Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04411-8) contains supplementary material, which is available to authorized users.
Keywords: Stir bar sorptive extraction, Electrospinning, Coating, Polycyclic aromatic hydrocarbons, Non-alcoholic beer
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds that adversely affect human beings, and they have raised many concerns over recent years (Mohamadi et al. 2019; Saberi et al. 2018; Varjani et al. 2018). PAHs can be found in various types of foodstuffs such as beers (Singh et al. 2016). Alcohol is outlawed in some regions such as Middle Eastern countries, so non-alcoholic beers are very popular in these countries. Thus, the extraction and determination of PAHs in that kind of drink are important. There are various techniques to extract PAHs from drinks such as liquid–liquid extraction (LLE) (Silva et al. 2011), solid-phase extraction (SPE) (Londoño et al. 2015), soxhlet extraction (Fiedler et al. 2002), solid-phase microextraction (SPME) (Robles-Molina et al. 2011), stir bar sorptive extraction (SBSE) (Zuin et al. 2005). SBSE was introduced in 1999 for the extraction of volatile and semivolatile compounds from aqueous samples (Baltussen et al. 1999). In SBSE, the analytes are eliminated from aqueous solution and attracted to a coating by stirring. One of the disadvantages of the method is the physical damage of the coating due to the friction between the container and the coating (Jachero et al. 2014). In recent years, for decreasing the coating deterioration, some methods such as rotating disk sorptive extraction (RDSE), stir rod sorptive extraction (SRSE) and mechanical stir bar sorptive extraction (MSBSE) have been introduced (Luo et al. 2010; Mollahosseini et al. 2016; Richter et al. 2009). In MSBSE, the stirring system was developed to increase the longevity of the coating and enhance the performance of the extraction (Mollahosseini et al. 2016). Parameters including stirring speed, sample volume, ionic strength, pH of the solution, temperate, extraction time and desorption time have significant effects on the extraction efficiency of the SBSE method (Gorji et al. 2019; Jafari et al. 2018b).
One of the important parameters that plays a substantial role in the extraction efficiency of SBSE is the coating type because it can affect the partitioning coefficient of the analyte between the coating and the sample (David and Sandra 2007). Polydimethylsiloxane (PDMS) is broadly used in SBSE as the coating since it is commercially available (Prieto et al. 2010). However, introducing new coatings has been taken into consideration over the last few years (Jafari et al. 2018a; Jillani et al. 2018; Mollahosseini et al. 2020; Taghvimi et al. 2019). Among different methods to prepare coatings, electrospinning is one of the versatile, simple and cost-effective methods (Castro-Mayorga et al. 2017; Cavaliere et al. 2011). PDMS has a low molecular weight, so it is not suitable for electrospinning (Yang et al. 2009); in contrast, polyacrylonitrile (PAN) has a suitable molecular weight that can be used as a carrier polymer in electrospinning (Rahaman et al. 2007). PAN and PDMS are resistant polymers; moreover, they are inexpensive and widely used as membranes for chemical separation (Liang and Chung 2018). Also, the π–π interaction can be formed between PAN and PAHs (Karbownik et al. 2019).
The purpose of this study is to introduce a PAN/PDMS coating for the extraction of PAHs by the MSBSE method. The PAN/PDMS coating was fabricated on the shaft of the MSBSE device by the electrospinning technique. The effective parameters on the extraction were optimized by response surface methodology (RSM) based on the central composite design (CCD). Afterward, the target analytes including naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), phenanthrene (Phe) and anthracene (Ant) were extracted from non-alcoholic beer sample. The analytes were detected by gas chromatography/flame ionization detector (GC/FID), and the performance of MSBSE coated with electrospun PAN/PDMS and coupled with GC/FID (MSBSE-PAN/PDMS-GC/FID) was compared with previous studies.
Materials and methods
Chemicals
Acetone (C3H6O), dimethyl formaldehyde (C3H7NO), Nap (C10H8), Acy (C12H8), Ace (C12H10), Ant (C14H10) and Phe (C14H10) were bought from Merk Company, Germany. PAN (C3H3N) and PDMS ((C2H6OSi)n, the viscosity of 1000 cS at 25 °C) were obtained from Nanomeghyas-Iran and Kcc-Korea, respectively. For preparing the stock solution, 10 mg of every analyte was dissolved in 100 mL acetone. The obtained solution was stored at 4 °C to prevent any unwanted chemical reactions.
Preparation of the coating
The PAN/PDMS coating was synthesized by the electrospinning technique. PAN was dissolved in dimethyl formaldehyde 15% (w/v), and the mixture was continuously stirred by a magnetic stirrer since a homogenous solution was obtained. Then, 22% (w/v) PDMS was added to the solution and again stirred to reach a homogenous solution. The obtained solution was inserted in the syringe for the electrospinning process. The electrospinning setup consists of three parts: a 5 mL syringe, two electrodes and a DC power. The syringe and the shaft of the MSBSE device were connected to the positive and negative electrode of the electrospinning setup, respectively. The effective parameters including applied voltage, the electrospinning distance and electrospinning time were optimized by the Taguchi design method in three levels (Table S1). The obtained coatings were washed with deionized water and then methanol to remove any impurities.
The MSBSE method
The MSBSE setup (Fig. 1) employed during the present study was firstly introduced in 2016 as a novel experimental setup (Mollahosseini et al. 2016). The mini stainless steel shaft (2 cm length and 3.3 mm diameter) of the setup is coated with the PAN/PDMS coating.
Fig. 1.
The MSBSE setup
The optimization of four parameters including extraction time (A), desorption time (B), stirring speed (C), and sample volume (D) were conducted by CCD to obtain the best response (Table S2). The concentration of 1 ppm of all five PHAs (naphthalene, acenaphthene, phenanthrene, anthracene, and acenaphthylene) was added to the real sample (non-alcoholic beer), and the sum of the peak areas is selected as the response (Table S3).
A determined volume of the sample was placed in the 20 mL container and the stainless steel shaft coated with PAN/PDMS was completely immersed in the solution to extract PAHs. In the second stage, stainless steel was washed with 1.5 mL acetone to desorb the analyte. Then acetone was completely evaporated with nitrogen purge; afterward, 100 µL acetone was added, and 2 µL of the added acetone was removed and injected to GC/FID.
Apparatus
Fourier-transform infrared spectroscopy (FTIR-8400S, Shimadzu, Kyoto, Japan) at a wavelength range of 400 and 4500 cm−1 was used to identify the functional groups of the PAN/PDMS coating. Moreover, a scanning electron microscope (SEM-Vega II, TESCAN, Brno, Czech Republic) was employed to observe the surface morphology of coating fibers, and the Image J software (National Institutes of Health, USA) was utilized to measure the diameter of fibers. The concentration of the PAHs was determined by GC/FID (GC/FID-2010, Shimadzu, Kyoto, Japan) equipped with a BP-5 fused silica capillary column. More details about the operating conditions of GC/FID are available in our previous work (Mollahosseini et al. 2020).
Results and discussion
Electrospinning optimization
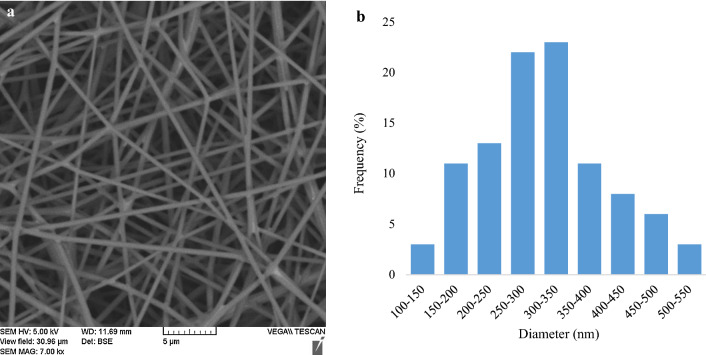
The Taguchi method suggested nine different runs for the electrospinning process (Table S4). The voltage was changed from 7 to 15 kV. Increasing the voltage caused the fiber diameter to decrease at first and to increase then. The evaporation of the solvent also increased with increasing voltage. These findings are in line with previous studies (CHEN et al. 2006; Shao et al. 2015; Wang et al. 2013). To achieve the minimum diameter of fibers followed by a maximum specific surface area, which causes the extraction efficiency to enhance, the voltage of 11 kV was chosen as an optimum voltage. The electrospinning distance was optimized under three different distances between 10 and 20 cm. At the electrospinning distance of 15 cm, the solvent had sufficient time to evaporate from the fibers, and fibers had enough space to stretch. Therefore, 15 cm was chosen as the optimum electrospinning distance. The electrospinning time was increased from 7 to 13 min. The thicker coating was obtained by increasing the electrospinning time as reported in previous studies (Choi et al. 2019; Jang et al. 2019). In lower electrospinning time, the fabricated coating was too thin; consequently, the mechanical strength of the coating was not enough, and it was deformed during the extraction by MSBSE. Furthermore, a direct relationship exists between the thickness of the coating and its adsorption capacity. The low coating thickness causes less amount of the adsorbate to adsorb and consequently the preconcentration factor to decrease. In higher electrospinning time, the uniformity of the coating thickness decreased (Choi et al. 2019; Jang et al. 2019). In electrospinning time of 10 min, a coating with relatively uniform thickness and a strong structure was obtained; as a result, 10 min was selected as the optimum electrospinning time. Figure 2a presents the SEM image of the PAN/PDMS coating, which was electrospun under the optimum condition. The fibers were bead-free; moreover, the coating had a uniform morphology (Fig. 2a). The average diameter of PAN/PDMS electrospun fibers was about 300 nm, and more than 40% of fibers had the diameters between 250 to 350 nm (Fig. 2b).
Fig. 2.
a The SEM images of the PAN/PDMS coating electrospun under the optimum condition. b The fiber diameter distribution of the PAN/PDMS coating
Coating FTIR
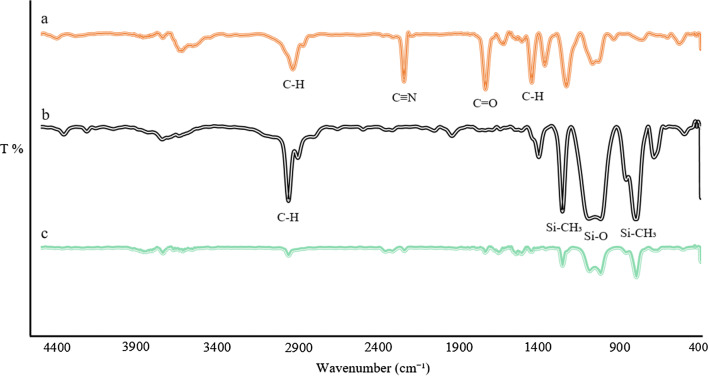
The FTIR spectra of PAN and PDMS polymers next to the PAN/PDM coating were depicted in Fig. 3. In the PAN spectrum, peaks detected at 1454 and 1733 cm−1 are attributed to C−H bending of −CH2− groups and C=O stretching of carbonyl groups, respectively (Zhao et al. 2016). The peak at 2243 cm−1 is owing to C≡N stretching and at 2940 cm−1 is related to C−H asymmetrical stretching of −CH2− groups (Deng et al. 2003). In PDMS, the peak at the region of 800 cm−1 is due to Si–CH3 rocking, and peaks at 1020 and 1093 cm−1 are because of Si–O–Si asymmetric stretching (Sankır et al. 2003). Moreover, the band observed at 2962 cm−1 is on account of C–H stretching in methyl groups (Mollahosseini et al. 2019). The peaks of PAN and PDMS are present in the PAN/PDMS coating spectrum, which is indicative of the successful synthesize of the coating by PAN and PDMS.
Fig. 3.
The FTIR spectra of a PAN, b PDMS, c PAN/PDMS coating
Optimization
The optimization of affecting variables was performed by CCD. Equation 1 reveals the quadratic equation obtained from the coded data.
| 1 |
The analysis of variance (ANOVA) was carried out to estimate the interactions and the significance of each factor (Table S5). The lack-of-fit over 0.05 and the P-value below 0.05 were considered to be statistically significant. The model can satisfactorily predict the response because the P-value was below 0.0001, and the F-value was 180.49. Also, the coefficient of determination (R2) was 0.9554, and the lack-of-fit (P-value = 0.3658) was not significant. Therefore, the developed model properly fits all data.
The effect of two variables on the response was investigated by three-dimensional (3D) graphs. Fig. S1 indicates that increasing the desorption time and the extraction time caused the performance of the extraction to improve; however, at higher extraction time, changing the other parameter did not have a significant effect on the extraction efficiency. A similar trend was observed for the interactive effect of stirring speed and the extraction time on response (Fig. S2), and the simultaneous change of the sample volume and the extraction time on efficiency (Fig. S3). Fig. S4 and Fig. S5 pinpoint there is a direct relationship between the combined effect of the two assessed parameters (Stirring speed-desorption time and sample volume-desorption time) and the response, but at higher desorption time, the extraction efficiency is almost constant with stirring speed and sample volume changes. It is evident from Fig. S6 that the extraction of the target analytes enhanced when stirring speed and sample volume increased. Taken together, the overall results of the optimization indicated that the optimal condition was the extraction time of 60 min, desorption time of 10 min, stirring speed of 1300 rpm and the sample volume of 16 mL.
Method validation
The validation of MSBSE-PAN/PDMS-GC/FID was investigated according to some criteria including precision (repeatability and reproducibility), recovery, linearity, the limits of detection (LOD) and limits of qualification (LOQ) under the optimum conditions. Knoll’s method was used to calculate LOD and LOQ (Knoll 1985). The figures of merit were listed in Table 1. The calibration curves were linear in the appropriate concentration range of 5–1000 ng mL−1 with R2 over 0.9438 for all target analytes. The obtained LOD at a signal-to-noise ratio (S/N) of 3 was between 0.009 (Nap) and 0.51 ng mL−1 (Acy), and LOQ at S/N of 10 was 0.03–1.5 ng mL−1, which the lowest value was related to Nap and the highest value was for Acy. The interday and intraday relative standard deviations (RSDs) were less than 11.4% and 10.2%, respectively. The enrichment factor (EF) of Nap, Acy, Ace, Ant, Phe was 126, 117, 124, 120 and 120, respectively.
Table 1.
The merit figures of MSBSE-PAN/PDMS-GC/FID for PAH extraction
| Analyte | LOD* | LOQ* | LDR* | R2 | RSD % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Interday | Intraday | |||||||||
| 10* | 500* | 900* | 10* | 500* | 900* | |||||
| Nap | 0.009 | 0.03 | 5–1000 | 0.9438 | 5.9 | 11.4 | 5.6 | 5.9 | 4.9 | 3.7 |
| Acy | 0.51 | 1.5 | 5–1000 | 0.9823 | 6.6 | 9.3 | 10.2 | 4.1 | 3.2 | 9.9 |
| Ace | 0.43 | 1.2 | 5–1000 | 0.9890 | 6.8 | 10.8 | 8.1 | 6.0 | 10.2 | 8.0 |
| Ant | 0.18 | 0.5 | 5–1000 | 0.9745 | 10.6 | 7.3 | 8.7 | 10.2 | 7.0 | 8.0 |
| Phe | 0.10 | 0.6 | 5–1000 | 0.9665 | 9.9 | 9.1 | 9.3 | 9.8 | 9.0 | 9.1 |
*ng mL−1
Real samples analysis
The applicability of MSBSE-PAN/PDMS-GC/FID was evaluated by the extraction and determination of PAHs in non-alcoholic beer under the optimum condition. The brand of non-alcoholic beer used in this study was JoJo, which has been produced by Tehran Govar Food & Beverage Industries. The real sample was provided from the city markets of Tehran, located in Iran. As Table 2 shows, the identified concentration of the target analytes in the real sample ranged between 5.1 and 84 ng mL−1. The highest concentration was related to Nap and Phe had the lowest concentration in the real sample between the studied analytes. The real samples were spiked with two concentration levels (60 and 700 ng mL−1) of the target analytes. The relative recoveries obtained by the proposed method were in the range of 94–98%. The results show that changing the concentration of the target analytes in the real sample has no significant effect on their relative recoveries achieved by the proposed method.
Table 2.
The concentration (ng mL−1) and relative recovery (%) of PAHs
| Sample | Analyte | Primary value | Spiked amount | Recovery percentage |
|---|---|---|---|---|
| Non-alcoholic beer | Nap | 84 | 60 | 95 |
| 700 | 94 | |||
| Acy | 6 | 60 | 98 | |
| 700 | 97 | |||
| Ace | 5.5 | 60 | 94 | |
| 700 | 96 | |||
| Ant | 43 | 60 | 96 | |
| 700 | 95 | |||
| Phe | 5.1 | 60 | 97 | |
| 700 | 96 |
Method performance
The results obtained from MSBSE-PAN/PDMS-GC/FID were compared with other methods (Table 3). The analytes could be detected in a wider linear range by the MSBSE-PAN/PDMS-GC/FID method compared with the MSBSE-PPY/PANI-GC/FID. Also, the obtained LODs for Nap, Ant and Phe by the proposed method were lower than MSBSE-PPY/PANI-GC/FID. The highest value of LDR achieved by MSBSE-PPY/PANI-GC/FID was 1000 ng mL−1, which is much higher than the previously proposed methods. The RSDs of the MSBSE-PPY/PANI-GC/FID method were < 11.4% that is in an acceptable range in comparison with the listed methods in Table 3. To sum up, MSBSE-PPY/PANI-GC/FID is an efficient method to extract the PAHs from drinks.
Table 3.
Analytical parameters of the proposed method and comparison of the model with other methods
| Analyte | LOD (ng mL−1) | LDR (ng mL−1) | RSD (%) | Method | Coating | References |
|---|---|---|---|---|---|---|
| Nap | 0.0093 | 5–1000 | 3.7–11.4 |
MSBSE GC/FID |
PAN/PDMS | Present work |
| Acy | 0.51 | 5–1000 | 3.2–10.2 | |||
| Ace | 0.43 | 5–1000 | 6.0–10.8 | |||
| Ant | 0.18 | 5–1000 | 7.0–10.6 | |||
| Phe | 0.10 | 5–1000 | 9.0–9.9 | |||
| Nap | 0.04 | 40–400 | 9.6 |
MSBSE GC/FID |
PPY/PANI | (Mollahosseini et al. 2016) |
| Acy | 0.03 | 40–450 | 10.0 | |||
| Ace | 0.02 | 40–350 | 1.6 | |||
| Ant | 1.03 | 40–450 | 3.0 | |||
| Phe | 1.1 | 40–400 | 1.9 | |||
| Nap | 0.044 | 0.1–500 | < 3 |
SBSE/MD HPLC/UV |
PEG/g/MWCNTs | (Ekbatani Amlashi and Hadjmohammadi 2016) |
| Ant | 0.046 | 0.1–400 | ||||
| Phe | 0.013 | 0.05–500 | ||||
| Nap | 0.010 | 0.007–0.022 | 3–17 |
SBSE GC/QqQMS/MSa |
PDMS | (Barco-Bonilla et al. 2011) |
| Acy | 0.010 | 0.007–0.022 | 2–15 | |||
| Ace | 0.010 | 0.007–0.022 | 10–13 | |||
| Ant | 0.002 | 0.007–0.022 | 4–16 | |||
| Phe | 0.002 | 0.007–0.022 | 4–21 | |||
| Nap | – | 0.005–0.450 | 14 |
SBSE GC/MS/MS |
PDMS | (Guart et al. 2014) |
| Acy | – | 0.005–0.450 | 15 | |||
| Ace | – | 0.005–0.450 | 10 | |||
| Ant | – | 0.005–0.450 | 5 | |||
| Phe | – | 0.005–0.450 | 7 |
aGas chromatography triple quadrupole mass spectrometry
Conclusion
The MSBSE-PAN/PDMS-GC/FID method was developed to determine PAHs in non-alcoholic beer samples. A PAN/PDMS coating was electrospun on the shaft of the MSBSE device. Then, the performance of the proposed method has been examined by extracting and determining PAHs in the non-alcoholic beer sample by MSBSE-PAN/PDMS-GC/FID. The results indicated that the method can detect PAHs in a broad range of concentrations. In addition, the method has advantages such as high enrichment factor, high relative recovery, repeatability (RSD < 11.4%). The performance of MSBSE-PAN/PDMS-GC/FID also is acceptable in comparison with the previously proposed methods.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the Iran University of Science and Technology (IUST). Also, the authors acknowledge the contributions of Ali Khadir in preparing this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baltussen E, Sandra P, David F, Cramers C. Stir bar sorptive extraction (SBSE) a novel extraction technique for aqueous samples: theory and principles. J Microcolumn Sep. 1999;11:737–747. doi: 10.1002/(SICI)1520-667X(1999)11:10<737::AID-MCS7>3.0.CO;2-4. [DOI] [Google Scholar]
- Barco-Bonilla N, Romero-González R, Plaza-Bolaños P, Fernández-Moreno JL, Frenich AG, Vidal JLM. Comprehensive analysis of polycyclic aromatic hydrocarbons in wastewater using stir bar sorptive extraction and gas chromatography coupled to tandem mass spectrometry. Anal Chim Acta. 2011;693:62–71. doi: 10.1016/j.aca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Castro-Mayorga J, Fabra M, Cabedo L, Lagaron J. On the use of the electrospinning coating technique to produce antimicrobial polyhydroxyalkanoate materials containing in situ-stabilized silver nanoparticles. Nanomaterials. 2017;7:4. doi: 10.3390/nano7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere S, Subianto S, Savych I, Jones DJ, Rozière J. Electrospinning: designed architectures for energy conversion and storage devices. Energy Environ Sci. 2011;4:4761–4785. doi: 10.1039/c1ee02201f. [DOI] [Google Scholar]
- Chen M, Patra PK, Warner SB, Bhowmick S. Optimization of electrospinning process parameters for tissue engineering scaffolds. Biophys Rev Lett. 2006;1:153–178. doi: 10.1142/S1793048006000148. [DOI] [Google Scholar]
- Choi S, Moon SH, Kim TK, Kim HS. Fabrication of capacitive yarn torsion sensors based on an electrospinning coating method. Polym Int. 2019;68:1921–1927. doi: 10.1002/pi.5902. [DOI] [Google Scholar]
- David F, Sandra P. Stir bar sorptive extraction for trace analysis. J Chromatogr A. 2007;1152:54–69. doi: 10.1016/j.chroma.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Deng S, Bai R, Chen J. Behaviors and mechanisms of copper adsorption on hydrolyzed polyacrylonitrile fibers. J Colloid Interface Sci. 2003;260:265–272. doi: 10.1016/S0021-9797(02)00243-6. [DOI] [PubMed] [Google Scholar]
- Ekbatani Amlashi N, Hadjmohammadi MR. Sol–gel coating of poly (ethylene glycol)-grafted multiwalled carbon nanotubes for stir bar sorptive extraction and its application to the analysis of polycyclic aromatic hydrocarbons in water. J Sep Sci. 2016;39:3445–3456. doi: 10.1002/jssc.201600416. [DOI] [PubMed] [Google Scholar]
- Fiedler H, Cheung C, Wong M. PCDD/PCDF, chlorinated pesticides and PAH in Chinese teas. Chemosphere. 2002;46:1429–1433. doi: 10.1016/S0045-6535(01)00264-8. [DOI] [PubMed] [Google Scholar]
- Gorji S, Bahram M, Biparva P. Optimized stir bar sorptive extraction based on self-magnetic nanocomposite monolithic kit for determining Bisphenol A in bottled mineral water and bottled milk samples. Anal Bioanal Chem. 2019;6:137–156. [Google Scholar]
- Guart A, Calabuig I, Lacorte S, Borrell A. Continental bottled water assessment by stir bar sorptive extraction followed by gas chromatography-tandem mass spectrometry (SBSE-GC-MS/MS) Environ Sci Pollut Res. 2014;21:2846–2855. doi: 10.1007/s11356-013-2177-9. [DOI] [PubMed] [Google Scholar]
- Jachero L, Ahumada I, Richter P. Rotating-disk sorptive extraction: effect of the rotation mode of the extraction device on mass transfer efficiency. Anal Bioanal Chem. 2014;406:2987–2992. doi: 10.1007/s00216-014-7693-z. [DOI] [PubMed] [Google Scholar]
- Jafari MT, Rezaei B, Bahrami H. Zirconium dioxide-reduced graphene oxide nanocomposite-coated stir-bar sorptive extraction coupled with ion mobility spectrometry for determining ethion. Talanta. 2018;182:285–291. doi: 10.1016/j.talanta.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Jafari MT, Rezayat MR, Mossaddegh M. Design and construction of an injection port for coupling stir-bar sorptive extraction with ion mobility spectrometry. Talanta. 2018;178:369–376. doi: 10.1016/j.talanta.2017.09.061. [DOI] [PubMed] [Google Scholar]
- Jang S, Kim Y, Lee S, Oh JH. Optimization of electrospinning parameters for electrospun nanofiber-based triboelectric. Int J Pr Eng Man-GT. 2019;6:731–739. [Google Scholar]
- Jillani SMS, Ganiyu SA, Alhooshani K. Development of a SBSE-HPLC method using sol-gel based germania coated twister for the analysis of 4-chloro-1-naphthol in biological and water samples. Arab J Chem. 2018;13:3440–3447. doi: 10.1016/j.arabjc.2018.11.016. [DOI] [Google Scholar]
- Karbownik I, Rac-Rumijowska O, Fiedot-Toboła M, Rybicki T, Teterycz H. The preparation and characterization of polyacrylonitrile-polyaniline (PAN/PANI) Fibers Polym. 2019;12:664. doi: 10.3390/ma12040664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll JE. Estimation of the limit of detection in chromatography. J Chromatogr Sci. 1985;23:422–425. doi: 10.1093/chromsci/23.9.422. [DOI] [Google Scholar]
- Liang CZ, Chung T-S. Robust thin film composite PDMS/PAN hollow fiber membranes for water vapor removal from humid air and gases. Sep Purif Technol. 2018;202:345–356. doi: 10.1016/j.seppur.2018.03.005. [DOI] [Google Scholar]
- Londoño VAG, Reynoso CM, Resnik SL. Polycyclic aromatic hydrocarbons (PAHs) survey on tea (Camellia sinensis) commercialized in Argentina. Food Control. 2015;50:31–37. doi: 10.1016/j.foodcont.2014.07.036. [DOI] [Google Scholar]
- Luo Y-B, Ma Q, Feng Y-Q. Stir rod sorptive extraction with monolithic polymer as coating and its application to the analysis of fluoroquinolones in honey sample. J Chromatogr A. 2010;1217:3583–3589. doi: 10.1016/j.chroma.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Mohamadi S, Saeedi M, Mollahosseini A. Enhanced electrokinetic remediation of mixed contaminants from a high buffering soil by focusing on mobility risk. J Environ Chem Eng. 2019;7:103470. doi: 10.1016/j.jece.2019.103470. [DOI] [Google Scholar]
- Mollahosseini A, Elyasi Y, Rastegari M. An improvement of electrospun membrane reusability via titanium dioxide nanoparticles and silane compounds for the electromembrane extraction. Anal Chim Acta. 2019;1088:168–177. doi: 10.1016/j.aca.2019.08.050. [DOI] [PubMed] [Google Scholar]
- Mollahosseini A, Rastegari M, Hatefi N (2020) Electrospun polyacrylonitrile as a new coating for mechanical stir bar sorptive extraction of polycyclic aromatic hydrocarbons from water samples. Chromatographia pp. 1–10 [DOI] [PMC free article] [PubMed]
- Mollahosseini A, Rokue M, Mojtahedi MM, Toghroli M, Kamankesh M, Motaharian A. Mechanical stir bar sorptive extraction followed by gas chromatography as a new method for determining polycyclic aromatic hydrocarbons in water samples. Microchem J. 2016;126:431–437. doi: 10.1016/j.microc.2016.01.001. [DOI] [Google Scholar]
- Prieto A, Basauri O, Rodil R, Usobiaga A, Fernández L, Etxebarria N, Zuloaga O. Stir-bar sorptive extraction: a view on method optimisation, novel applications, limitations and potential solutions. J Chromatogr A. 2010;1217:2642–2666. doi: 10.1016/j.chroma.2009.12.051. [DOI] [PubMed] [Google Scholar]
- Rahaman MSA, Ismail AF, Mustafa A. A review of heat treatment on polyacrylonitrile fiber. Polym Degrad Stabil. 2007;92:1421–1432. doi: 10.1016/j.polymdegradstab.2007.03.023. [DOI] [Google Scholar]
- Richter P, Leiva C, Choque C, Giordano A, Sepúlveda B. Rotating-disk sorptive extraction of nonylphenol from water samples. J Chromatogr A. 2009;1216:8598–8602. doi: 10.1016/j.chroma.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Robles-Molina J, Gilbert-López B, García-Reyes JF, Martos NR, Molina-Díaz A. Multiclass determination of pesticides and priority organic pollutants in fruit-based soft drinks by headspace solid-phase microextraction/gas chromatography tandem mass spectrometry. Anal Methods. 2011;3:2221–2230. doi: 10.1039/c1ay05303e. [DOI] [Google Scholar]
- Saberi N, Aghababaei M, Ostovar M, Mehrnahad H. Simultaneous removal of polycyclic aromatic hydrocarbon and heavy metals from an artificial clayey soil by enhanced electrokinetic method. J Environ Manage. 2018;217:897–905. doi: 10.1016/j.jenvman.2018.03.125. [DOI] [PubMed] [Google Scholar]
- Sankır M, Küçükyavuz Z, Küçükyavuz S. Synthesis and characterization of poly (dimethylsiloxane)–polythiophene composites. J Appl Polym Sci. 2003;87:2113–2119. doi: 10.1002/app.11504. [DOI] [Google Scholar]
- Shao H, Fang J, Wang H, Lin T. Effect of electrospinning parameters and polymer concentrations on mechanical-to-electrical energy conversion of randomly-oriented electrospun poly (vinylidene fluoride) nanofiber mats. RSC adv. 2015;5:14345–14350. doi: 10.1039/C4RA16360E. [DOI] [Google Scholar]
- Silva FS, Cristale J, Ribeiro ML, de Marchi MRR. Polycyclic aromatic hydrocarbons (PAHs) in raw cane sugar (rapadura) in Brazil. J Food Compos Anal. 2011;24:346–350. doi: 10.1016/j.jfca.2010.08.012. [DOI] [Google Scholar]
- Singh L, Varshney JG, Agarwal T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food chem. 2016;199:768–781. doi: 10.1016/j.foodchem.2015.12.074. [DOI] [PubMed] [Google Scholar]
- Taghvimi A, Dastmalchi S, Javadzadeh Y. Novel ceramic carbon-coated magnetic nanoparticles as stir bar sorptive extraction coating for simultaneous extraction of amphetamines from urine samples. Arab J Sci Eng. 2019;44:6373–6380. doi: 10.1007/s13369-019-03810-0. [DOI] [Google Scholar]
- Varjani SJ, Joshi RR, Kumar PS, Srivastava VK, Kumar V, Banerjee C, Kumar RP (2018) Polycyclic aromatic hydrocarbons from petroleum oil industry activities: effect on human health and their biodegradation. In: Waste Bioremediation. Springer, pp 185–199
- Wang N, et al. Tailored fibro-porous structure of electrospun polyurethane membranes, their size-dependent properties and trans-membrane glucose diffusion. J Membr Sci. 2013;427:207–217. doi: 10.1016/j.memsci.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Liu X, Jin Y, Zhu Y, Zeng D, Jiang X, Ma H. Electrospinning of poly (dimethylsiloxane)/poly (methyl methacrylate) nanofibrous membrane: fabrication and application in protein microarrays. Biomacromol. 2009;10:3335–3340. doi: 10.1021/bm900955p. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang J, Zhou T, Liu X, Yuan Q, Zhang A. New understanding on the reaction pathways of the polyacrylonitrile copolymer fiber pre-oxidation: online tracking by two-dimensional correlation FTIR spectroscopy. RSC Adv. 2016;6:4397–4409. doi: 10.1039/C5RA24320C. [DOI] [Google Scholar]
- Zuin VG, Montero L, Bauer C, Popp P. Stir bar sorptive extraction and high-performance liquid chromatography–fluorescence detection for the determination of polycyclic aromatic hydrocarbons in Mate teas. J Chromatogr A. 2005;1091:2–10. doi: 10.1016/j.chroma.2005.07.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.