Abstract
In the present study, modified solvent evaporation assisted methanolic Trametes versicolor extract was evaluated for its antimicrobial and anti-inflammatory efficacy. Mushroom extract showed significantly (p < 0.05) higher total phenolic content (48.71 mg/g) followed by total flavonoid content (13.13 mg/g), ascorbic acid content (11.03 mg/g), β-carotene content (8.34), and lycopene content (6.85). Fourier transform infrared spectroscopy revealed the functional groups of the observed bioactive compounds, while HPLC chromatogram showed significantly (p < 0.05) higher gallic acid content (45.72 mg/g) as compared to rutin content (12.50 mg/g). Statistically, mushroom extract and artificial antioxidant (BHA) showed a non-significant (p < 0.05) difference in terms of percentage inhibition during DPPH and N2O2 scavenging assay. During the time-kill kinetics, the mushroom extract significantly inhibited the growth of S. aureus in comparison with the growth of P.aeruginosa, K. pneumonia, and E.coli. Mushroom extract showed effective anti-inflammatory activity during membrane stabilization (33.71–73.24%) and protein denaturation (23.11–74.56%) assays.
Keywords: Mushroom, Trametes versicolor, HPLC, FTIR, Anti-inflammatory
Introduction
Nowadays, wild mushrooms gained tremendous interest in the field of pharmacology and food science due to their potential nutritional and medicinal properties (Reis et al. 2017). Globally, about 12,000 species of mushrooms are known and among them, about 2000 species are edible (Garofalo et al. 2017). Also, almost 200 species of mushrooms are commercially cultivated for medicine preparation and human consumption (Zhang et al. 2007). Moreover, the wild mushrooms, due to excellent nutritional value, can be compared with meat, fish, egg, and milk (Kalač 2009). Besides, nutritional value, mushrooms are well known for their bioactive compounds which are responsible for different biological and pharmaceutical activities including antimicrobial, antioxidant, anti-inflammatory, antidiabetic, and anticancerous properties (Ma et al. 2013). Among all wild mushrooms, Trametes versicolor (commonly known as turkey tail) mushrooms have gained remarkable popularity due to its broad spectrum utilization in food and pharma industries. It is a white-rot lignicolous fungus commonly grow on pine and oak trees and it is widely used as folk medicine in various countries such as China, Japan, and other parts of Asia and Africa (Lorenzo et al. 2002). However, it is a polypore in nature and it cannot be consumed directly as food due to its peculiar woody taste; therefore, its dried powder added as an ingredient in green tea for its consumption (Shavit et al. 2009). Furthermore, the extract of Trametes versicolor contains the number of polysaccharide fractions including ß-glucans, d-glucose polymer along with units of glucuronic acids, arabinose, mannose, fucose, galactose, and xylose and these polysaccharides are responsible for several biological activities (Thatoi et al. 2018). Also, Trametes versicolor consists of 18 different amino acids viz aspartic acid, threonine, serine, glutamic acid, glycine, alanine, valine, and leucine, and all these amino acids contribute to several potential applications (Yeung and Or 2012). Therefore, in the studies encoded by different scientists, it was observed that mushroom extracts revealed good antimicrobial and anti-inflammatory properties. Although inflammation is a body defensive phenomenon, insufficient regulation, and unsuitable as in disparity to self-tissue, it could be the reason for severe diseases and injuries (Chung et al. 2018). To combat inflammation, steroidal and non-steroidal drugs (NSAID) are most frequently used. However, these allopathic medicines have numerous adverse effects such as gastric ulcers, and tissue irritations (Adnan et al. 2019). Therefore, natural bioactive components and phytochemicals obtained from mushrooms with anti-inflammatory activity have gained great interest in recent years. In addition to this mushroom extract have antimicrobial properties and, therefore, susceptible to both Gram-positive and Gram-negative bacteria and the susceptibility is primarily due to the presence of different bioactive compounds (Asri et al. 2019). The mechanism of action of these hydroxyl groups includes the production of oxidized compounds that inhibits the membrane-bound enzyme’s activities. Among phenolic compounds, flavonoids are the secondary metabolites that are synthesized by the mushrooms that act as antimicrobial agents. They can form a complex with membrane proteins. Some of them are lipophilic and their presence results in disruption of cell wall membrane, they may also result in nucleic acid synthesis and deteriorate cell metabolism. Another class of bioactive compounds present in the mushroom is represented by terpenes that are lipophilic, hence disrupt membrane (Matijašević et al. 2016). Therefore, all these antimicrobial peptides are responsible for the antimicrobial activity of mushrooms in several ways that include deterioration of cell wall membrane, inhibition of proteins, and DNA synthesis (Strempel et al. 2015). Furthermore, among several existing methods, the organic solvent extraction method is a broadly used method for the extraction of extracts from mushrooms. To remove the organic solvent various methods such as distillation, open dish evaporation, and reduced-pressure method (vacuum oven and rotatory evaporator) can be used; however, heat, temperature, and oxygen during evaporation can cause deteriorative effects to the bioactivity of extracts (Chawla et al. 2019). Therefore, to achieve the desirable bioactivity of compounds, the modified solvent evaporation technique (evaporation of organic solvent at refrigerated temperature) could be a better approach. In this process, at refrigerated temperature (4–7 ℃), polyphenolic components dispersed in methanol and molecules of methanol or organic solvent gather enough kinetic energy from its exchange with neighbor molecules to escape from the bonds with another molecule, hence molecules leave the mass of liquids and join the air as a vapor (Gupta et al. 2015). Till now, no reports have been published on the modified solvent evaporation technique used for the mushroom extract preparation. Therefore, the present study was carried out with the following objectives: (1) Collection, taxonomical identification, and physicochemical evaluation of Trametes versicolor, (2) in-vitro antioxidant, anti-inflammatory, and antimicrobial efficacy of modified solvent evaporated extract of Trametes versicolor and (3) confirmation of bioactive components using High-pressure liquid chromatography technique.
Materials and methods
Materials
Mushroom samples were collected from the forest of Chail, Solan, Himachal Pradesh, India. The identification of the collected mushroom sample was carried out by the conventional method and the fruiting body was submitted (herbarium voucher number 27216) to the Department of Botany, Punjab University, Chandigarh, Punjab, India. All analytical reagent grade and HPLC grade chemicals such as Muller Hinton Agar, Malt extract broth, methanol, Folin-Ciocalteu reagent, phosphate buffer, NaCl, DMSO, l-ascorbic acid, Diclofenac sodium salt, Aluminium chloride, sodium carbonate, metaphosphoric acid, 2,6 dichlorophenol, DPPH, α- naphthyl ethylenediamine, sulphanilic acid, H3PO4 were procured from Hi-Media Private Limited, Mumbai, India. Gallic acid and Rutin were procured from Sigma Aldrich St. Louis, USA. Gram-positive and Gram-negative bacterial strains, i.e., Staphylococcus aureus (MTCC 3160), Pseudomonas aeruginosa (MTCC 424), Klebsiella pneumoniae (MTCC 3384) and Escherichia coli (MTCC 443), were obtained from the Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh, India. Triple distilled and HPLC grade water and acid-washed glassware were used during experimentation.
Methods
Preparation of methanolic extract from the mycelial culture of Trametes versicolor using modified evaporation technique
The mycelial culture of mushroom was inoculated in a 250 ml conical flask containing Malt extract broth and kept for incubation at 30 ℃ for 3 weeks. The culture thus obtained was filtered and dried in an oven at 30 ℃ for 48 h. The dried mycelial culture was then ground using a mechanical grinder (Remi High Speed 950, Mumbai, India). For extract preparation, a method proposed by (Chawla et al. 2019) was used and the modified solvent evaporation technique was used to evaporate the methanol. Briefly, a 5 g powdered mycelial sample was dispersed in 25 ml of absolute methanol in a conical flask and kept the mixture in an orbital shaker (Orbitek LT, Scigenics Biotech Pvt. Ltd., Chennai, India) for 72 h. The sample was then filtered using Whatman no 1 filter paper. Methanol was evaporated to dryness at a refrigerated temperature from 4–7 ℃. The obtained extract was filled into the glass vials and stored at – 20 ℃ for further characterization.
Characterization of extract
Mushroom extracts contain various bioactive components having several functional groups. Therefore, Fourier Transform Infrared Spectroscopy (Agilent Cary 630, Mumbai, India) was used to evaluate the functional groups present in the methanol extract of Trametes versicolor. Spectra were obtained in mid-infrared region 4000-600 cm−1 using air as background and data were obtained in terms of transmittance (55 scans). For the quantification of bioactive compounds, High-pressure liquid chromatography was used. Here, the HPLC system (Waters 515) was used for the estimation of phenol and flavonoids from Trametes versicolor extract. The system consisted of a high-pressure gradient binary pump system, manual injector, temperature-controlled column chamber, and 2998 Photodiode Array Detector detector was used for the detection and quantification of phenols and flavonoids. Data thus obtained was collected and analyzed using Empower2 software. The analytic column employed for analysis of gallic acid and rutin was C18 (4.6 × 250 mm, 33 cm) column (Waters) with a flow rate of 60 ml/h. The quantification of gallic and rutin was done at 272 and 357 nm (Kaushik et al. 2014).
Total phenolic content of Trametes versicolor extract
Total phenolic content was determined by following the method of (Sadh et al. 2018). A stock solution (10 mg/10 ml methanol) was prepared before analysis. Briefly, 200 µl of methanol extract solution was mixed with 1 ml of 1 N Folin-Ciocalteu reagent and 2 ml 7.5% sodium carbonate (Na2CO3) and after vortex shaking, the mixture was kept undisturbed for 30 min in dark condition. The absorbance of the sample was then measured at 760 nm and total phenolic content was calculated GAE/g from the regression equation value (y = 0.0106x + 0.041) obtained from the calibration curve of gallic acid.
Estimation of flavonoid contents:
Total flavonoid content was evaluated by aluminum chloride colorimetric assay as described by (Sadh et al. 2018). Briefly, a 2 ml extract solution was mixed with 200 µl of 5% sodium nitrite and kept constant for 5 min. After that, 200 µl of 10% aluminum chloride (AlCl3) was added and mixed well. The reaction mixture was kept for 6 min followed by the addition of 2 ml of 1 M NaOH. The absorbance was measured at 510 nm and total flavonoid content was calculated using a standard curve of rutin.
Estimation of ascorbic acid:
Ascorbic acid content was calculated by the method proposed by (Klein and Perry 1982). Herein, a 100 mg methanol extract was mixed with 1% of metaphosphoric acid and kept constant for 45 min at 30 ℃. The mixture was then filtered through Whatman no. 1 filter paper and the filtrate thus obtained mixed with 2,6 dichlorophenol. Absorbance was measured within 30 min at 515 nm. The total amount of ascorbic acid was calculated using the standard curve of l-ascorbic acid.
Estimation of β-carotene and lycopene content:
Briefly, 100 mg methanol extract of mushroom was dissolved in 10 ml of the acetone-hexane mixture (4:6) and kept constant for 1 min at room temperature. The mixture was then filtered through Whatman no. 4 filter paper and absorbance was measured at 453, 505, and 663 nm (Nagata and Yamashita 1992). Total β-carotene and lycopene contents were measured by applying the following equations:
In-vitro antioxidant efficacy:
HRBC membrane stabilization assay:
For the HRBC membrane stabilization assay, 5 ml blood was collected from a healthy human volunteer who did not intake NSAID (nonsteroidal anti-inflammatory drugs) for 14 days. The collected blood was dissolved in sterilized Alsever solution (20.5 g Dextrose, 8 g sodium citrate, 0.55 g citric acid, and 4.2 g sodium chloride in 1000 ml water) in an equal amount. The mixture was then centrifuged at 3000 × g for 15 min. and Packed cells were obtained and washed with isosaline. The assay mixture was prepared that contains 500 µl extract 1 ml 0.15 M phosphate buffer with pH 7.4, 2 ml 0.36% hyposaline solution, and 500 µl HRBC suspension. The whole mixture was then incubated in the BOD incubator at 37ºC for 30 min followed by the centrifugation at 3000 × g for 20 min. Diclofenac sodium was used as a reference standard and 2 ml of distilled water was used as a negative control. The content of hemoglobin was estimated through a spectrophotometer at 560 nm (Bains and Tripathi 2017).
HRBC membrane stabilization percentage can be calculated as follows:
Albumin denaturation assay:
To perform albumin denaturation assay a mixture containing 200 µl of fresh egg albumin, 2.8 ml of phosphate-buffered saline (pH6.4), and 2 ml of methanol extract was taken so that final volume becomes 5 ml. Deionized water served as control. The reaction mixture was kept for incubation at 37 ℃ in the BOD incubator for 15 min followed by heating up to 70 ℃ for 5 min. The absorbance was measured at 660 nm. Diclofenac sodium was used as a positive control. Albumin denaturation percentage inhibition was calculated as follows
here, VT = absorbance of the test sample. VC = absorbance of the control (Bains and Tripathi 2017).
Antioxidant assay
DPPH free radical scavenging activity:
Antioxidant activity of methanol extract of Trametes versicolor was determined by following the method of (Sadh et al. 2018). Briefly, 200 µl extract was added in 2 ml of 0.1 mM DPPH solution and the mixture was then kept constant for 30 min in dark condition. Change in DPPH color from purple to pale yellow was observed and absorbance was measured at 517 nm using a UV–Visible spectrophotometer. The percentage of free radical scavenging activity was calculated using the following formula:
where AD is the absorbance of DPPH. AS is the absorbance of the sample.
Nitric Oxide scavenging assay
The nitrite detection method was carried out using the proposed method of Bains and Tripathi (20). Briefly, 10 mM of Sodium nitroprusside dissolve in 0.5 M phosphate buffer (pH 7.4) was used as a chemical source of nitric oxide. The solution was mixed well and kept for 5 h at 37 ℃. After five h of incubation 500 µl of Griess reagent (α-naphthyl ethylenediamine 0.1% in water and sulphanilic acid 1% in 5% H3PO4) was added. The absorbance of the solution was measured at 546 nm (Tewari et al. 2015).
Antimicrobial efficacy of methanol extract of Trametes versicolor
Antimicrobial susceptibility of methanol extract against S. aureus, E.coli, Pseudomonas aeruginosa, and K. pneumonia was determined by the agar well diffusion method proposed by (Chawla et al. 2020). Muller Hinton agar plate enriched with 4% of NaCl was prepared and inoculated with test organisms (approximately 1.5 × 108 cells/ml). The plates were allowed to dry and wells were made using a cork borer. 25 µl of methanol extract dissolved in 5% DMSO was introduced into bore agar wells. Ciprofloxacin antibiotic and DMSO were taken as a positive and negative control. The plates were incubated at 37 ℃ for 24 h and the zone of inhibition (mm) was measured.
Time Kill study
A time-kill study was performed by following the method proposed by (Majeed et al. 2016). A 100 µl of the extract solution was taken for each sample after a time interval of 0, 18, 24, and 48 h. The sample was serially diluted and was spread on plates containing Muller-Hinton agar. The plates were then incubated in an incubator at 37 ℃ for 24 h and Log CFU/ml was calculated.
Statistcal analysis
Statistical analysis was carried out for the obtained results by following the method proposed by (Kaushik et al. 2018). Standard error mean was calculated by Microsoft excel office, 2013. One way analysis of variance was used to calculate the statistical difference and critical difference value was used to calculate the comparison between mean.
Results and discussion
Bioactive compounds and characterization of Trametes versicolor extract
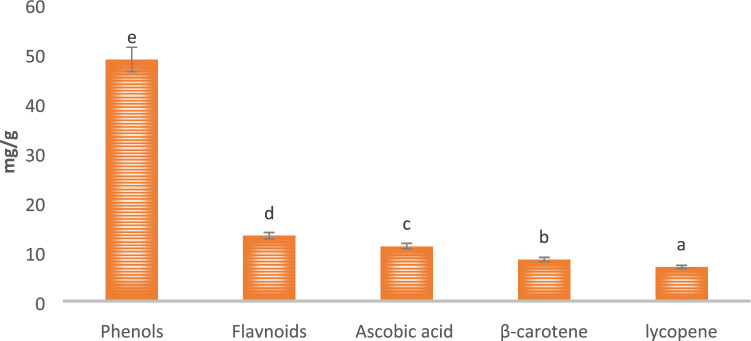
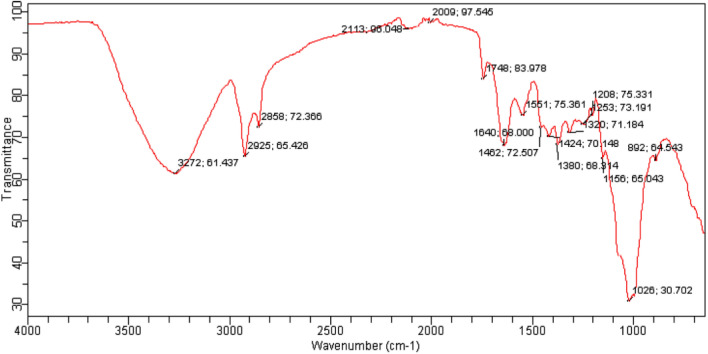
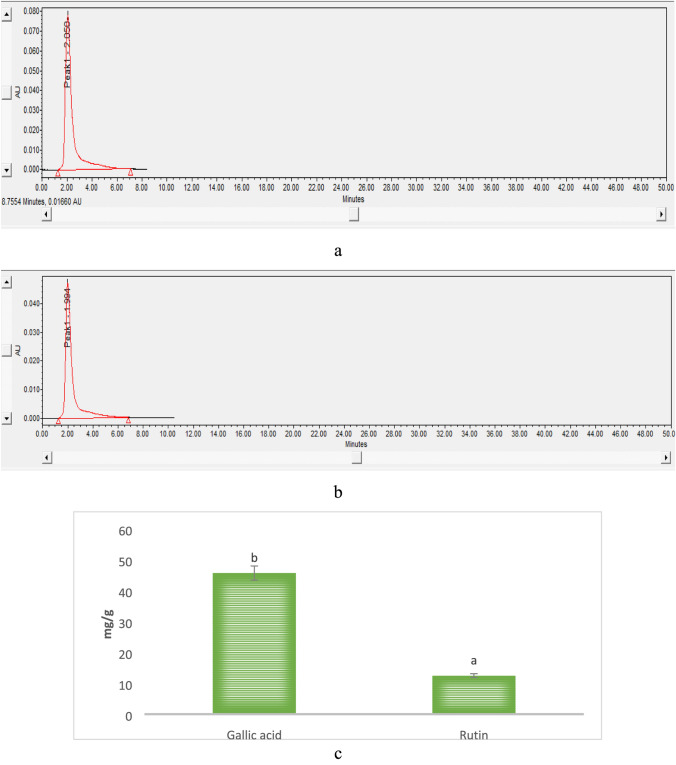
Total phenol, flavonoid, ascorbic acid, β-carotene, and lycopene were determined as major bioactive compounds and the results are represented in Fig. 1. Herein, the mushroom extract showed significantly (p < 0.05) higher total phenolic content (48.71 mg/g) followed by total flavonoid content (13.13 mg/g), ascorbic acid content (11.03 mg/g), β-carotene content (8.34), and lycopene content (6.85), respectively. The functional groups of these bioactive compounds were confirmed by the FTIR and results are represented in Fig. 2. Herein, the stretching peak at 3272 cm−1 confirmed the vibrational stretching of –OH group. The peak at 2925.65 cm−1, 1380 cm−1 of carotenoid was due to C-H bending, and at 1640 cm−1 was due to C = C stretching of the conjugated double bond (Diaz et al. 2011). The stretching of ascorbic acid at 1748 cm−1 was due to the stretching vibration of C = O of a five-membered lactone ring (Kim et al. 2008). The vibrational band at 2858.72 cm−1 confirmed the presence of a pyranose ring. Also, stretching at 892 cm−1 revealed the presence of β-glycosidic linkage. Stretching at 1320 cm−1 and 1547 cm−1 confirmed the presence of secondary amines (N–H bending), whereas H-C-H symmetric or asymmetric stretch indicated the presence of alkanes (Andrew et al. 2018). The vibrational band at 1156 cm−1 attributes to the presence of glycosidic bonds (Thenmozhi et al. 2013). HPLC analysis of methanol extract was carried out for the quantification of gallic acid and rutin. HPLC chromatogram of gallic acid and rutin present in extract showed the peak with retention time 2.050 and 1.991 min, respectively, as shown in Fig. 3a–c. Herein, HPLC chromatogram revealed significantly (p < 0.05) higher gallic acid content (45.72 mg/g) as compared to rutin content (12.50 mg/g). In conclusion, the obtained FTIR spectra and HPLC chromatogram of mushroom extract justified and supported the presence of varied bioactive components and their activity (Kim et al. 2008; Khatua et al. 2015; Yildirim et al. 2012).
Fig. 1.
Total phenol, Flavonoid, Ascorbic acid content, total β-carotene, and lycopene content of modified solvent extraction assisted Trametes versicolor extract. Data are presented as means ± SEM (n = 3). a−e Means within the column with different lowercase superscript are significantly different (p < 0.05) from each other
Fig. 2.
FTIR spectra of modified solvent extraction assisted Trametes versicolor extract
Fig. 3.
HPLC chromatogram of a gallic acid, b rutin content observed in modified solvent extraction assisted Trametes versicolor extract and c quantitative analysis of gallic acid and rutin of modified solvent extraction assisted Trametes versicolor extract. Data are presented as means ± SEM (n = 3). a−b Means within the column with different lowercase superscript are significantly different (p < 0.05) from each other
Antioxidant activity
Antioxidant activity in comparison with natural and artificial standard antioxidants was evaluated and results are represented in Fig. 4a, b. Here, all concentration of l-Ascorbic acid showed significantly (p < 0.05) higher percentage inhibition in comparison with varying concentrations of BHA and mushroom extract. Ascorbic acid showed significantly (p < 0.05) higher inhibition ranges from 51.63–82.50% for DPPH and 42.33- 82.92% for N2O2. Furthermore, methanol extract of Trametes versicolor showed percentage inhibition ranges from 32.62–72.32% for DPPH and 34.31–62.30% for N2O2, whereas BHA showed 33.14–72.91% for DPPH and 34.92–63.03% for N2O2. Statistically, the extract of Trametes versicolor and BHA showed a non-significant (p < 0.05) difference in terms of percentage inhibition during DPPH and N2O2 scavenging assay. The ability of the extract to scavenge free radicals was similar to the artificial anti-oxidant BHA and was more effective at higher concentrations. Therefore, it showed that the activity of bioactive components correspondingly increased with increasing concentration and if their pure form is isolated they may show more effective results than that of positive control (Olugbami et al. 2015). The present results were in agreement with some of the previous findings (Keleş et al. 2011; Puia et al. 2018; Taofiq et al. 2016). The presence of a good amount of gallic acid and rutin confirmed the efficient antioxidant properties of mushroom extract in comparison with artificial antioxidants. Also, HPLC results justified and well supported the in vitro antioxidant activity of mushroom extract obtained during DPPH and N2O2 assay.
Fig. 4.
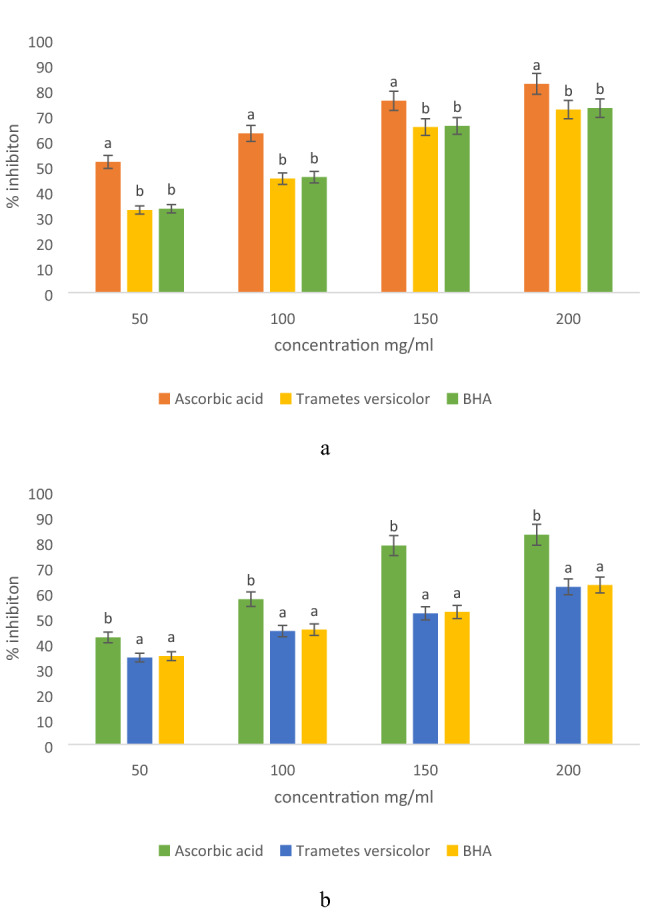
Antioxidant activity of modified solvent extraction assisted Trametes versicolor extract using a DPPH free radical scavenging assay, and b nitric oxide free radical scavenging assay
Antimicrobial properties
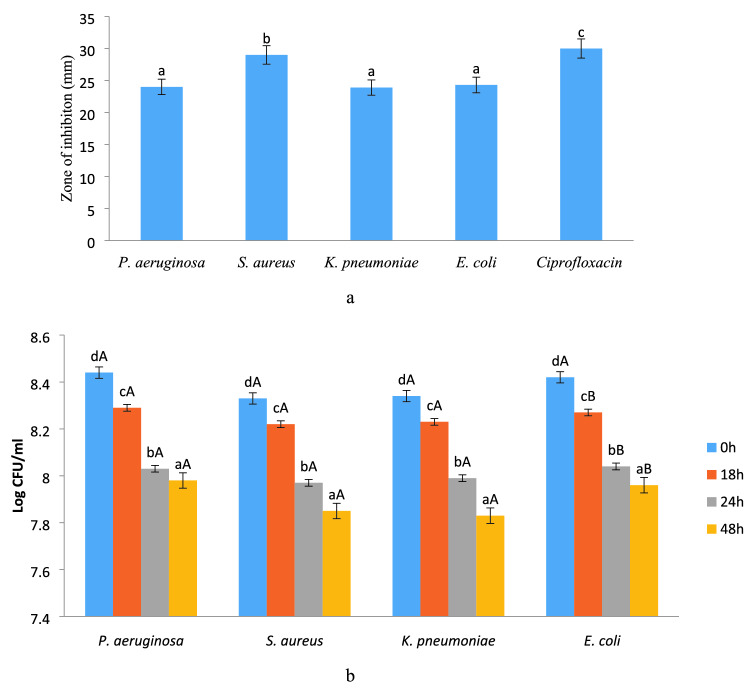
Antimicrobial activity of methanol extract was evaluated against both Gram-positive and Gram-negative bacteria and the zone of inhibition was observed and the results are presented in Fig. 5a. Herein, the zone of inhibition corresponded to the range 24.14—30.18 mm for all the bacterial strains. The extract showed a significantly (p < 0.05) higher zone of inhibition against S. aureus (29.14 mm), whereas for E. coli, K. pneumonia and P. aeruginosa, significantly (p < 0.05) lower zone of inhibition was observed. However, E. coli, K. pneumonia, and P. aeruginosa revealed non-significant (p < 0.05) differences with each other in terms of zone of inhibition. According to literature, the susceptibility of the extract against S. aureus was significantly (p < 0.05) higher due to the absence of membrane-bound periplasm in the cell membrane which consists of peptidoglycans like teichoic or teichuronic acid. Furthermore, the outer cell wall of S. aureus formulates a thick hydrophobic porous structure that can bind a large number of proteins and lipids and the porous membrane could be a reason for increased permeability of chemotherapeutic agents and mushroom peptidoglycans (Chawla et al. 2020; Lule et al. 2020). On the other hand, Gram-negative bacteria contain lipopolysaccharides in the outer membrane that act as an effective permeability barrier to the bioactive components of the mushroom; therefore, extract showed the least susceptibility against these microorganisms. Results were well supported by the findings from previous literature (Appiah et al. 2017; Silva et al. 2010; Boakye et al. 2016) who observed similar trends for mushroom extract against Gram-positive and Gram-negative microorganisms.
Fig. 5.
Antimicrobial activity of modified solvent extraction assisted Trametes versicolor extract evaluated by a zone of inhibition against pathogenic microorganism and b Time kill assay at a different time interval. Data are presented as means ± SEM (n = 3). a−dMeans within the column with different lowercase superscript are significantly different (p < 0.05) from each other. A−BMeans within the row with different uppercase superscript are significantly different (p < 0.05) from each other
Time kill study
Time killed study of the mushroom extract against pathogenic bacteria was carried out and results are represented in Fig. 5b. Methanol extract strongly inhibits the growth of S. aureus as compared to P.aeruginosa, K. pneumonia, and E.coli. Here, in the case of S. aureus, the log CFU/ml value significantly (p < 0.05) reduced from 8.33 to 7.85 in 48 h. Whereas, in the case of E.coli log CFU/ml was significantly (p < 0.05) reduced from 8.42 to 7.96, in the case of K. pneumonia log CFU/ml significantly (p < 0.05) reduced from 8.45 to 7.95, and in the case of P.aeruginosa log CFU/ml significantly (p < 0.05) reduced from 8.44 to 7.98. Statistically, the mushroom extract showed significantly (p < 0.05) lower log CFU/ml for S. aureus than that of P.aeruginosa, K. pneumonia, and E.coli. However, all Gram-negative bacteria showed a non-significant (p < 0.05) difference with each in terms of antimicrobial efficiency of mushroom extract. Moreover, with increasing time all the bacteria showed significant (p < 0.05) difference with each other in terms of antimicrobial efficiency of mushroom extract. The inhibition in the growth of S. aureus is due to the permeability of the peptidoglycan layer that allows antibiotics and other mushroom peptidoglycans to enter inside the cell and results in protein denaturation and disruption in the cell membrane. The log CFU/ml value for P.aeruginosa was significantly higher than that of other micro-organisms due to the efflux mechanism, in which active component pump out via active transport (Chika et al. 2016). The present results were in line with the findings of (Appiah et al. 2017; Silva et al. 2010; Boakye et al. 2016) who observed the time-kill effect of S.commune, Trametes gibbosa, Trametes elegans, S.commune and V. volvacea against E.coli and S. aureus.
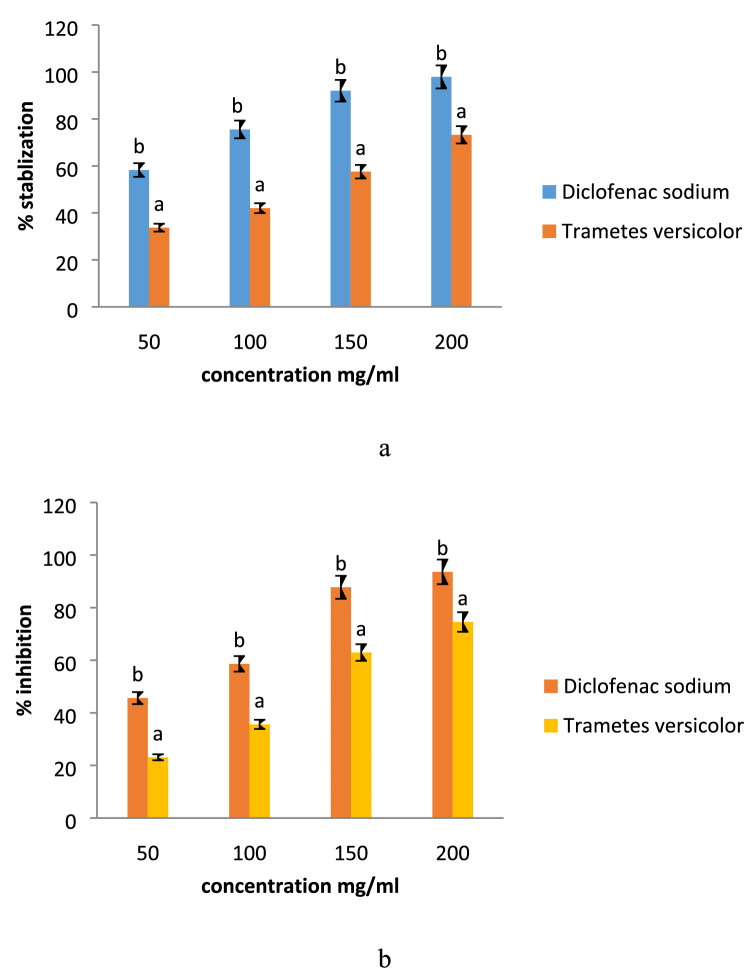
Anti-inflammatory activity
The anti-inflammatory activity was carried out by HRBC membrane stabilization and albumin denaturation assay and results are represented in Fig. 6a, b. Here, diclofenac sodium salt was used as standard, and mushroom extracts’ anti-inflammatory activity was compared with standard. Significant (p < 0.05) difference was observed in the anti-inflammatory activity of mushroom extract as compared to standard during membrane stabilization and protein denaturation assay. However, all concentrations of diclofenac sodium showed significantly (p < 0.05) higher percentage stabilization and percentage inhibition in comparison with varying concentrations of the mushroom extract. Diclofenac showed higher percent stabilization ranges from 58.26–97.91% for the HRBC membrane stabilization test and 45.61–93.61% for albumin denaturation test. The methanol extract showed significantly (p < 0.05) lower percentage stabilization of membrane ranges from 33.71–73.24% and percentage protein denaturation ranges from 23.11–74.56%. The anti-inflammatory activity of the mushroom extract was exerted due to the presence of glycopeptides and other bioactive components. The present results were in line with the findings of (Bains and Tripathi 2017) who observed efficient anti-inflammatory activity of mushroom extract and results were significantly comparable with the positive control.
Fig. 6.
Effect of modified solvent extraction assisted Trametes versicolor extract on a membrane stabilization and b on albumin denaturation. Data are presented as means ± SEM (n = 3). a−bMeans within the column with different lowercase superscript are significantly different (p < 0.05) from each other
Conclusion
Modified solvent evaporation assisted methanolic mushroom extract was evaluated for its antimicrobial and anti-inflammatory efficacy. The mushroom extract showed significantly (p < 0.05) higher total phenolic content than that of other bioactive components. FTIR confirmed the presence of the functional groups of l-ascorbic acid, β-carotene, gallic acid, and rutin. HPLC chromatogram revealed significantly (p < 0.05) higher gallic acid content than that of rutin content. Statistically, the extract of Trametes versicolor and BHA showed a non-significant (p < 0.05) difference in terms of percentage inhibition during DPPH and N2O2 scavenging assay. The mushroom extract significantly inhibited (p < 0.05) higher growth of S. aureus. The mushroom extract showed effective anti-inflammatory activity during membrane stabilization and albumin denaturation assay. Therefore, in conclusion, the mushroom extract with effective antimicrobial and anti-inflammatory activity could be used as an active ingredient for the preparation of antimicrobial hand sanitizer and anti-inflammatory drugs.
Acknowledgements
Authors are grateful acknowledged to Shoolini University, Solan, Himachal Pradesh
Abbreviations
- DPPH
2,2 Diphenyl-1-picrylhydrazyl
- N2O2
Nitric oxide
- BHA
Butylated hydroxyanisole
- HPLC
High performance liquid chromatography
- FTIR
Fourier transform infrared spectroscopy
Author contributions
AB did all practical and Laboratory work. PC in writing paper provided the research idea.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest between the authors.
Ethical approval
No animal trial was done during this research project.
Contributor Information
Aarti Bains, Email: aarti05888@gmail.com.
Prince Chawla, Email: princefoodtech@gmail.com.
References
- Adnan M, Chy MNU, Kamal AM, Barlow JW, Faruque MO, Yang X, Uddin SB. Evaluation of anti-nociceptive and anti-inflammatory activities of the methanol extract of Holigarna caustica (Dennst.) Oken leaves. J Ethnopharmacol. 2019;236:401–411. doi: 10.1016/j.jep.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Andrew, K., Andrew, K. A., Thomas, M., Pesterfield, A., Lineberry, Q., Steinfelds, E. V. (2018). Raman Detection Threshold Measurements for Acetic Acid in Martian Regolith Simulant JSC-1 in the Presence of Hydrated Metallic Sulfates. arXiv preprint arXiv:1801.06870.
- Appiah T, Boakye YD, Agyare C. Antimicrobial activities and time-kill kinetics of extracts of selected ghanaian mushrooms. Evidence-Based Complement Altern Med. 2017;2017:15. doi: 10.1155/2017/4534350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asri RM, Yahya H, Rehan MM, Yahya HN. Antibacterial properties of ethanolic extract of mushrooms sold in malaysian local market. East Afr J Agri Life Sci. 2019;2:516–522. [Google Scholar]
- Bains A, Tripathi A. Evaluation of antioxidant and anti-inflammatory properties of aqueous extract of wild mushrooms collected from Himachal Pradesh. Asian J Pharm Clin Res. 2017;10(3):467–472. [Google Scholar]
- Boakye YD, Agyare C, Hensel A. Anti-infective properties and time-kill kinetics of Phyllanthus muellerianus and its major constituent, geraniin. Med Chem Curr Res. 2016;6(2):96–104. [Google Scholar]
- Chawla P, Kumar N, Bains A, Dhull SB, Kumar M, Kaushik R, Punia S. Gum arabic capped copper nanoparticles: Synthesis, characterization, and applications. Int J Biol Macromol. 2020;146:232–242. doi: 10.1016/j.ijbiomac.2019.12.260. [DOI] [PubMed] [Google Scholar]
- Chawla P, Kumar N, Kaushik R, Dhull SB. Synthesis, characterization and cellular mineral absorption of nanoemulsions of Rhododendron arboreum flower extracts stabilized with gum arabic. J Food Sci Technol. 2019;56(12):5194–5203. doi: 10.1007/s13197-019-03988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chika E, Charles E, Ifeanyichukwu I, Chigozie U, Chika E, Carissa D, Michael A. Phenotypic detection of AmpC beta-lactamase among anal Pseudomonas aeruginosa isolates in a Nigerian abattoir. Arch Clin Microbiol. 2016;7((2:14)):1–5. [Google Scholar]
- Da Silva RF, de Almeida Barros AC, Pletsch M, Argolo ACCM, de Araujo BS. Study on the scavenging and anti-Staphylococcus aureus activities of the extracts, fractions, and subfractions of two Volvariella volvacea strains. World J Microbiol Biotechnol. 2010;26(10):1761–1767. [Google Scholar]
- Garofalo C, Osimani A, Milanović V, Taccari M, Cardinali F, Aquilanti L, Clementi F. The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol. 2017;62:15–22. doi: 10.1016/j.fm.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Gupta C, Chawla P, Arora S, Tomar SK, Singh AK. Iron microencapsulation with blend of gum arabic, maltodextrin and modified starch using modified solvent evaporation method–milk fortification. Food Hydrocolloids. 2015;43:622–628. [Google Scholar]
- Chung JH, Kong JN, Choi HE, Kong KH. Antioxidant, anti-inflammatory, and anti-allergic activities of the sweet-tasting protein brazzein. Food Chem. 2018;267:163–169. doi: 10.1016/j.foodchem.2017.06.084. [DOI] [PubMed] [Google Scholar]
- Kalač P. Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem. 2009;113(1):9–16. [Google Scholar]
- Kaushik R, Chawla P, Kumar N, Janghu S, Lohan A. Effect of premilling treatments on wheat gluten extraction and noodle quality. Food Sci Technol Int. 2018;24(7):627–636. doi: 10.1177/1082013218782368. [DOI] [PubMed] [Google Scholar]
- Kaushik R, Sachdeva B, Arora S, Wadhwa BK. Development of an analytical protocol for the estimation of vitamin D2 in fortified toned milk. Food Chem. 2014;151:225–230. doi: 10.1016/j.foodchem.2013.11.085. [DOI] [PubMed] [Google Scholar]
- Keleş A, Koca I, Gençcelep H. Antioxidant properties of wild edible mushrooms. J Food Process Technol. 2011;2(6):2–6. [Google Scholar]
- Khatua S, Dutta AK, Acharya K. Prospecting Russula senecis: a delicacy among the tribes of West Bengal. PeerJ. 2015;3:e810. doi: 10.7717/peerj.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Seguin P, Ahn JK, Kim JJ, Chun SC, Kim EH, Ro HM. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J Agri Food Chem. 2008;56(16):7265–7270. doi: 10.1021/jf8008553. [DOI] [PubMed] [Google Scholar]
- Klein BP, Perry AK. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J Food Sci. 1982;47(3):941–945. [Google Scholar]
- Lorenzo M, Moldes D, Couto SR, Sanroman A. Improving laccase production by employing different lignocellulosic wastes in submerged cultures of Trametes versicolor. Biores Technol. 2002;82(2):109–113. doi: 10.1016/s0960-8524(01)00176-6. [DOI] [PubMed] [Google Scholar]
- Lule VK, Tomar SK, Chawla P, Pophaly S, Kapila S, Arora S. Bioavailability assessment of zinc enriched lactobacillus biomass in a human colon carcinoma cell line (Caco-2) Food Chem. 2020;309:125583. doi: 10.1016/j.foodchem.2019.125583. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen H, Dong P, Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013;139(1–4):503–508. doi: 10.1016/j.foodchem.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Majeed H, Liu F, Hategekimana J, Sharif HR, Qi J, Ali B, Zhong F. Bactericidal action mechanism of negatively charged food-grade clove oil nanoemulsions. Food Chem. 2016;197:75–83. doi: 10.1016/j.foodchem.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Matijašević D, Pantić M, Rašković B, Pavlović V, Duvnjak D, Sknepnek A, Nikšić M. The antibacterial activity of Coriolus versicolor methanol extract and its effect on ultrastructural changes of Staphylococcus aureus and Salmonella Enteritidis. Front Microbiol. 2016;7:1226. doi: 10.3389/fmicb.2016.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi. 1992;39(10):925–928. [Google Scholar]
- Olugbami JO, Gbadegesin MA, Odunola OA. In vitro free radical scavenging and antioxidant properties of ethanol extract of Terminalia glaucescens. Pharmacogn Res. 2015;7(1):49. doi: 10.4103/0974-8490.147200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia IC, Aida PUIA, Chedea VS, Leopold N, Bocsan IC, Buzoianu AD. Characterization of Trametes versicolor: medicinal mushroom with important health benefits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2018;46(2):343–349. [Google Scholar]
- Reis FS, Martins A, Vasconcelos MH, Morales P, Ferreira IC. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci Technol. 2017;66:48–62. [Google Scholar]
- Rubio-Diaz DE, Francis DM, Rodriguez-Saona LE. External calibration models for the measurement of tomato carotenoids by infrared spectroscopy. J Food Compos Anal. 2011;24(1):121–126. [Google Scholar]
- Sadh PK, Chawla P, Duhan JS. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Biosci. 2018;22:113–120. [Google Scholar]
- Shavit E, Rose D, French A, Vellinga EC, Schaechter E, Wood M, Evans L. Over-the-Counter Medicinal Mushrooms. Fungi. 2009;2:15–19. [Google Scholar]
- Strempel N, Strehmel J, Overhage J. Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr Pharm Des. 2015;21(1):67–84. doi: 10.2174/1381612820666140905124312. [DOI] [PubMed] [Google Scholar]
- Taofiq O, González-Paramás AM, Martins A, Barreiro MF, Ferreira IC. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—a review. Ind Crops Prod. 2016;90:38–48. [Google Scholar]
- Tewari R, Horemans N, Nauts R, Wannijn J, Van Hees M, Vandenhove H. Uranium exposure induces nitric oxide and hydrogen peroxide generation in Arabidopsis thaliana. Environ Exp Bot. 2015;120:55–64. [Google Scholar]
- Thatoi H, Singdevsachan SK, Patra JK. Prebiotics and their production from unconventional raw materials (Mushrooms) In: Grumezescu AM, Holban AM, editors. Therapeutic, Probiotic, and Unconventional Foods. Cambridge: Academic Press; 2018. pp. 79–99. [Google Scholar]
- Thenmozhi N, Gomathi T, Sudha P. Preparation and characterization of biocomposites: chitosan and silk fibroin. Der Pharmacia Lettre. 2013;5(4):88–97. [Google Scholar]
- Yeung JH, Or PM. Polysaccharide peptides from Coriolus versicolor competitively inhibit model cytochrome P450 enzyme probe substrates metabolism in human liver microsomes. Phytomedicine. 2012;19(5):457–463. doi: 10.1016/j.phymed.2011.09.077. [DOI] [PubMed] [Google Scholar]
- Yildirim NC, Turkoglu S, Yildirim N, Ince OK. Antioxidant properties of wild edible mushroom Pleurotus eryngii collected from Tunceli province of Turkey. Dig J Nanomaterials Biostructures (DJNB) 2012;7(4):1647–1654. [Google Scholar]
- Zhang M, Cui SW, Cheung PCK, Wang Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Technol. 2007;18(1):4–19. [Google Scholar]