Abstract
Bovine skin was incubated with plant enzymes bromelain (B) and zingibain (Z) at the level of 0, 5, 10, 15, 20 and 25 unit/g of skin and gelatin was extracted at 60 °C for 6 h. Control gelatin was extracted without enzymatic pretreatment. The yield and gel strength were 17.90% and 283.35 g for the control samples and 22.26% and 160.88 g for B20 samples. The zingibain extracted gelatin (GEZ) samples failed to form gel. Viscosities of GEZ gelatins were significantly (P < 0.05) lower than the gelatins extracted using bromelain (GEB). β and α chains were absolutely degraded in all GEB and GEZ samples. Only smear bands were observed in GEZ gelatins whereas GEB samples revealed presence of low molecular weight polypeptides. Loss of molecular order was noticed in Z5 as elaborated by Fourier transform infrared (FTIR) spectroscopy. Larger particle size, denser and inter-connected irregular network was observed in B20 under scanning electron microscopy. Based on the results obtained, bromelain, particularly at level 20, could be used to obtain a better quality gelatin with higher yield compared to zingibain.
Keywords: Gelatin, Bovine skin, Gelatin extraction, Bromelain, Zingibain
Introduction
Gelatin is an important food additive for providing texture, chewiness and foam stability to confectionery products, imparting mouth feel and chewiness to jelly products as well as it is added to dairy products for its ability to provide texture and stability (Nishimoto et al. 2005). Further use includes instant sauces and soups, edible film for confectionery items and pastry fruit toppings (Karim and Bhat 2008). It is also used as clarifying agent in juice and beverage industry and provides consistency to yoghurt and desserts. In meat industry, gelatin is utilized as coating substance to provide glaze and retard color deterioration (Tyburcy et al. 2010). Besides, being good in heat transfer and holding juice during cooking, it is used in meat canning industry (Mariod and Adam 2013). Stabilizing property of gelatin is utilized in food foams, cream cheese, cottage cheese, ice cream and in fruit salads. Its property of forming an oil-in-water emulsion could be used to partially replace the fat to produce low-fat and reduced-calorie products (GME 2020).
Gelatin is gel forming protein obtained from denaturation of collagen by its partial hydrolysis. Insoluble collagen is turned soluble by pretreating it with either acid or alkali. During the gelatin extraction, covalent and hydrogen bonds are broken down by heat leading to conversion of collagen into gelatin via a transition from helical to coil structure (Ahmad et al. 2019). Collagen cross-linkages being resistant to acid and thermal action (Fan et al. 2017) result in low yield of gelatin (Nalinanon et al. 2008). In the past, some protein degrading enzymes that have the ability to break the cross-links of collagen were used to improve the gelatin recovery (Nalinanon et al. 2008). The gelatins yield, gel strength and viscosity were found to be relatively low when gelatin from cattle skin was recovered by using proctase derived from Aspergillus niger and pepsin (Chomarat et al. 1994).
Collagenous sources were initially pretreated with various protease enzymes before actual gelatin extraction (Balti et al. 2011; Chomarat et al. 1994; Lassoued et al. 2014). They added the skin to the enzyme solutions and allowed pretreatment time duration for skin materials to be acted upon by the employed enzymes. In contrast, Pitpreecha and Damrongsakkul (2006) and Damrongsakkul et al. (2008) did not pretreat the bovine skin with papain enzyme before extracting gelatin by giving pretreatment time duration. Enzyme was dissolved, skin was mixed in it and gelatin was extracted from the samples without providing pretreatment time period. Although, this resulted in higher gelatin recovery, the gel strength was low and β and α protein chains were broken down completely (Pitpreecha and Damrongsakkul 2006). Gelatin extracted from rawhide splits using papain exhibited low viscosity as well as gel strength (Damrongsakkul et al. 2008).
The aforesaid examples evidently demonstrated that the functional properties of the extracted gelatin were negatively affected when enzymes were used to extract gelatin. Gelatin with less degraded peptides having high molecular weight protein chains are thought to possess better functional characteristics (Badii and Howell 2006; Gómez-Guillén et al. 2002). This situation validates the search for new enzymes that can cleave long gelatin chains at few locations so as to obtain long peptide chain gelatin possessing better functional characteristics (Ahmad et al. 2017). Zingibain preparation was found to be most efficient protease in hydrolyzing protein of beef connective tissue among enzymes papain, bromelain, actinidin and zingibain (Ha et al. 2012). Pineapple and zinger, the source of enzymes bromelain and zingibain, respectively, are cultivated globally and overall pineapple and ginger production in the year 2017 amounted to 27.40 (Statista 2020a) and 3 million metric tons (Statista 2020b), respectively. The worldwide production of these two crops ensures commercial availability of bromelain and zingibain in the market (Ha et al. 2012). Therefore, in this study bromelain and one more enzyme, zingibain from ginger, used to extract gelatin and the obtained gelatin was characterized.
Materials and methods
Chemicals
Acrylamide, sodium dodecyl sulphate (SDS), 2-mercaptoethanol, coomassie brilliant blue R-250 and N,N,N′,N′-tetramethyl ethylene diamine (TEMED) were procured from Merck (Darmstadt, Germany). For amino acid analysis, chromatographic column, reagents, mobile phase, and amino acid standards were obtained from Waters Corporation (MA, USA). Agilent Technologies (CA, USA) supplied hydroxyproline standard. In all other cases, analytical grade chemicals and reagents were used. Merck (Darmstadt, Germany) supplied bromelain enzyme (EC 3.4.22.32; ≥ 2.0 mAnsonU/mg) extracted from pineapple and Biohawk (QLD, Australia) a commercial company, gifted enzyme zingibain (> 12 units/mg) extracted from zinger (dry powder blend of Zingiberaceae species).
Preparation of skin
Bovine skin was obtained from the neck region of the female Brahma (age 3–4 years) cattle. The skin was transported to laboratory by keeping it in ice filled box from Shah Alam abattoir situated at Shah Alam, Selangor, Malaysia. Upon arrival, skin was thoroughly cleaned and washed with tap water and stored under low temperature (− 20 °C). The skin was thawed overnight at 4 °C before experiment was performed.
Extraction of gelatin from bovine skin with bromelain and zingibain
Initially, non-collagenous substances were removed from skin by treating it with 0.1 M NaOH (w/v) solution with stirring at room temperature (25 ± 1 °C) for 6 h. A ratio of 1:5 (w/v) of skin to solution was used and the solution was changed at every 2 h interval. Thereafter, skin was cleaned thoroughly with distilled water until neutral wash water is obtained. The hairs on the skin were removed by scraping. The skin was cut into pieces of 2 × 1 cm approximate size and washed thoroughly.
Hereafter, the skin was immersed in HCl solution (1%) at room temperature at 1:10 (w/v) ratio (one part skin and ten part solution) for 20 h with discontinuous stirring to swell the skin. Upon completion, the skin samples were washed carefully till neutral wash water was achieved. The swollen skin samples were incubated with enzymes at various levels (0, 5, 10, 15, 20 and 25 units per g of wet skin) for 48 h at the optimum pH and temperature of the enzymes (pH 6.0 and 6.5 and temperature 35.5 and 50 °C, respectively for bromelain and zingibain) as specified by the supplier firms.
To prepare the enzymatic solutions, optimum pHs solutions (pH 6.0 and 6.5) were prepared using distilled water and required amount of enzymes were dissolved into it. The swollen skins were added to these solutions having enzymes at the ratio of 1:3 (w/v) (one part skin and three part solution). It was transferred to water bath and incubated for 48 h at the corresponding optimum temperatures (35.5 and 50 °C) under continuous stirring. After incubation, the enzymatic activity was stopped by transferring the mixture to water bath maintained at 90 °C for 15 min and subsequently, gelatin was extracted for 6 h at 60 °C in water bath under continuous stirring. The solution mixture (having solubilized gelatin and left over skin) was filtered through cheese cloth to separate the left over skin from gelatin and thereafter gelatin in solution was centrifuged for 20 min at 12,800 × g (Beckman Coulter Avanti J-26 XPI). Labconco FreeZone18, USA freeze drier was employed to dry the supernatant obtained after centrifugation and the dry gelatin was obtained. Same procedure as mentioned above was followed without any enzyme addition to obtain control gelatin from the swollen skin. Triple replication was performed for the extraction.
Analyses of gelatin
Gelatin yield
Weight of the wet skin was taken into consideration while determining the gelatin yield (Balti et al. 2011; Ktari et al. 2014; Lassoued et al. 2014):
Amino acid profile of gelatin
Amino acid (AA) analysis of the gelatin samples was done using high performance liquid chromatography (HPLC) purchased from Waters Corporation, Milford, MA, USA. 6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate (Waters Corporation, Milford, MA, USA) was used for pre-column derivatization. Approximately, 0.1–0.2 g of sample was hydrolyzed with 6 N HCl (5 ml) at 110 °C for 24 h. Fluorescent detector (2475; Waters Corporation, Milford, MA, USA) was employed to detect the peaks and triplicate determinations were done to arrive at mean values. In all the cases, standard deviations were found to be lower than 2%.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) of gelatin
Determination of molecular weight distributions of the recovered gelatins was performed by SDS-PAGE. Stacking gels of 4% and resolving gels of 7.5% were used to load the samples. The sample solution was added to loading buffer (5-times-concentrated) in a ratio of 1:2 (v/v) and loaded onto gels. Mini-PROTEAN Tetra System obtained from Bio-Rad Laboratories, CA, USA was used to provide constant current (15 mA/gel for 15 min) and later current of 25 mA/gel was provided till the bromophenol blue reached the lower end of the gel. Prestained protein ladder BLUeye from GeneDireX, Taiwan was also run concurrently to determine the molecular weights of the gelatins protein bands.
Gel strength measurement
Fernandez-Dıaz et al. (2001) method was slightly altered for gel strength estimation. Gelatin solutions of concentration 6.67% (w/v) was made and gels were matured at 7 °C for time duration of 16–18 h. Texture analyzer of Stable Micro Systems, Surrey, UK (Model TA-XT2i) was employed to determine the gel strength. The plunger speed was kept at 0.5 mm/s and gel strength was recorded as the maximum force (in grams) needed to penetrate 4 mm inside the gel sample. Triplicate determination was done to arrive at the means.
Gelatin viscosity
Gelatin (1.34 g) was dissolved in distilled water (20 ml) to obtain gelatin concentration of 6.67%. The mixture was heated to 60 °C and the gelatin viscosity was measured at 25 °C using RheolabQC viscometer (Anton Paar, Graz, Austria). The viscosity data show the mean of triplicate measurement.
FTIR spectroscopy
Shimadzu (IRTracer-100, Kyoto, Japan) FTIR spectrometer fitted with a detector deuterated l-alanine triglycine sulphate (DLATGS) was used to acquire the spectra. CsI internal reflection crystal was used at room temperature to gain the spectra in the mid-IR region (4000–650 cm−1) having 4 cm−1 resolution. Sixteen scans were done to collect the automatic signals. Clean and empty cell at 25 °C was used to normalize the signals against a background spectrum.
Microstructure analysis of gelatin
JEOL (JSM-IT100 InTouchScope) scanning electron microscope (SEM) obtained from Tokyo, Japan was employed to study the microscopic structures of gelatin samples at 30 × using 10 kV of acceleration voltage. 2–3 mm thick dried gelatin samples were prepared, mounted on bronze stub and sputter-coated with gold using BAL-TEC SCD 005 from Schalksmühle, Germany.
Statistical analysis
Statistical analyses were performed by Statistical Analysis System (SAS) using version 9.4 software developed by SAS Institute Inc., Cary, NC, USA. The two enzymes and their different levels were fixed as the main effects. Data were subjected to analysis of variance (ANOVA) using the GLM. The treatment means were compared by Duncan’s multiple range test (P < 0.05).
Results and discussion
Effect of bromelain and zingibain on gelatin extraction
The effects of different levels (0, 5, 10, 15, 20 and 25 unit/g of skin) of bromelain and zingibain on gelatin yield and pH are shown in the Table 1. Use of bromelain and zingibain significantly (P < 0.05) increased the gelatin yield than the control. Yields of gelatin at B15 and B25 (23.25% and 23.49%, respectively) were significantly (P < 0.05) higher than all other treatment levels of bromelain. The highest yield of gelatin obtained from zingibain was 23.52% at level 15. The gelatin yield for zingibain increased up to level 15 and then decreased at successive levels. Proteolytic enzymes have been reported to give higher gelatin yield (Balti et al. 2011; Lassoued et al. 2014). Extraction yield of 7.84% (on wet weight basis from cuttle fish skin) was obtained using crude acid protease used at level 15 unit/g extracted from cuttle fish and yield increased with increase in the enzyme level (Balti et al. 2011). Pepsin pretreatment of skin of thornback ray (Raja clavata) gave 30% gelatin yield (Lassoued et al. 2014).
Table 1.
Yield, gel strength and viscosity of the bovine skin gelatin extracted using enzymes bromelain and zingibain at different levels
| Treatment levels | Yield (%) of gelatin extracted using enzyme | Gel strength of gelatin extracted using enzyme | Viscosity of gelatin extracted using enzyme | |||
|---|---|---|---|---|---|---|
| Bromelain | Zingibain | Bromelain (g) | Zingibain (g) | Bromelain (mPa s) | Zingibain (mPa s) | |
| Control | 17.90 ± 0.19e | 17.90 ± 0.19e | 283.35 ± 1.84a | 283.35 ± 1.84 | 12.10 ± 0.23a | 12.10 ± 0.23a |
| 0 | 20.43 ± 0.26cA | 20.76 ± 0.36cA | 214.50 ± 1.09b | Not detected | 11.77 ± 0.09abA | 6.37 ± 0.07bcB |
| 5 | 19.36 ± 0.19dB | 22.61 ± 0.26bA | 197.05 ± 0.77c | Not detected | 11.57 ± 0.09bA | 6.13 ± 0.09bcdB |
| 10 | 19.11 ± 0.14dB | 20.69 ± 0.29cA | 140.42 ± 1.39e | Not detected | 11.07 ± 0.15cA | 6.40 ± 0.17bB |
| 15 | 23.25 ± 0.22aA | 23.52 ± 0.22aA | 111.56 ± 0.72f | Not detected | 9.13 ± 0.26eA | 5.87 ± 0.03dB |
| 20 | 22.26 ± 0.15bA | 20.66 ± 0.25cB | 160.88 ± 1.65d | Not detected | 10.20 ± 0.12dA | 5.80 ± 0.06dB |
| 25 | 23.49 ± 0.26aA | 18.82 ± 0.33dB | 140.77 ± 1.12e | Not detected | 10.83 ± 0.07cA | 6.00 ± 0.06cdB |
Values are given as mean ± SD from triplicate determination
Means with different superscripts in the same column (small letter) and row (capital letter) indicate significant difference in the gelatin yields at P < 0.05. Treatment levels 0, 5, 10, 15, 20 and 25 show the unit of enzymes used per gram of skin
Amino acid profile of the gelatin
Amino acid content of skin (S), HCl pretreated skin (PS) samples, control, GEB and GEZ gelatins are given in Table 2. As gelatin is partially denatured collagen, the gelatin amino acid composition remains very similar to collagen from which it is derived with few variations due to the manufacturing process (Duconseille et al. 2015). Glycine, proline and hydroxyproline values of halal bovine gelatin were reported to be 34.1, 12.3 and 9.6%, 34.48, 13.39 and 9.54%, respectively by Balti et al. (2011) and Lassoued et al. (2014). However in the present study, quantities of different amino acids were expressed as mg of amino acid per 100 mg of gelatin sample. This led to differences in the amount of different amino acids reported in this study and the earlier published studies. In addition to this, the differences in source of skin and gelatin manufacturing processes could also contribute towards the differences in amino acids content (Zhou and Regenstein 2006).
Table 2.
Amino acid composition (% of sample) of skin, HCl treated skin sample (PS), control gelatin and gelatin extracted using bromelain and zingibain enzymes from the bovine skin
| Amino acids | Skin | PS | Control | Levels of enzyme bromelain | Levels of enzyme zingibain | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 0 | 5 | 10 | 15 | 20 | 25 | ||||
| Hydroxyproline (Hyp) | 15.26 | 15.13 | 14.14 | 15.03 | 14.93 | 14.82 | 14.31 | 13.60 | 14.49 | 14.65 | 14.36 | 14.84 | 14.69 | 14.00 | 15.42 |
| Aspartic acid (Asp) | 4.91 | 4.77 | 4.06 | 3.45 | 3.61 | 3.49 | 3.50 | 3.37 | 3.54 | 3.58 | 3.92 | 4.08 | 3.82 | 4.00 | 3.57 |
| Serine (Ser) | 3.35 | 3.11 | 2.82 | 3.02 | 3.14 | 3.10 | 2.96 | 2.82 | 3.03 | 3.08 | 3.00 | 3.19 | 3.23 | 3.08 | 3.27 |
| Glutamic acid (Glu) | 8.74 | 8.52 | 7.81 | 7.00 | 7.29 | 7.01 | 6.96 | 6.69 | 7.01 | 7.11 | 7.86 | 8.00 | 7.64 | 7.75 | 7.11 |
| Glycine (Gly) | 23.18 | 21.88 | 19.87 | 21.75 | 21.41 | 21.63 | 20.87 | 19.78 | 20.84 | 20.73 | 21.74 | 21.38 | 21.65 | 20.27 | 21.65 |
| Histidine (His) | 0.98 | 0.89 | 0.82 | 0.86 | 0.85 | 0.85 | 0.83 | 0.78 | 0.85 | 0.89 | 0.98 | 1.01 | 0.99 | 0.96 | 0.94 |
| Arginine (Arg) | 7.75 | 7.43 | 6.87 | 7.26 | 7.35 | 7.21 | 7.08 | 6.64 | 7.13 | 7.01 | 7.09 | 7.13 | 7.36 | 6.90 | 7.29 |
| Threonine (Thr) | 2.41 | 2.35 | 1.63 | 1.72 | 1.73 | 1.72 | 1.69 | 1.62 | 1.74 | 1.75 | 1.69 | 1.73 | 1.76 | 1.71 | 1.78 |
| Alanine (Ala) | 7.20 | 7.03 | 6.50 | 7.00 | 7.03 | 6.89 | 6.79 | 6.56 | 6.79 | 6.71 | 7.40 | 7.35 | 7.27 | 6.70 | 6.68 |
| Proline (Pro) | 11.30 | 11.01 | 10.29 | 10.75 | 10.70 | 10.55 | 10.21 | 9.85 | 10.40 | 10.09 | 10.32 | 10.08 | 10.58 | 9.82 | 10.08 |
| Tyrosine (Tyr) | 0.99 | 0.99 | 0.62 | 0.65 | 0.67 | 0.69 | 0.69 | 0.68 | 0.78 | 0.78 | 0.75 | 0.79 | 0.77 | 0.73 | 0.80 |
| Valine (Val) | 2.65 | 2.59 | 2.11 | 2.12 | 2.12 | 2.09 | 2.06 | 2.00 | 2.14 | 2.11 | 2.18 | 2.21 | 2.21 | 2.17 | 2.13 |
| Lysine (Lys) | 3.47 | 3.40 | 3.13 | 2.85 | 2.93 | 2.85 | 2.90 | 2.74 | 2.91 | 2.97 | 3.17 | 3.22 | 3.06 | 3.16 | 2.84 |
| Isoleucine (Ile) | 1.73 | 1.69 | 1.30 | 1.38 | 1.38 | 1.38 | 1.36 | 1.32 | 1.43 | 1.41 | 1.39 | 1.43 | 1.42 | 1.41 | 1.44 |
| Leucine (Leu) | 3.37 | 3.31 | 2.66 | 2.81 | 2.80 | 2.74 | 2.70 | 2.60 | 2.78 | 2.85 | 2.76 | 2.82 | 2.80 | 2.81 | 2.89 |
| Phenylalanine (Phe) | 2.23 | 2.16 | 1.78 | 1.91 | 1.91 | 1.88 | 1.83 | 1.77 | 1.93 | 1.90 | 1.88 | 1.90 | 1.93 | 1.85 | 2.01 |
| Imino acids (Pro + Hyp) | 26.56 | 26.14 | 24.43 | 25.78 | 25.63 | 25.38 | 24.52 | 23.45 | 24.89 | 24.74 | 24.68 | 24.92 | 25.27 | 23.81 | 25.50 |
All the data were expressed in the unit of mg/100 mg of gelatin sample. Measurements were performed in triplicate and data correspond to mean values. Standard deviations were in all cases were lower than 2%
Balti et al. (2011) and Lassoued et al. (2014) found that bovine gelatin contained 21.90% imino acid. Hyp content in control (14.14%), GEB and GEZ (varied from 13.60 to 15.03% and 14.00 to 15.42%, respectively) were higher compared to Hyp content of 9.6 and 9.54%, respectively as recorded by Balti et al. (2011) and Lassoued et al. (2014) in halal bovine gelatin. Imino acids content is directly responsible for the triple helical strands stability of the renatured gelatin as regions high in Pro + Hyp most probably take part in the nucleation zones formation (Ledward 1986). In particular, Hyp, through its hydroxyl group, is believed to take part in hydrogen bond formation and thus provide stability to the triple-helical structure of collagen (Ledward 1986). The higher imino acid content found in the present study does not commensurate with the observed gel strength as the amino acid composition is not the sole determinant of gelatin viscoelastic properties (Giménez et al. 2005) but is also influenced by the molecular weight distribution. Occurrence of high molecular weight polypeptides in GEB and absence of even low molecular protein bands in GEZ samples might be responsible for the higher gel strength as well as viscosity of GEB than GEZ gelatins.
SDS-PAGE analysis of the gelatin
Besides amino acids, the gelatin functional qualities are dependent on molecular weights distribution, structure and composition of its subunits to great extent.(Balti et al. 2011) SDS-PAGE image for HCl pretreated skin sample (PS), GEB and GPZ gelatin samples are shown in Fig. 1. α (α1 and α2) as well as its dimer, β chains, were observed in the SDS-PAGE result of PS. All GEB and GPZ gelatins revealed complete destruction of β and α chains. Lower molecular weight protein bands which were degradation products of β and α chains were observed in GEB samples. GEZ samples revealed only smear bands indicating over hydrolysis by zingibain.
Fig. 1.
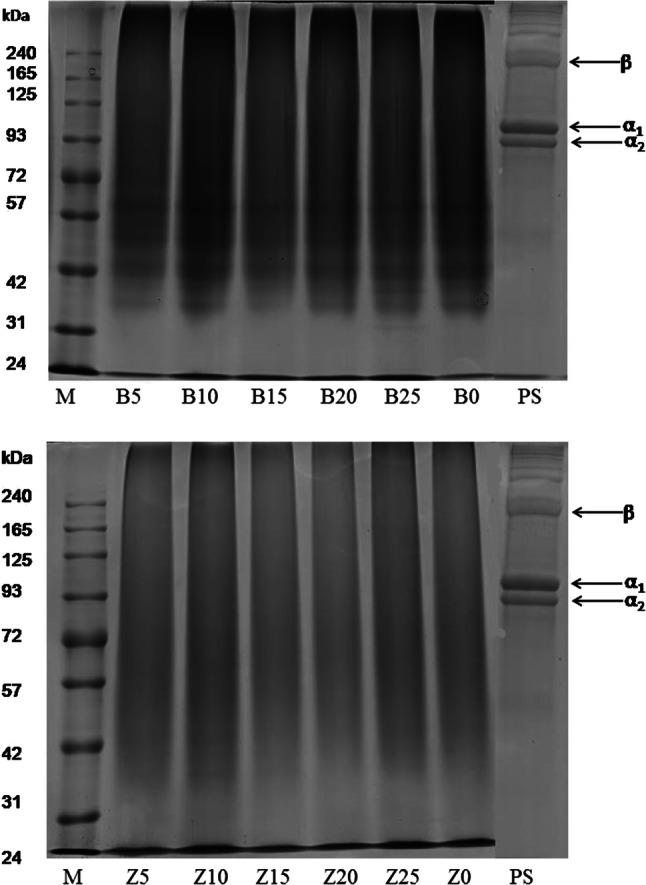
SDS-PAGE pattern of pretreated skin (PS) along with gelatin samples extracted using different levels of enzymes bromelain (B) and zingibain (Z). 0, 5, 10, 15, 20 and 25 refer to corresponding enzymes level of 0, 5, 10, 15, 20 and 25 unit/g of skin; M denotes high molecular marker
Peptide chains of varying mass is the result of cleavage of inter- and intra chains peptide linkages and some undesirable cleavage of inter-chain cross links (Zhou and Regenstein 2006). Occurrence of lower molecular weight protein fractions in the zebra blenny skin gelatin obtained using zebra blenny crude acid protease (ZBCAP) was ascribed to the extensive degradation of gelatin protein chains by ZBCAP (Ktari et al. 2014). Complete absence of major protein components (β, α1 and α2 chains) and appearance of smear band was observed in gelatin extracted using bigeye snapper pepsin from its skin (Nalinanon et al. 2008). Pitpreecha (2005) reported low molecular weight bands of 21 kDa and 14 kDa in the gelatin extracted from raw skin using commercial papain enzyme.
Gel strength of gelatin
Normally, gelatins possessing high molecular weight components exhibits higher gel strength than the gelatins revealing low molecular weight protein chains (Badii and Howell 2006). The gel strength for control was 283.35 g and 197.05 and 160.88 g for B5 and B20, respectively (Table 1). Hafidz et al. (2011) obtained gel strength of 193.49 [force (g)] at pH 3 and 270.35 [force (g)] at pH 9 for bovine gelatin obtained from Sigma (St. Louis, USA). The gel strength of bovine gelatin obtained from Halalgel Sdn. Bhd., Malaysia and gelatin recovered from surimi processing wastes using bromelain were 161 and 62.9 g, respectively (Norziah et al. 2014).
All the GEZ samples failed to form gel and therefore, gel strength was not detectable (Table 1). The gel strength of gelatin extracted from bovine skin using enzymes pepsin and proctase (source A. niger) were 140 and 29 g, respectively (Chomarat et al. 1994). Gelatin gel strength obtained from rawhide splits using papain at pH 6, 7 and 8 at 70 °C after 90 min extraction were 75.4, 146.5 and 200.6 g, respectively (Damrongsakkul et al. 2008). Low gel strength gelatin of bloom less than 125, 75 and 200 g were recovered from the raw hide extracted with the aid of crude proteolytic enzyme derived from papaya latex at different temperatures of 65, 75 and 85 °C, respectively (Pitpreecha and Damrongsakkul 2006). Comparable results were obtained with commercial papain enzyme. The results indicated that the enzymes broke down the peptide bonds more emphatically and harshly than by lime or acid (Simeonova and Dalev 1996). Enzymatic extraction of gelatin from collagen using crude enzyme obtained from papaya latex resulted in low gel strength gelatin than simple extraction using acid or lime (Pitpreecha and Damrongsakkul 2006). Over hydrolysis leading to formation of much shorter chains (Norziah et al. 2014) due to severe breakdown of collagen chains by zingibain during pretreatment resulted in failure to form gel by GEZ samples. Inspite of having high imino acid, occurrence of protein degraded components resulted in very low gel strength of less than 25 g of squid gelatin (Gómez-Guillén et al. 2002).
Viscosity
Viscosity is determined by molecular weight as well as polydispersity of the gelatin polypeptides (Jamilah et al. 2011). Control gelatin exhibited viscosity of 12.10 mPa s whereas B20 viscosity was 10.20 mPa s (Table 1). Similar viscosity of 9.80 cP for the commercial grade bovine gelatin was reported by Mohtar et al. (2010). All the GEZ samples showed significantly (P < 0.05) lower viscosity than the control and GEB. Gelatins recovered from rawhide using papain at pH 6, 7 and 8 at 70 °C for 90 min exhibited 3.5, 4.5 and 5.6 Pa s−1 viscosities, respectively (Damrongsakkul et al. 2008). Viscosity of gelatin extracted adding pepsin and proctase from bovine skin was 3.43 and 1.11 (centipoise units), respectively (Chomarat et al. 1994). High viscosity could be attributed to the difference in the molecular weight and molecular size distribution of the protein molecules (Mohtar et al. 2010). Control and GEB samples had higher molecular weight protein compared to GEZ samples which showed only band smear.
FTIR spectra
B20 and Z5 were selected for FTIR analysis as these gelatins gave better yield, gel strength and viscosity. FTIR spectra of control, B20 and Z5 have been presented in Fig. 2 and corresponding spectral peaks were shown in Table 3. Amide I bands of control, B20 and Z5 were displayed at the wavenumbers 1631.78, 1629.85 and 1631.78 cm−1, respectively. Shift to higher wavenumber as well as higher amplitude of amide I pointed to higher molecular order as a result of bonding of C=O group with neighboring chains through H-bonds (Ahmad and Benjakul 2011). Although control and Z5 displayed their amide I band at higher wavenumber compared to B20 but only Z5 showed higher amplitude also signifying the loss of molecular order in Z5. B20 showed relatively lower amide I band wavenumber with lower amplitude than Z5 indicative of higher molecular order than Z5 gelatin.
Fig. 2.
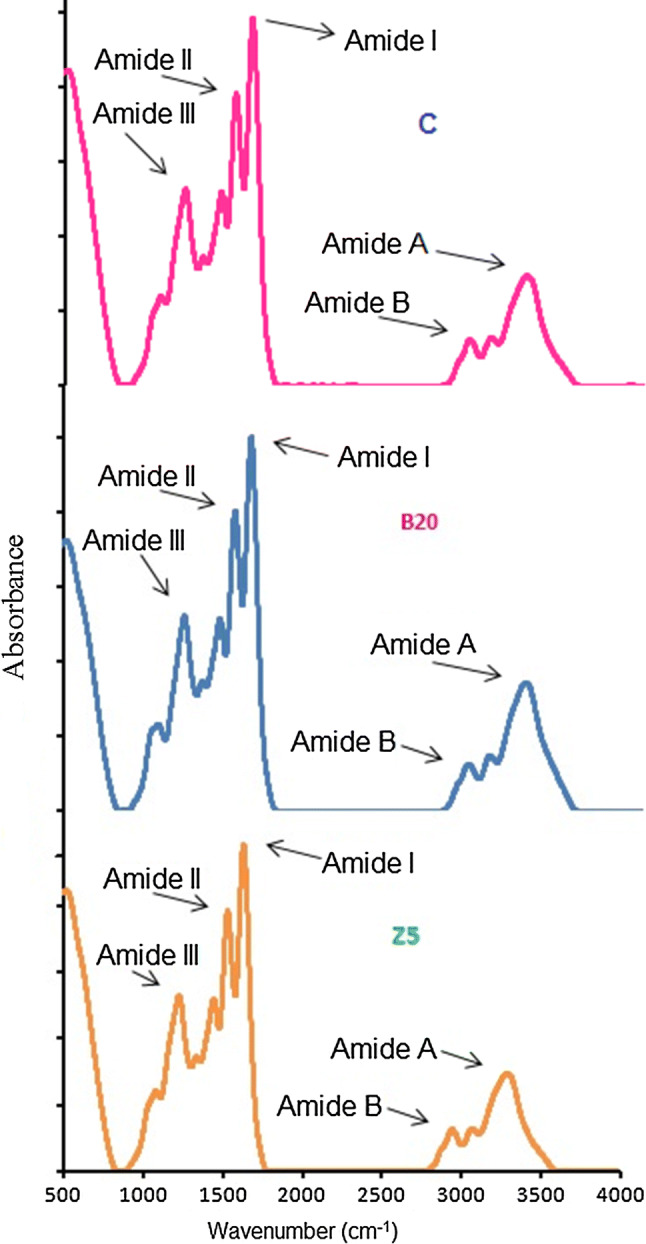
Fourier transform infrared spectra of gelatin samples extracted using bromelain and zingibain at level of 20 (B20) and 5 (Z5) unit of enzymes/g of wet skin, respectively. Control gelatin (C) was extracted without enzyme pretreatment
Table 3.
FTIR spectra peak position of gelatin samples extracted using bromelain and zingibain at level of 20 (B20) and 5 (Z5) unit of enzymes/g of wet skin, respectively
| Band | Peak wavenumber (cm−1) | ||
|---|---|---|---|
| Control | B20 | Z5 | |
| Amide I | 1631.78 | 1629.85 | 1631.78 |
| Amide II | 1537.27 | 1541.12 | 1543.05 |
| Amide III | 1234.44 | 1234.44 | 1236.37 |
| Amide A | 3294.42 | 3302.13 | 3296.35 |
| Amide B | 2931.80 | 2937.59 | 2939.52 |
Control gelatin was extracted without enzyme pretreatment
Dry collagen displayed its characteristic amide II band between wavenumber 1530 and 1540 cm−1 (Tu et al. 2015). Shift to lower wavenumber with low amplitude signified NH group is involved more forming hydrogen bond with adjoining molecules (Ahmad et al. 2011). The amide-II absorption spectral peaks of control, B20 and Z5 gelatins were observed at the wavenumbers of 1537.27, 1541.12 and 1543.05 cm−1, respectively. Comparatively lower amide II wavenumber with lower amplitude of control and B20 than Z5 suggested greater NH group participation in H-bond formation with the side chain molecules.
Amide III band detected about wavenumber 1233–1234 cm−1 specified to disordered gelatin molecules (Sinthusamran et al. 2014). The amide III was displayed at the wavenumbers 1234.44, 1234.44 and 1236.37 cm−1 -for control, B20 and Z5, respectively which indicated random coiled structure. Lower amplitude of amide III suggested disorder in the indigenous α helix structure of gelatin during collagen conversion into gelatin resulting in the destruction of triple helical configuration (Muyonga et al. 2004). Although all the three samples displayed low amide III amplitude but the lowest amplitude of control compared to B20 and Z5 indicated greatest destruction of triple helix configuration.
Formation of hydrogen bonds through the participation of N–H group of a peptide results in shifting of amide A to lower wavenumbers of around 3300 cm−1 (Tu et al. 2015). For gelatin control, B20 and Z5, amide A band was noticed at 3294.42, 3302.13 and 3296.35 cm−1, respectively. Greater participation of a N–H group in peptide chains in hydrogen bond formation of control gelatin caused lower amide A wavenumber compared to B20 and Z5 sample. Among B20 and Z5, N–H group in α chains of Z5 were found to be involved more in H bonding.
The amide B spectral bands were observed at 2931.80, 2937.59 and 2939.52 cm−1 for control, B20 and Z5, respectively. Comparatively, lower wavenumbers of control than B20 and Z5 suggested greater interaction of –NH3 groups between peptide chains in control sample (Ahmad and Benjakul 2011). –NH3 groups of B20 were more involved in the interaction between different α chains than Z5 as amide B band of B20 were detected at lower wavenumber than Z5.
Microstructure of gelatin
SEM images of control, B20 and Z5 gelatin samples are shown in Fig. 3. The Z5 sample showed sheet structure having abundant small size void spaces resulting in failure to form gel and low viscosity. Gelatin samples exhibiting higher gel strength showed denser and finer gel network (Tu et al. 2015). It is posited that zingibain enzyme pretreatment led to severe breakdown of protein chains. B20 sample exhibited larger particle size and comparatively denser inter-connected gelatin network with less number of irregular shape void spaces than Z5 sample. The differences in microstructure were reflected in the inability to form gel and low viscosity of the GEZ samples. Control gelatin showed honeycomb pattern with comparatively less disorganization, higher density and higher inter-connected network than B20 and Z5 reflecting in its higher gel strength than the other two samples.
Fig. 3.
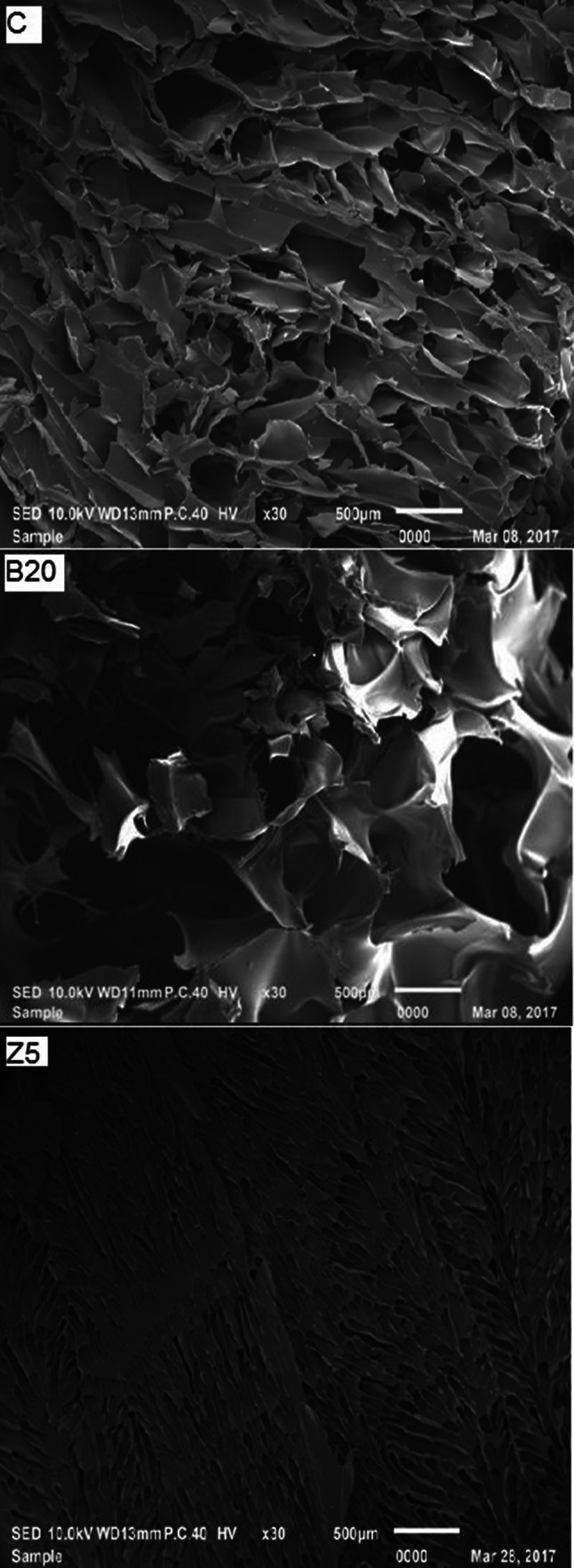
SEM images of gelatin samples extracted using bromelain and zingibain enzymes at level of 20 (B20) and 5 (Z5) unit of enzymes/g of wet skin, respectively. Control gelatin (C) was extracted without enzyme pretreatment. Magnification: × 30
Conclusion
Bromelain enzyme gave better result, particularly at level 20 unit/g, in term of yield and quality characteristics of gelatin in comparison to zingibain enzyme. GEB samples exhibited medium quality gelatin in term of gel strength whereas GEZ samples failed to form gel indicating that the product obtained from the zingibain pretreatment was collagen hydrolysate. Higher viscosity was obtained for GEB than GEZ samples. SDS-PAGE images revealed that the of β and α chains were entirely absent in both GEB and GEZ samples. Although lower molecular bands protein was revealed by GEB samples, only smear bands were visible in GEZ samples suggesting over hydrolysis by the zingibain enzyme. Higher incubation temperature for long time (50 °C for 48 h) in case of zingibain enzyme might have resulted in complete cleavage of protein chains resulting in the failure to form gel, low viscosity and presence of smear bands.
Acknowledgements
The first author is obliged to ICAR (Indian Council of Agricultural Research) (Grant no. F. No. 29-1/2009-EQR/Edn (pt.III)), New Delhi, India for awarding ICAR-International Fellowship and Department of Agricultural Research & Education (DARE), Ministry of Agriculture and Farmers Welfare, Government of India, New Delhi for granting him permission to pursue Ph.D. in Malaysia. Biohawk, QLD, Australia is duly recognized for gifting enzyme zingibain. The research work was supported by Putra Grant (no. UPM/700-2/1/GP-IPS/2015/9467000) given by Universiti Putra Malaysia, Malaysia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad M, Benjakul S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocoll. 2011;25(3):381–388. doi: 10.1016/j.foodhyd.2010.07.004. [DOI] [Google Scholar]
- Ahmad M, Benjakul S, Ovissipour M, Prodpran T. Indigenous proteases in the skin of unicorn leatherjacket (Aluterus monoceros) and their influence on characteristic and functional properties of gelatin. Food Chem. 2011;127(2):508–515. doi: 10.1016/j.foodchem.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Ismail A, Ahmad SA, Khalil KA, Kumar Y, Adeyemi KD, Sazili AQ. Recent advances on the role of process variables affecting gelatin yield and characteristics with special reference to enzymatic extraction: a review. Food Hydrocoll. 2017;63:85–96. doi: 10.1016/j.foodhyd.2016.08.007. [DOI] [Google Scholar]
- Ahmad T, Ismail A, Ahmad SA, Khalil KA, Teik Kee L, Awad EA, Sazili AQ. Physicochemical characteristics and molecular structures of gelatin extracted from bovine skin: effects of actinidin and papain enzymes pretreatment. Int J Food Prop. 2019;22(1):138–153. doi: 10.1080/10942912.2019.1576731. [DOI] [Google Scholar]
- Badii F, Howell NK. Fish gelatin: structure, gelling properties and interaction with egg albumen proteins. Food Hydrocoll. 2006;20(5):630–640. doi: 10.1016/j.foodhyd.2005.06.006. [DOI] [Google Scholar]
- Balti R, Jridi M, Sila A, Souissi N, Nedjar-Arroume N, Guillochon D, Nasri M. Extraction and functional properties of gelatin from the skin of cuttlefish (Sepia officinalis) using smooth hound crude acid protease-aided process. Food Hydrocoll. 2011;25(5):943–950. doi: 10.1016/j.foodhyd.2010.09.005. [DOI] [Google Scholar]
- Chomarat N, Robert L, Seris JL, Kern P. Comparative efficiency of pepsin and proctase for the preparation of bovine skin gelatin. Enzyme Microb Technol. 1994;16(9):756–760. doi: 10.1016/0141-0229(94)90032-9. [DOI] [Google Scholar]
- Damrongsakkul S, Ratanathammapan K, Komolpis K, Tanthapanichakoon W. Enzymatic hydrolysis of rawhide using papain and neutrase. J Ind Eng Chem. 2008;14(2):202–206. doi: 10.1016/j.jiec.2007.09.010. [DOI] [Google Scholar]
- Duconseille A, Astruc T, Quintana N, Meersman F, Sante-Lhoutellier V. Gelatin structure and composition linked to hard capsule dissolution: a review. Food Hydrocoll. 2015;43:360–376. doi: 10.1016/j.foodhyd.2014.06.006. [DOI] [Google Scholar]
- Fan H, Dumont MJ, Simpson BK. Extraction of gelatin from salmon (Salmo salar) fish skin using trypsin-aided process: optimization by Plackett–Burman and response surface methodological approaches. J Food Sci Technol. 2017;54(12):4000–4008. doi: 10.1007/s13197-017-2864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Dıaz MD, Montero P, Gomez-Guillen MC. Gel properties of collagens from skins of cod (Gadus morhua) and hake (Merluccius merluccius) and their modification by the coenhancers magnesium sulphate, glycerol and transglutaminase. Food Chem. 2001;74(2):161–167. doi: 10.1016/S0308-8146(01)00110-8. [DOI] [Google Scholar]
- Giménez B, Turnay J, Lizarbe MA, Montero P, Gómez-Guillén MC. Use of lactic acid for extraction of fish skin gelatin. Food Hydrocoll. 2005;19(6):941–950. doi: 10.1016/j.foodhyd.2004.09.011. [DOI] [Google Scholar]
- GME-Gelatin Manufacturers of Europe (2020) Gelatine is indispensable for the food industry and for nutrition. https://www.gelatine.org/applications/food-industry.html. Accessed 04 Mar 2020
- Gómez-Guillén MC, Turnay J, Fernández-Dıaz MD, Ulmo N, Lizarbe MA, Montero P. Structural and physical properties of gelatin extracted from different marine species: a comparative study. Food Hydrocoll. 2002;16(1):25–34. doi: 10.1016/S0268-005X(01)00035-2. [DOI] [Google Scholar]
- Ha M, Bekhit AE-DA, Carne A, Hopkins DL. Characterisation of commercial papain, bromelain, actinidin and zingibain protease preparations and their activities toward meat proteins. Food Chem. 2012;134(1):95–105. doi: 10.1016/j.foodchem.2012.02.071. [DOI] [Google Scholar]
- Hafidz RMRN, Yaakob CM, Amin I, Noorfaizan A. Chemical and functional properties of bovine and porcine skin gelatin. Int Food Res J. 2011;18:813–817. [Google Scholar]
- Jamilah B, Tan KW, Umi Hartina MR, Azizah A. Gelatins from three cultured freshwater fish skins obtained by liming process. Food Hydrocoll. 2011;25(5):1256–1260. doi: 10.1016/j.foodhyd.2010.11.023. [DOI] [Google Scholar]
- Karim AA, Bhat R. Gelatin alternatives for the food industry: recent developments, challenges and prospects. Trends Food Sci Technol. 2008;19:644–656. doi: 10.1016/j.tifs.2008.08.001. [DOI] [Google Scholar]
- Ktari N, Bkhairia I, Jridi M, Hamza I, Riadh BS, Nasri M. Digestive acid protease from zebra blenny (Salaria basilisca): characteristics and application in gelatin extraction. Food Res Int. 2014;57:218–224. doi: 10.1016/j.foodres.2014.01.041. [DOI] [Google Scholar]
- Lassoued I, Jridi M, Nasri R, Dammak A, Hajji M, Nasri M, Barkia A. Characteristics and functional properties of gelatin from thornback ray skin obtained by pepsin-aided process in comparison with commercial halal bovine gelatin. Food Hydrocoll. 2014;41:309–318. doi: 10.1016/j.foodhyd.2014.04.029. [DOI] [Google Scholar]
- Ledward DA. Gelation of gelatin. In: Mitchell JR, Ledward DA, editors. Functional properties of food macromolecules. London: Elsevier Applied Science Publishers; 1986. pp. 171–201. [Google Scholar]
- Mariod AA, Adam HF. Review: gelatin, source, extraction and industrial. Acta Sci Pol Technol Aliment. 2013;12(2):135–147. [Google Scholar]
- Mohtar NF, Perera C, Quek S-Y. Optimisation of gelatine extraction from hoki (Macruronus novaezelandiae) skins and measurement of gel strength and SDS-PAGE. Food Chem. 2010;122(1):307–313. doi: 10.1016/j.foodchem.2010.02.027. [DOI] [Google Scholar]
- Muyonga JH, Cole CGB, Duodu KG. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus) Food Chem. 2004;86(3):325–332. doi: 10.1016/j.foodchem.2003.09.038. [DOI] [Google Scholar]
- Nalinanon S, Benjakul S, Visessanguan W, Kishimura H. Improvement of gelatin extraction from bigeye snapper skin using pepsin-aided process in combination with protease inhibitor. Food Hydrocoll. 2008;22(4):615–622. doi: 10.1016/j.foodhyd.2007.01.012. [DOI] [Google Scholar]
- Nishimoto M, Sakamoto R, Mizuta S, Yoshinaka R. Identification and characterization of molecular species of collagen in ordinary muscle and skin of the Japanese flounder Paralichthys olivaceus. J Food Chem. 2005;90:151–156. doi: 10.1016/j.foodchem.2004.03.034. [DOI] [Google Scholar]
- Norziah MH, Kee HY, Norita M. Response surface optimization of bromelain-assisted gelatin extraction from surimi processing wastes. Food Biosci. 2014;5:9–18. doi: 10.1016/j.fbio.2013.10.001. [DOI] [Google Scholar]
- Pitpreecha S (2005) Gelatin production from large animal raw hide using proteolytic enzyme extracted from papaya latex. Mater thesis, Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Thailand, ISBN:974-53-2940-1
- Pitpreecha S, Damrongsakkul S. Hydrolysis of raw hide using proteolytic enzyme extracted from papaya latex. Korean J Chem Eng. 2006;23(6):972–976. doi: 10.1007/s11814-006-0017-z. [DOI] [Google Scholar]
- Simeonova L, Dalev P. Utilization of a leather industry waste. Waste Manag. 1996;16(8):765–769. doi: 10.1016/S0956-053X(97)00020-2. [DOI] [Google Scholar]
- Sinthusamran S, Benjakul S, Kishimura H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014;152:276–284. doi: 10.1016/j.foodchem.2013.11.109. [DOI] [PubMed] [Google Scholar]
- Statista (2020a) Pineapple production worldwide from 2002 to 2018. https://www.statista.com/statistics/298505/global-pineapple-production/. Accessed 06 Mar 2020
- Statista (2020b) Production of ginger worldwide from 2010 to 2017. https://www.statista.com/statistics/1064660/ginger-production-volume-worldwide/. Accessed 06 Mar 2020
- Tu Z-C, Huang T, Wang H, Sha X-m, Shi Y, Huang X-q, Man Z-z, Li D-j. Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. J Food Sci Technol. 2015;52(4):2166. doi: 10.1007/s13197-013-1239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburcy A, Wasiak P, Cegiełka A. Application of composite protective coatings on the surface of sausages with different water content. Acta Sci Pol Technol Aliment. 2010;9(2):151–159. [Google Scholar]
- Zhou P, Regenstein JM. Determination of total protein content in gelatin solutions with the Lowry or Biuret assay. J Food Sci. 2006;71(8):C474–C479. doi: 10.1111/j.1750-3841.2006.00151.x. [DOI] [Google Scholar]


