters show significant differences (p < 0.05) among concentrations. Values are the means of three replicates
Emulsifying Stability Index (ESI) of SPI-tea polyphenol conjugates is shown in Fig. 4b. Both SPI-black tea polyphenol conjugates and SPI-green tea polyphenol conjugates showed significantly higher ESI than that of underivatized SPI but there were no significant changes among the samples. SPI-green tea polyphenol 0.75% (w/w) showed the highest ESI value (61.98 ± 10.54 min) which was 59% higher than that of the underivatized SPI (39.08 ± 3.64 min), but it was still 41% lower than that of lecithin (104.94 ± 8.34 min). Thus, the conjugation with polyphenols increases emulsifying stability of SPI but it is still inferior to the stabilizing effect of lecithin, a commercial food emulsifier.
In order to better understand the behavior of SPI-tea polyphenol conjugates emulsion, we measured an important emulsion attribute, the surface charge of droplets in the emulsion (zeta potential). Zeta potential value of emulsions prepared with SPI-tea polyphenol conjugates are shown in Table 1. Emulsion with SPI-green tea polyphenol conjugates showed higher negative zeta potential values than underivatized SPI, whereas zeta potential value of SPI-black tea polyphenol conjugates shows no significant difference (p < 0.05) compared with the underivatized SPI. The value of zeta potential indicates degree of repulsion force between charged particles in the colloidal system, where higher negative or positive zeta potential value means stronger repulsion force between the droplets. As reported earlier, high zeta potential value in the range between − 41 and − 50 mV showed better emulsion stability (van Nieuwenhuyzen and Szuhaj 1998). The high negative zeta potential values in emulsion with SPI-green tea polyphenol conjugates (− 44.9 mV and − 41.0 mV) indicate that the droplets had strong repulsion forces. High repulsion forces prevent the droplets from flocculating or breaking, and finally resulting in a stable emulsion system. This result was in agreement with the high ESI value of SPI-green tea polyphenol conjugates (Fig. 4b).
Table 1.
Zeta potential and mean droplet size of SPI-tea polyphenols conjugates
| Sample code | Zeta potential (mV) | Mean droplet size (nm) | Polydispersity Index |
|---|---|---|---|
| Underivatized SPI | − 34.15 ± 2.16c | 1155.83 ± 37.12c | 0.44 ± 0.02c |
| SPI-GT 0.10% w/w | − 44.93 ± 1.29a | 1416.13 ± 54.19d | 0.47 ± 0.01c |
| SPI-GT 0.75% w/w | − 41.02 ± 1.89b | 495.77 ± 80.37a | 0.27 ± 0.09a |
| SPI-BT 0.10% w/w | − 36.19 ± 3.86c | 736.87 ± 158.73b | 0.30 ± 0.07b |
| SPI-BT 1.00% w/w | − 34.34 ± 0.73c | 775.43 ± 239.26b | 0.32 ± 0.11b |
Different superscript letters in a column show significant differences (p < 0.05) among the samples. Values are the means of three replicates
Other factors that contribute to the emulsion stability were mean droplets size of emulsion and polydispersity index (PdI) of emulsion. The mean droplets size defines a diameter of emulsion particles size, while PdI of emulsion defines degree of droplets size distribution in the dispersion system.
The range of PdI value is from 0 to 1, where 0 means a monodisperse particle dispersion system and 1 means a highly polydisperse particle dispersion system (Baboota et al. 2007). As shown in Table 1, both mean droplets size and PdI of SPI-green tea polyphenol 0.75% (w/w) emulsion was about two times smaller than those of underivatized SPI, indicating that the attachment of green tea polyphenol at that concentration greatly improved emulsifying properties of soy protein. Interestingly, when low green tea polyphenol concentration (0.10%, w/w) was used, the mean droplets size and PdI of the emulsion were larger than those of underivatized SPI. It suggests that the conjugation with green tea polyphenol at very low polyphenol concentration may inhibit soy protein molecules to form such compact adsorption layer to cover oil droplets, leading broader droplets size distribution by coalescence of droplets. In the case of black tea treatments, mean size and PdI of droplets formed with SPI with polyphenols at the lowest (0.10%, w/w) and the highest (1.00%, w/w) polyphenol concentration were smaller than those of the underivatized SPI, indicating that the conjugation with black tea polyphenol improves emulsifying properties of soy protein. Thus, the modifications with tea polyphenols improved emulsifying properties of soy protein emulsion. This is because the structural changes of protein in the presence of tea polyphenols increased protein ability to rapidly adsorb at the oil–water interface and reduce the surface tension between oil and water fraction in the system. This effect was dependent on the concentration of tea polyphenol used.
In summary, covalent modification of SPI-tea polyphenols gave positive impact on emulsifying properties of SPI. Emulsion with SPI-green tea polyphenol conjugates 0.75% (w/w) showed the highest emulsifying ability, highest emulsifying stability with strong repulsion force, smaller emulsion droplets size, and lower PdI. Alkaline treatment of SPI with 0.75% (w/w SPI) green tea extract was considered as the best condition for improving emulsifying properties of native SPI.
Despite the favourable and improvement functional characteristics of soy protein with addition of both tea extract in the alkaline treatment, there is limitation in the application of this product. Due to the generally low absorption of phenolic compounds after food intake, most of the consumed polyphenols remain in the gastrointestinal tract, where they then can exert inhibitory effects on enzymes involved in the degradation of saccharides, lipids, and proteins. While the inhibitory effects of phenolics on the digestion of energy-rich food components (saccharides and lipids) may be regarded as beneficial, primarily in the weight-control diets, their inhibitory effects on the digestion of proteins are not desirable for the reason of reduced utilization of amino acids (Velickovic and Stanic-Vucinic 2018). However, since utilization of the soy protein–polyphenol as emulsifier in the food product require only a small amount (less than 2%), then the unfavourable health effect of phenolic attachment in the soy protein may be neglected. In addition, there is another health benefit in using SPI-tea polyphenol, namely its antioxidant activity as discussed in the following paragraph.
Antioxidant activity of SPI-tea polyphenols conjugates
Figure 5 shows DPPH scavenging activity of SPI-tea polyphenol conjugates. The SPIs treated with 0.50% and higher concentrations of green tea polyphenol extracts showed higher antioxidant activity than underivatized SPI, eventhough lower concentration of tea extracts reduced the antioxidant activity of SPI, which may attributed to the masking effect of soy protein at lower concentrations of tea polyphenol. In case of black tea, antioxidant activity of SPI-tea polyphenols conjugates at 0.25% and higher concentrations of extracts were higher than that of underivatized SPI. The highest value of DPPH scavenging activity (38.76 ± 5.49 µmol TE/g) was achieved in the treatment of black tea polyphenol 1.00% w/w, which was 65% higher than that of underivatized SPI. These results imply that conjugation at higher polyphenol concentrations of both green tea and black tea extracts improve soy protein ability to scavenge DPPH free radicals. Similar studies showed that covalent attachment between protein and a tea polyphenol such as catechin, EGCG, or epigallocatechin exhibited stronger scavenging activity against DPPH radicals than native protein (Feng et al. 2018; Gu et al. 2017). Our experiment with the whole extract of green tea and black tea polyphenols, showed that various phenolic compounds in the tea extract enhance the protein antioxidant properties.
Fig. 5.
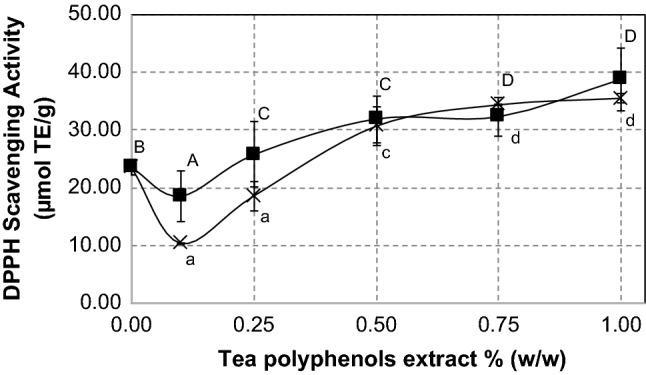
DPPH scavenging activity of SPI-green tea polyphenols conjugates (×) and SPI-black tea polyphenols conjugates (filled square) at several levels of tea polyphenols extract concentration. Different superscript letters show significant differences (p < 0.05) among concentrations. Values are the means of three replicates
We suggest that different type of polyphenols in green tea and black tea influence the binding affinity of polyphenols toward proteins, improved the protein structure and functionalities, and at the same time increased the antioxidant activity. Recent evidences revealed that larger molecular size of polyphenol and/or higher hydroxylation level in the polyphenol strongly increase the binding affinity between polyphenol and protein (Yildirim-Elikoglu and Erdem 2018; Czubinski and Dwiecki 2017).
Conclusion
SPI-tea polyphenol conjugates were successfully formed by addition of green and black tea extract under alkaline treatment. The SPI structure was modified, giving better functionalities. The SPI-tea conjugates showed higher emulsifying activity and higher antioxidant activity than underivatized SPI. SPI-green tea polyphenols 0.75% (w/w) showed the best quality as emulsifier as tested by various parameters. Although the emulsion stability is still inferior to lecithin, the SPI-tea polyphenol conjugates offer high antioxidant activity as emulsifier.
These findings suggest that the conjugation of SPI with polyphenols in the tea extract provide a new food emulsifier with good characters.
Funding
The funding was received from Bogor Agricultural University-Kagawa University Research Internship Program.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Salem FM, Mahmoud MH, El-Kalyoub MH, Gibriel AY, Abou-Arab A. Characterization of antioxidant peptides of soybean protein hydrolysate. World Acad Sci Eng Technol. 2013;79:249–253. [Google Scholar]
- Aewsiri T, Benjakul S, Visessanguan W, Wierenga PA, Gruppen H. Emulsifying property and antioxidative activity of cuttlefish skin gelatin modified with oxidized linoleic acid and oxidized tannic acid. Food Bioprocess Technol. 2013;6(4):870–881. doi: 10.1007/s11947-011-0636-1. [DOI] [Google Scholar]
- Babji AS, Fatimah S, Ghassem M, Abolhassani Y. Protein quality of selected edible animal and plant protein sources using rat bio-assay. Int Food Res J. 2010;17(2):303–308. [Google Scholar]
- Baboota S, Shakeel F, Ahuja A, Ali J, Shafiq S. Design development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007;57:315–332. doi: 10.2478/v10007-007-0025-5. [DOI] [PubMed] [Google Scholar]
- Bate-Smith EC, Swain T. Flavonoid compounds. In: Mason HS, Florkin AM, editors. Comparative biochemistry. Cambridge: Academic Pres; 1962. [Google Scholar]
- Curcio M, Parisi OI, Puoci F, Altimari I, Spizzirri UG, Picci N. Antioxidant polymers by free radical grafting on natural polymers. Antioxidant polymers: synthesis, properties, and applications. New York: Wiley; 2012. pp. 153–178. [Google Scholar]
- Czubinski J, Dwiecki K. A review of methods used for investigation of protein–phenolic compound interactions. Int J Food Sci Technol. 2017;52(3):573–585. doi: 10.1111/ijfs.13339. [DOI] [Google Scholar]
- Feng J, Cai H, Wang H, Li C, Liu S. Improved oxidative stability of fish oil emulsion by grafted ovalbumin-catechin conjugates. Food Chem. 2018;241:60–69. doi: 10.1016/j.foodchem.2017.08.055. [DOI] [PubMed] [Google Scholar]
- Fling SP, Gregerson DS. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris-buffer system without urea. Anal Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Gu L, Peng N, Chang C, McClements DJ, Su Y, Yang Y. Fabrication of surface-active antioxidant food biopolymers: conjugation of catechin polymers to egg white proteins. Food Biophys. 2017;12(2):198–210. doi: 10.1007/s11483-017-9476-5. [DOI] [Google Scholar]
- International Organization for Standardization (ISO) 14502-1 (2005) Determination of substances characteristic of green and black tea—part 1 content of total polyphenols in tea—colorimetric method using Folin–Ciocalteu reagent. https://www.iso.org/standard/31356.html. Accessed 20 Jan 2017
- Kanakis CD, Hasni I, Bourassa P, Tarantilis PA, Polissiou MG, Tajmir-Riahi HA. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011;127(3):1046–1055. doi: 10.1016/j.foodchem.2011.01.079. [DOI] [PubMed] [Google Scholar]
- Kempka AP, Honaiser TC, Fagundes E, Prestes RC. Functional properties of soy protein isolate of crude and enzymatically hydrolysed at different times. Int Food Res J. 2014;21(6):2229–2236. [Google Scholar]
- Kroll J, Rawel HM, Rohn S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci Technol Res. 2003;9:205–218. doi: 10.3136/fstr.9.205. [DOI] [Google Scholar]
- Le Bourvellec C, Renard CMGC. Interactions between polyphenols and macromolecules: quantification methods and mechanisms. Crit Rev Food Sci Nutr. 2012;52:213–248. doi: 10.1080/10408398.2010.499808. [DOI] [PubMed] [Google Scholar]
- Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen Z. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131:2248–2251. doi: 10.1093/jn/131.9.2248. [DOI] [PubMed] [Google Scholar]
- Liang JY, Wu JY, Yang MY, Hu A, Chen LY. Photocatalytic polymerization of catechin molecules in alkaline aqueous. J Photochem Photobiol B. 2016;165:115–120. doi: 10.1016/j.jphotobiol.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Liu F, Sun C, Yang W, Yuan F, Gao Y. Structural characterization and functional evaluation of lactoferrin polyphenol conjugates formed by free-radical graft copolymerization. RSC Adv. 2015;156(5):41–51. doi: 10.1039/C4RA10802G. [DOI] [Google Scholar]
- Liu F, Ma C, Gao Y, McClements DT. Food-grade covalent complexes and their application as nutraceutical delivery systems: a review. Compr Rev Food Sci Food Saf. 2016;16:76–95. doi: 10.1111/1541-4337.12229. [DOI] [PubMed] [Google Scholar]
- Market Research (2020) Tea extract market to 2027. https://www.reportlinker.com/p05875920. Accessed 20 Mar 2020
- Oliveira FCD, Coimbra JSDR, de Oliveira EB, Zuniga ADG, Rojas EEG. Food protein–polysaccharide conjugates obtained via the Maillard reaction: a review. Crit Rev Food Sci Nutr. 2016;56:1108–1125. doi: 10.1080/10408398.2012.755669. [DOI] [PubMed] [Google Scholar]
- Ozdal T, Capanoglu E, Altay F. A review on protein–phenolic interactions and associated changes. Food Res Int. 2013;51:954–970. doi: 10.1016/j.foodres.2013.02.009. [DOI] [Google Scholar]
- Perez-Jimenez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the phenol-explorer database. Eur J Clin Nutr. 2010;64:112–120. doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- Prodpran T, Benjakul S, Phatcharat S. Effect of phenolic compounds on protein cross-linking and properties of film from fish myofibrillar protein. Int J Biol Macromol. 2012;51(5):774–782. doi: 10.1016/j.ijbiomac.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Rawel HM, Czajka D, Rohn S, Kroll J. Interactions of different phenolic acids and flavonoids with soy proteins. Int J Biol Macromol. 2002;30:137–150. doi: 10.1016/S0141-8130(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Rohn S. Possibilities and limitations in the analysis of covalent interactions between polyphenols and proteins. Food Res Int. 2014;65:13–19. doi: 10.1016/j.foodres.2014.05.042. [DOI] [Google Scholar]
- Singh P, Kumar R, Sabapathy SN, Bawa AS. Functional and edible uses of soy protein products. Compr Rev Food Sci Food Saf. 2008;7:14–28. doi: 10.1111/j.1541-4337.2007.00025.x. [DOI] [Google Scholar]
- Tsuji H, Bando N, Hiemori M, Yamanishi R, Kimoto M, Nishikawa K, Ogawa T. Purification of characterization of soybean allergen Gly m Bd 28K. Biosci Biotechnol Biochem. 1997;61:942–947. doi: 10.1271/bbb.61.942. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhuyzen W, Szuhaj BF. Effects of lecithins and proteins on the stability of emulsions. Lipid/Fett. 1998;100(7):282–291. doi: 10.1002/(SICI)1521-4133(199807)100:7<282::AID-LIPI282>3.0.CO;2-W. [DOI] [Google Scholar]
- Velickovic TDC, Stanic-Vucinic DJ. The role of dietary phenolic compounds in protein digestion and processing technologies to improve their antinutritive properties. Compr Rev Food Sci Food Saf. 2018;17(1):82–103. doi: 10.1111/1541-4337.12320. [DOI] [PubMed] [Google Scholar]
- Wang H, Provan GJ, Helliwell K. Tea flavonoids, their functions, utilization and analysis. Trends Food Sci Technol. 2000;11:152–160. doi: 10.1016/s0924-2244(00)00061-3. [DOI] [Google Scholar]
- Wang X, Zhang J, Lei F, Liang C, Yuan F, Gao Y. Covalent complexation and functional evaluation of (–)-epigallocatechin gallate and α-lactalbumin. Food Chem. 2014;150:341–347. doi: 10.1016/j.foodchem.2013.09.127. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li Y, Jiang L, Qi B, Zhou L. Relationship between secondary structure and surface hydrophobicity of soybean protein isolate subjected to heat treatment. J Chem. 2014;2014:1–10. doi: 10.1155/2014/475389. [DOI] [Google Scholar]
- Yashin AY, Nemzer BV, Combet E, Yashin YI. Determination of the chemical composition of tea by chromatographic methods: a review. J Food Res. 2015;4(3):56–87. doi: 10.5539/jfr.v4n3p56. [DOI] [Google Scholar]
- Yildirim-Elikoglu S, Erdem YK. Interactions between milk proteins and polyphenols: binding mechanisms, related changes, and the future trends in the dairy industry. Food Rev Int. 2018;34(7):665–697. doi: 10.1080/87559129.2017.1377225. [DOI] [Google Scholar]
- Zhu C, Zhang W, Kang Z, Zhou G, Xu X. Stability of an antioxidant peptide extracted from Jinhua ham. Meat Sci. 2014;96(2):783–789. doi: 10.1016/j.meatsci.2013.09. [DOI] [PubMed] [Google Scholar]