Abstract
The leaves of Ocimum sanctum were extracted in methanol (OsM) and sequentially fractionated with n-hexane (OsH), ethylacetate (OsE) and butanol (OsB) to find the best extraction solvent for antioxidants from the herb known for its medicinal values. OsB was rich in both total polyphenolic content (TPC) (212.26 ± 6.3 mg GAE/g extract) and total flavonoid contents (TFC) (54.51 ± 3.5 mg QE/g extract). OsE also had significantly high TPC (202.71 ± 5.5 mg GAE/g extract). The EC50 based on DPPH (3.91 ± 0.3 μg/ml), ABTS (1.6 ± 0.1 μg/ml) and phosphomolybdate (2.31 ± 0.1 μg/ml) for OsB; hydroxyl (5.3 ± 0.4 μg/ml), superoxide (7.32 ± 0.9 μg/ml) radicals for OsM and DPPH (8.61 ± 0.6 μg/ml), phosphomolybdate (2.43 ± 0.1 μg/ml) and ABTS (5.3 ± 0.4 μg/ml) for OsE were lower than ascorbic acid showing potential antioxidant properties. EC50 values of different fractions for DPPH anion, ABTS cation free radical scavenging and phosphomolybdate reducing property were significantly and positively correlated with TPC and TFC. LC–MS analysis of OsB and OsE showed the presence of luteolin, apigenin, rosmarinic, chlorogenic, caffeic acid and their derivatives. Quercetin is extracted in ethylacetate fraction. Overall data revealed that O. sanctum leaf extracts in butanol and ethylacetate with high polyphenolics and flavonoids, had strong antioxidant potential.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04417-2) contains supplementary material, which is available to authorized users.
Keywords: Ocimum sanctum, LC–MS, Total phenolic, Total flavonoid, Antioxidant assay
Introduction
Reactive oxygen species are essentially produced as the intermediate metabolites in a number of physiological processes like energy production in mitochondria, phagocytosis, cell growth regulation, intracellular signaling and detoxification of xenobiotics in the human body. Exposure to UV irradiations, chemical pollutants, organic solvents, tobacco smoke and pesticides are some of the environmental factors contributing to ROS over production. Stress generated due to current life style further adds to the overproduction of free radicals and causing tissue damage leading to deteriorating health conditions. High and chronic stress is related to a large number of pathological conditions like atherosclerosis, arthritis, ischemia, cancer and injuries to many tissues including central nervous system (Mazandarani 2012). Human health is the primary concern as it influences the efficiency and indirectly affects the economic status of individual, family, society and nation. Use of antioxidants to combat increasing oxidative stress under normal and pathological conditions is exponentially rising. Application of antioxidants finds a new dimension in food technology to improve the shelf life of eatables. Synthetic antioxidant are reported to have various side effects including carcinogenicity and potential risks to human health (Nunes et al. 2012), hence research on natural antioxidants and their applications is the need of the hour. Polyphenols are the secondary metabolites of plants, synthesized under environmental stress (Nunes et al. 2012). The phytochemicals and secondary metabolites of plants contribute to their medicinal values. Polyphenols absorb, quench and neutralize free radicals, act as reducing agents, metal chelators and can efficiently protect biological systems from degeneration under high oxidative stress (Lobo et al. 2010). Antioxidants from plant sources, especially polyphenolic compounds effectively impede rancidity due to lipid oxidation in foods as well as development of oxidative stress related diseases (Sarija et al. 2014). Industrial use of phenolics to improve food quality, shelf life and nutritional value (Naczk and Shahidi 2004) is attracting researchers to find natural, safe components with better efficiency from plants.
Flavonoids, a class of polyphenolics with free radical scavenging properties are known to inhibit hydrolytic and oxidative enzymes, reduce blood glucose and lipids, exhibit anti-inflammatory effect and enhance immunity in human beings (Atoui et al. 2005). Therefore, flavonoids have attracted attention as a possible reply to various health issues especially related to oxidative stress.
Holy basil (Ocimum sanctum L), commonly grown in Indian subcontinent for its religious sanctity, is mentioned in the Charaka Samhita (an Indian traditional medicine scripture) for its diverse medicinal properties including anti-hyperlipedimic, hypoglycemic, anxiolytic, hypotensive, anti-inflammatory and antimicrobial (Mondal et al. 2009; Satyamitra et al. 2014; Venuprasad et al. 2014). It is also considered to be an adaptogen being helpful for adapting to stress. In the traditional (ayurvedic and unani) medical systems, the extracts of holy basil leaves are used for common colds, inflammation and headaches. The leaves of Ocimum are the rich source of various compounds including β-caryophyllene, derivatives of eugenol, vanillin, rosmarinic acid, ursolic acid, gallic acid and vanillic acid (Ali and Ali 2012) but the extraction and identification of active flavonoids and their correlation with antioxidant properties is still a challenging issue. Iloki et al. (2015) reported water and alcohols as better solvents for flavones while Złotek et al. 2016 demonstrated acetone to extract more flavones as compared to other organic solvents (Iloki-Assanga et al. 2015; Złotek et al. 2016). Agarwal et al. 2017 illustrated about ethyl acetate for the better extraction of polyphenolics from holy basil (Agarwal et al. 2017). The studies depict that polarity of solvent is the key issue which affects the phytochemical extraction and their antioxidant activities. Sequential fractionation with solvents of different polarity may be an effective tool to find a fraction with maximum yield of polyphenols, flavonoids and the active antioxidant components. But differential extraction of the leaves of this herb is not on records. Thus, the aim of this study was to get maximum extraction of polyphenols and flavonoids from the leaves of O. sanctum by sequential fractionation with different solvents and to explore the correlation in the constituents and the antioxidant activity of the preparations. Through the present study, an attempt was made to identify major flavonoids and their derivatives in the most effective fractions.
Material and methods
Chemicals
Ascorbic acid, DPPH (2,2-Diphenyl-1-picrylhydrazyl), aluminium chloride, ferric chloride, nito blue tetrazolium (NBT), riboflavin, ammonium molybdate, trichloroacetic acid (TCA), thiobarbituric acid (TBA), ABTS (2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid), deoxyribose, Folin–Ciocalteu reagent, methanol, n-hexane, ethyl acetate and n-butanol were of analytical grade and procured from Merck (Mumbai, India). HPLC grade solvents were purchased from Merck (Mumbai, India). Other chemicals used were of analytical grade and purchased from Himedia (Mumbai, India). Milli-Q System (Merck, Mumbai, India) was used to prepare ultrapure water.
Plant material
O. sanctum seeds of genotype HOB15 were obtained from Medicinal, Aromatic and Potential Crops Section, department of genetics and plant breeding, college of agriculture, CCS HAU, Hisar (India). Smaller sized seeds were planted shallow (ca.0.3 cm) in plastic bags following all recommended procedures in literature. Soil was kept moist to hasten germination. Emergence occurred after 18–21 days. Germination was more than 85%. Seedlings were transplanted after 5 weeks of growth to earthen pots. When plants were about 12 cm in height, topping was done to encourage lateral branching. Plants were grown without herbicides for two months and young leaves were harvested after every 10 days. Leaves were dried in shade at room temperature for two days and at 45 °C in oven for seven days to obtain a constant dry weight.
Preparation of extract and fractionation
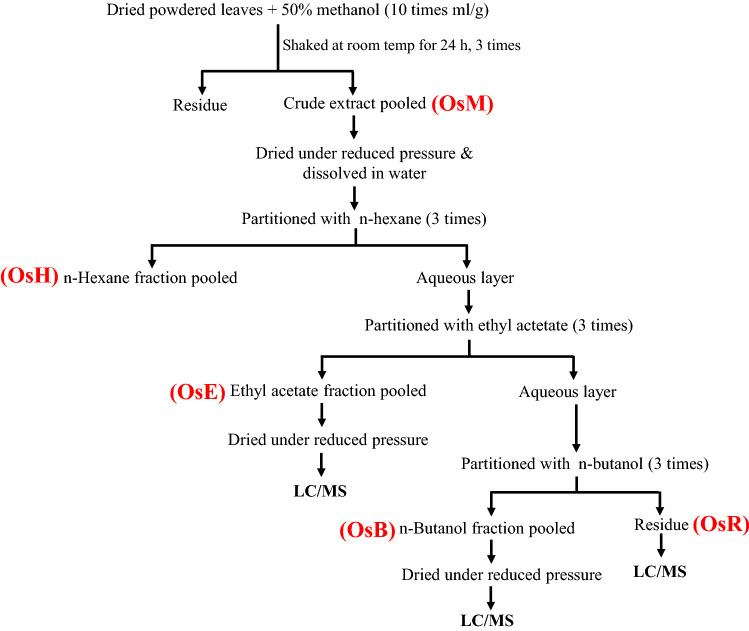
Coarsely powdered to 60-mesh size 100 g dry leaves were extracted thrice with 50% methanol (1 g/10 ml) for 24 h at room temperature in a shaker and pooled. The extract was centrifuged at 3000g for 5 min after passing through double-layered muslin cloth. The solvent in supernatant was evaporated at 40 °C in a rotary evaporator to get the crude dried extract OsM (19.27%). The crude extract was suspended in 200 ml of distilled water and successively partitioned with n-hexane (500 ml × 3), ethyl acetate (500 ml × 3) and n-butanol (500 ml × 3) to get OsH, OsE, OsB and OsR (Fig. 1). These fractions were stored at − 20 °C after drying under reduced pressure.
Fig. 1.
Scheme of extraction of leaves of O. sanctum
Determination of total polyphenolic content
To estimate the total phenolic content of the preparations 2 ml of 2% sodium carbonate was mixed with the extract aliquots. After 2 min, 100 µl of 1 N Folin–Ciocalteau was added and read at 750 nm after 30 min (Shan et al. 2005). Standard curve with gallic acid (0–80nmoles) was prepared and phenolic content was calculated as mg gallic acid equivalent (GAE).
Determination of total flavonoid content
To determine flavonoid content of the preparations, an aliquot of the extract was diluted to make volume 0.5 ml; 30 μl NaNO2 (5%) was added and mixed well. 300 μl of AlCl3 (10%) was added after 5 min and mixed. After another 5 min, 200 μl NaOH (1 N) was mixed with and absorbance was recorded at 510 nm (Dua et al. 2013). Standard curve was prepared with quercetin (0–100 nmoles) and flavonoids were reported as mg quercetin equivalents (QE).
In vitro antioxidant activity
DPPH radical scavenging activity
Extracts with different solvents (1–50 μg) were mixed with 1 ml of DPPH solution (50 × 10–5 M) in methanol (Brand-Williams et al. 1995). The absorbance was recorded at 517 nm after incubation for 15 min. The percentage decrease in scavenging of DPPH free radicals was determined as [(Ab − Aa)/Ab] × 100; Ab is optical density (OD) of control without extract; Aa is OD with extract in the reaction mixture. EC50, the volume of extract that scavenges 50% of free radicals was determined.
Superoxide radical scavenging activity
The activity was estimated by riboflavin-light-nitroblue tetrazolium system (Saeed et al. 2012). Reaction mixture with 0.5 ml of 50 mM phosphate buffer (pH 7.6), 0.3 ml riboflavin (50 mM), 0.25 ml phenanzine methosulphate (PMS, 20 mM) and varying amounts of sample (1–50 μg/ml) was illuminated using a fluorescent lamp for 20 min. The absorbance was recorded at 560 nm. The superoxide scavenging by the extract was calculated by the equation mentioned in previous section and EC50 was determined.
Phosphomolybdate assay
The MO(VI) reducing activity was performed as described. Ammonium molybdate reagent was prepared by mixing 0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate (Umamaheswari and Chatterjee 2007). An aliquot of sample fraction was mixed with 1.0 ml of ammonium molybdate reagent, incubated at 95 °C for 90 min and recorded the absorbance at 765 nm. The antioxidant capacity was calculated and EC50 was determined.
Hydroxyl radical scavenging assay
The sample fractions were included in Fenton reaction mixture to measure the hydroxyl radical scavenging (Ilavarasana et al. 2005). To 200 μl of reaction mixture containing 50 mM ferric chloride and 50 mM EDTA, 100 μl of 200 mM H2O2 and 50 μl of 2.8 mM 2-deoxyribose in phosphate buffer (50 mM, pH 7.4) extract (1–50 μg) was mixed. 100 μl of 300 mM ascorbic acid was added and incubated at 37 °C for 1 h. 0.5 ml of the mixture was mixed with 1 ml of 2.9% TCA and 1 ml of 1% TBA. The mixture was incubated at 100 °C for 15 min. The contents were cooled to room temperature and the absorbance was recorded at 532 nm. The hydroxyl radical scavenging activity was calculated and EC50 was determined.
ABTS radical scavenging activity
To determine cation radical scavenging activity 7 mM ABTS mixed with 2.45 mM potassium persulfate was kept overnight in the dark to yield cation radicals by the procedure reported in literature (Re et al. 1999). Varying amount of extracts was mixed with appropriately diluted solution and the decrease in absorbance was recorded up to 6 min. Scavenging activity and EC50 were determined.
LC–MS Analysis
LC–MS analysis was performed on a Q-TOF micromass spectrometer (Waters Corporation, Milford, MA, USA). Chromatographic separation was done by using Spherisorb 5 µm ODS2 column with the help of auto sampler at a flow rate of 0.2 mL/min, 280 nm wavelength and 20 μL injection volume (Venuprasad et al. 2014). Electrospray mass spectra data were recorded on positive and negative ionization mode for a mass range m/z 50–m/z 1000.
Statistical analysis
Results were presented as mean ± SD. The EC50values were determined on SPSS/Windows (SPSS 10.0.) software. One-way ANOVA test and Tukey’s test (P < 0.05) were performed to analyze the variations among EC50 of different fractions and correlation of EC50 with polyphenolic and flavonoid content.
Results and discussion
Extraction, total polyphenolic and flavonoid content
Extraction with aqueous methanol yielded the crude extract 19.27% of the dry weight of leaves. Methanol extract subjected to differential and sequential fractionation with n-hexane, ethyl acetate and n-butanol yielded 0.24–2.63% of the dry weight of leaves (Table 1) i.e. 1.22–13.66% of total extracted soluble components in methanol extract. Maximum 13.66% of soluble components were extracted with n-butanol followed by 4.46% with ethyl acetate. Only 1.22% of the soluble components could be extracted with n-hexane indicating that only a small fraction of methanol soluble compounds were non-polar. In plants this fraction mainly represents small chain essential oils and may contain the phenolics or other hydrophilic groups conjugated with hydrophobic lipids. Ali and Ali (2012) has characterized 1.93% fatty acids and derivatives in methanol extract of O. sanctum leaves (Ali and Ali 2012). The oil content in extract of plant materials varies with age and maturity stage. Total polyphenolic content (TPC) of the extract and fractions of O. sanctum estimated by Folin-Ciocalteu reagent varied significantly. TPC measured as Gallic acid equivalent (GAE) varied widely ranging from 212.26 ± 6.3 mg/g in OsB to 52.68 ± 1.8 mg/g in OsH (Table 1). TPC of fractions varies in descending order of OsB > OsE > OsM > OsR and OsH. The flavonoid content expressed as quercetin equivalents, varied from 54.51 ± 3.5 in OsB to 9.71 ± 0.6 mg QE/g in OsR. n-Butanol fraction contained maximum flavonoids while only a small fraction was left in the residue (OsR). We could extract almost equal amount of TPC (mg/g) with butanol and ethyl acetate but OsB fraction was more rich in TFC (mg/g) than OsE. The percent yield (g/100 g dry weight of leaves) of OsB was higher (2.63%) than OsE (0.86%) proving n-butanol as better solvent for fractionation of polyphenolics and flavonoids from aqueous methanol extract of O. sanctum. Although 67.87% of the soluble components extracted with methanol were still in residual fraction OsR, OsB and OsE were rich in polyphenolics. OsB was enriched with flavonoids. OsR fraction was left with only a small amount of flavonoids unextracted. Alcoholic extract of holy basil is reported to contain alkaloids, triterpenes, steroids, quinines, lectonic groups, amines, amino-acids, flavonoids and enthocyanins as ethyl/methyl esters, glucosides, glucuronides etc. (Mondal et al. 2009). Harichandan et al. 2019 have also reported that methanol extract of holy basil leaves contains alkaloids, terpenes, phenol, tenins, steroids etc. (Harichandan et al. 2019). OsR in the present study after extraction of major fractions of polyphenols and flavanoids with ethyl acetate and n-butanol may be containing tenins, terpenes, alkaloids with some residual phenols and glycosides. Earlier reports on differential extraction of polyphenols from other plant sources also exhibited similar results (Ao et al. 2008).
Table 1.
Total phenolics, flavonoid and extraction yield of methanol extract and soluble fractions of O. sanctum
| Extract/fraction | Total phenolic mg GAE/g | Flavonoid mg QE/g | Extraction yield (%) | % Extraction of soluble components from OsM |
|---|---|---|---|---|
| OsM | 87.13 ± 4.6b | 221.97 ± 4.6c | 19.27 ± 1.3e | 100 |
| OsH | 52.68 ± 1.8a | 32.86 ± 2.2b | 0.24 ± 0.04a | 1.22 |
| OsE | 202.71 ± 5.5c | 33.41 ± 2.7b | 0.86 ± 0.05b | 4.46 |
| OsB | 212.26 ± 6.3c | 54.51 ± 3.5d | 2.63 ± 0.4c | 13.66 |
| OsR | 52.99 ± 1.2a | 9.71 ± 0.6a | 13.08 ± 1.6d | 67.87 |
Each value in the table is represented as mean ± SD (n = 3). Values in the same column followed by a different letter (a–e) are significantly different (P < 0.05). OsM (50% Methanol extract), OsH (n-Hexane fraction), OsE (Ethyl acetate fraction), OsB (n-Butanol fraction), OsR (Residue fraction)
Chemical nature of the sample, nature of the solvent, mode, duration and temperature of extraction are the factors affecting the recovery of polyphenols and flavonoids. Methanol is reported to extract polar polyphenols and flavonoids to a better extent as compared to water and other solvents (Iloki-Assanga et al. 2015; Kchaou et al. 2013). There is a steady exploration for the sources of polyphenols including O. sanctum. Phenolic compounds in alcoholic extracts of O. sanctum have been examined (Shan et al. 2005; Sundaram et al. 2011) but differential extraction and concentration of polyphenolics or flavonoids are not on records. Agarwal et al (2017) have fractioned O. sanctum and O. kilimandscharicum root extract with different solvents and reported flavonoids and their derivatives in ethyl acetate fraction (Agarwal et al. 2017). Polyphenolic compounds are present in plants as various derivatives complexed with sugars and fatty acids as esters, glycosides, glucuronids etc. (Ali and Ali 2012; Harichandan et al. 2019). Differential proportions and nature of other biomolecules in complex may also affect the recovery and activity of polyphenols in different solvents. Though the structure and activity relationships of phenolic compounds in herbs, have not been thoroughly investigated, antioxidant properties are reported to be associated with phenolic content of the extracts (Agarwal et al. 2017; Saeed et al. 2012; Shan et al. 2005). Efficacy of these preparations to scavenge various free radicals and reduce metal ions was examined to assess their antioxidant potential.
In vitro antioxidant activity
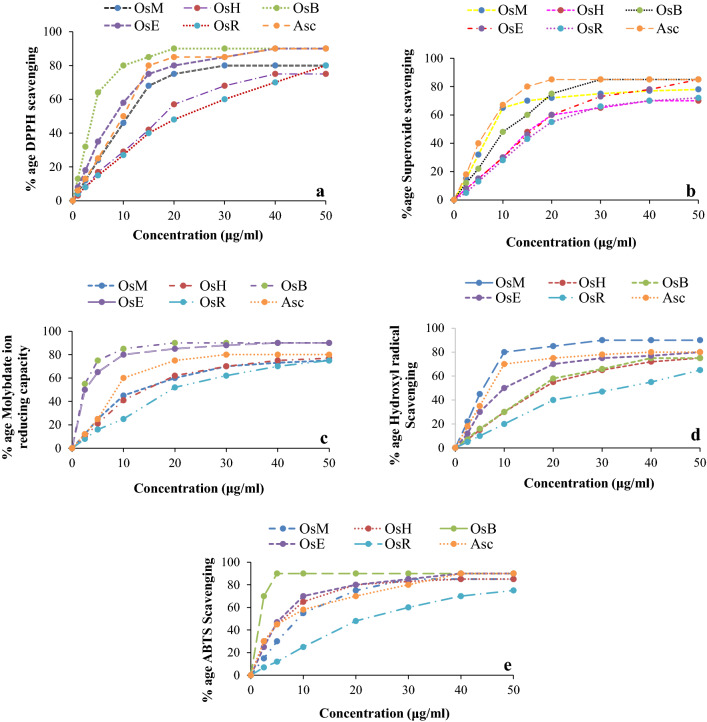
The holy basil extract and fractions in different solvents were examined by five different in vitro methods to assess their antioxidant potential and the results are shown in Fig. 2a–e. The EC50 values were determined to compare efficiency with the standards and among themselves (Table 2).
Fig. 2.
a DPPH free radical scavenging activity of different fractions. b Superoxide radical scavenging activity of different fractions. c Molybdate ion reducing activity of different fractions. d Hydroxyl radical scavenging activity of different fraction. e ABTS scavenging activity of different fractions
Table 2.
Radical scavenging activities of O. sanctum fractions
| Extract/fraction | DPPH radical | Superoxide radical | Phosphomolybdate assay | Hydroxyl radical | ABTS radical |
|---|---|---|---|---|---|
| EC50 values (μg/ml) of radical scavenging | |||||
| OsM | 10.0 ± 0.5b | 7.32 ± 0.9a | 13.60 ± 0.9c | 5.30 ± 0.4a | 9.2 ± 0.7d |
| OsH | 17.75 ± 0.6d | 15.62 ± 0.8c | 15.35 ± 0.8c | 18.51 ± 0.5c | 6.4 ± 0.5b |
| OsE | 8.61 ± 0.6b | 16.50 ± 0.9c | 2.43 ± 0.1a | 14.30 ± 0.2b | 5.3 ± 0.4b |
| OsB | 3.91 ± 0.3a | 10.32 ± 0.6b | 2.31 ± 0.1a | 17.90 ± 0.3c | 1.6 ± 0.1a |
| OsR | 22.02 ± 0.8c | 18.36 ± 0.8d | 19.51 ± 0.9d | 35.30 ± 1.6d | 20.3 ± 0.9e |
| Asc | 10.0 ± 0.3b | 6.8 ± 0.3a | 8.5 ± 0.5b | 6.0 ± 0.3a | 7.5 ± 0.1c |
Each value in the table is represented as mean ± SD (n = 3). Values in the same column followed by a different letter (a–e) are significantly different (P < 0.05). OsM (50% Methanol extract), OsH (n-Hexane fraction), OsE (Ethyl acetate fraction), OsB (n-Butanol fraction), OsR (Residue fraction), Asc (Ascorbic acid)
DPPH radical scavenging activity
The antioxidant activity of different fractions determined as DPPH free radical scavenging potential as shown in Fig. 2a was in the following order: OsB > OsE > OsM > OsH > OsR. The OsB fraction exhibit 50% of the DPPH scavenging at concentration of 3.91 ± 0.3 μg/ml (Table 2) which is significantly lower than the ethyl acetate and methanol extract (8.61 ± 0.6 and 10.0 ± 0.5 μg/ml respectively) indicating that antioxidants are concentrated in n-butanol fraction. OsH exhibited higher values of EC50 depicting less hexane extractable antioxidants in this plant material. Extraction of significant antioxidants by ethyl acetate and n-butanol resulted in higher EC50 of OsR due to presence of few residual active antioxidants. The EC50 value of ascorbic acid under similar conditions was 10.0 ± 0.3 μg/ml. Results of this study indicated that the leaf extract of O. sanctum contained active constituents that could impart hydrogen to a free radical and terminate the potential damaging entity. These observations further revealed that OsB and OsE fractions with high amount of polyphenolics and flavonoids fractioned (Table 1) had much of free radical scavenging potential indicating that both n-butanol and ethyl acetate could extract active antioxidants from holy basil leaves but n-butanol fraction exhibited better efficiency and higher yield. Polyphenols extracted with hexane may be complexed with non-polar constituents, were not efficient to scavenge DPPH free radicals, as evident from higher EC50 of OsH.
Superoxide radical scavenging activity
In the biological systems, superoxide are produced in immune response, mitochondrial respiration and as byproduct in reaction catalyzed by enzyme xanthine oxidase. Superoxide anion radical produces hydroxyl radicals and singlet oxygen to contribute to oxidative stress, damage various biomolecules and are related to various pathological conditions (Nita and Grzybowski 2016). The EC50 values determined by superoxide scavenging assay can be arranged as OsM < OsB < OsH ≤ OsE < OsR (Table 2). The superoxide scavenging capacity of the OsE, OsH and OsR fractions was low as compared to ascorbic acid. OsM (EC50 7.32 ± 0.9 μg/ml) and OsB (EC50 10.32 ± 0.6 μg/ml) could scavenge superoxide anion radicals quite efficiently. These observations indicated that antioxidant components in holy basil extract were fractioned with n-butanol in more active form as compared to other solvents.
Phosphomolybdate assay
The metal ion MO(VI) reducing potential of the fractions of the leaves of O. sanctum decreased in the following order: OsB ≥ OsE > > OsM ≥ OsH > OsR fraction (Table 2; Fig. 2c). OsB and OsE showed linear increase in activity upto 10 μg/ml, while other fractions exhibited increasing activity upto 40 μg/ml (Fig. 2c). The EC50 value of antioxidant activity for OsB (2.31 ± 0.1 μg/ml) and OsE (2.43 ± 0.1 μg/ml) were much lower (P < 0.05) than OsM (13.60 ± 0.9 μg/ml) and OsH (15.35 ± 0.8 μg/ml). Lower EC50 values observed with OsB and OsE indicated the presence of powerful reducing agents in these fractions. The significant metal ion reducing capacity observed in OsB and OsE might be due to high amount of polyphenols and flavonoids in these fractions. Polyphenol and flavonoid content of the extracts of many medicinal plants have been reported to be correlated with their phosphomolybdate reducing property significantly (Agarwal et al. 2017; Shafqatullah et al. 2014). Metal ion reducing capacity of plant polyphenols and flavonoids may play important role in modulating the activities of metallo-enzymes and regulating redox status of the cell (Schafer et al. 2003).
Hydroxyl radical scavenging activity
Hydroxyl and superoxide ions produce hydroxyl radicals in biological system. Hydroxyl radicals are also produced by immune system active against pathogens. Hydroxyl radical concentration in living organisms has been implicated in several neurological autoimmune diseases (Babu et al. 2001). Unlike superoxides, the hydroxyl radicals are not scavenged enzymatically and can cause more damage (Schafer et al. 2003). Although these radicals are short lived but are more dangerous, therefore, scavenging of these radicals is an important parameter indicating antioxidant potential of any preparation. The hydroxyl radical scavenging capablilty of O. sanctum extract and fractions observed here can be arranged as OsM > OsE > OsB ≥ OsH > > OsR (Table 2). Antioxidant activity increased linearly with all extracts at concentration 1–50 μg/ml. (Fig. 2d). The OsM could scavenge 50% hydroxyl radicals with concentrations 5.30 ± 0.5 μg/ml while 14.30 ± 0.2 μg/ml of OsE was required for the same (Table 2). Observed strong antioxidant potential of OsM, as compared to ascorbic acid projects O. sanctum as significant source of natural antioxidants. Considerable antioxidant activity of OsH fraction might be due to significant potential of hydrophobic small chain essential oils and associated polyphenols in this fraction to scavenge hydroxyl radicals. These results indicate that the essential oil fraction of this plant may protect lipids from hydroxyl radical damage. Ali and Ali 2012 had reported the presence of flavonids glycosides associated to fatty acids in methanol extract of O. sanctum leaves (Ali and Ali 2012). Ethanol extract of Ocimum leaves containing fatty acids, polyphenols and flavonoids exhibits good hydroxyl radical scavenging activity (Venuprasad et al. 2014).
ABTS radical scavenging activity
ABTS cation radical scavenging capacity of any preparation may indicate the antioxidant capability to react with organic cation radicals. Oxidative stress causes damage to the biomolecules hence this activity may mimic the protecting ability of any preparation against oxidative damage caused to biomolecules. Results of this study showed that the ABTS radical scavenging capability of OsB was much higher than other fractions (Fig. 2e). The ABTS cation radical scavenging potential of the fractions could be arranged as OsB > > OsE ≥ OsH > OsM > OsR. OsB, OsE and OsH exhibited prominent cation radical scavenging abilities with EC50 even lower than ascorbic acid indicating the presence of very efficient organic cation scavengers in these fractions (Table 2).
Antioxidants reported in holy basil include essential oils, terpenes, phenolics; phenolic acids and flavonoids (Agarwal et al. 2017; Cohen 2014; Kadian and Milind 2012). Antioxidant efficiency of these compounds depends upon their chemical structures as well as their binding to other biomolecules like lipids and carbohydrates (Montoro et al. 2005). Differential extraction of these antioxidant moieties and their complexes with solvents of different polarities might also be contributing to the observed differential antioxidant potential of the preparations.
Correlation of the antioxidant activities with polyphenol and flavonoid content
To explore the correlation between antioxidant potential and polyphenol/flavonoid content of different fractions statistical analysis was done. Total polyphenolic content of the fractions was positively and significantly correlated to EC50 values observed by phosphomolybdate assay with R2 > 0.961, DPPH scavenging activity with R2 > 0.754 and ABTS cation scavenging ability with R2 > 0.482 (Table 3). TFC is positively correlated to EC50 values for ABTS cation scavenging (R2 > 0.843), phosphomolybdate reaction (R2 > 0.661) and DPPH scavenging capacity (R2 > 0.611). However, weak but positive correlation was observed for superoxide and hydroxyl radical scavenging activity with TFC although the same was non-significantly correlated with TPC (Table 3). The data indicated that antioxidant activities were positively correlated with TPC and to a greater extent with TFC supporting the view that polyphenols particularly flavonoids significantly contribute to the antioxidant potential of the preparations. A number of reports of positive correlation between total polyphenolics and antioxidant properties of many medicinal herbs (Agarwal et al. 2017; Cai et al. 2004; Shan et al. 2005), has been proved for holy basil leaves also in this study.
Table 3.
Correlations between the EC50 values of antioxidant activities and phenolic and flavonoid content of O. sanctum
| Assays | Correlation R2 | |
|---|---|---|
| Phenolics | Flavonoid | |
| EC50 of DPPH radical scavenging ability | 0.754* | 0.611* |
| EC50 of superoxide radical scavenging ability | 0.042 | 0.134 |
| EC50 of phosphomolybdate ion reducing capacity | 0.961* | 0.661* |
| EC50 of hydroxyl radical scavenging ability | 0.099 | 0.142 |
| EC50 of ABTS radical scavenging ability | 0.482* | 0.843* |
Each value in the table is represented as mean ± SD (n = 3). *indicates significance at P < 0.05
Presence of multiple hydroxyl groups imparts free radical scavenging property to the phytochemicals. Hydroxyl groups also confer metal ion chelating property to polyphenols. The electrons of benzene rings in polyphenols also contribute to the antioxidant property. Flavonoids are the chief antioxidant compounds among polyphenolic compounds of plants since keto group at C4 and hydroxyl groups at ring A and C contribute to the antioxidant potential (Nunes et al. 2012). Flavonoids are reported to be strong free radicals scavengers active against singlet oxygen and other oxidizing molecules implicated in various diseases (Ranjit et al. 2016).
Reports in literature also underline the importance of antioxidants in in vivo protection against oxidative stress (Cohen 2014; Kadian and Milind 2012; Nita and Grzybowski 2016). The antioxidant properties of flavonoids and their relation to membrane protection have also been established (Ali and Ali 2012). In vivo protection of membranes and various biomolecules against damage due to oxidative stress by these preparations can be explored further to find easily available, efficient natural solution to combat damaging oxidative stress conditions.
Phytochemical analysis
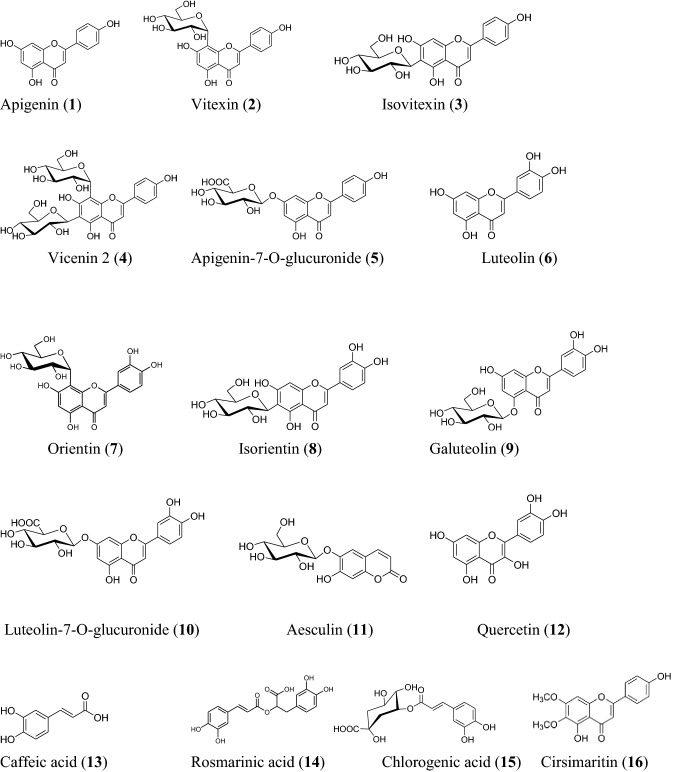
In the light of assumed benefits to human beings and the drift for natural polyphenols, there is a steady exploration for the sources of polyphenols with utilization of O. sanctum. Studies are available for isolation of various phenolic compounds from extracts of O. sanctum in different solvents but extract was never fractionated to get the polyphenolic enriched fraction containing almost all the reported phenolics. In the present study, different fractions of O. sanctum leaf extract were subjected to LC–MS analysis to identify major polyphenols and flavonoids in these preparations (supplementary Figs. 1–4). The results here depicted that these compounds tentatively belong to 3-substituted flavones or flavonol. The n-hexane fraction being non polar could not be processed for the analysis of polyphenols by LC/MS. Ali and Ali (2012) have also shown the presence of only small polyphenol like vanillic acid associated with fatty acids in methanol extract of this herb. The in vitro antioxidant potential of the fractions in present study indicated that ethyl acetate and n-butanol fractions were more efficient and had higher TPC and TFC extracted. Therefore, OsE and OsB fractions were subjected to mass spectroscopic studies for the identification of polyphenols. The LC–MS analysis of these fractions was done in both negative and positive ion modes (Supplementary Fig. 1, 2). The mass detection with negative mode was found better for identification of more number of polyphenols. A total of fifteen flavonoid compounds were identified (Table 4, Fig. 1).
Table 4.
Identification of polyphenolic compounds in O. sanctum leaves extract
| Fraction | Calculated MW | Negative ion mode | Positive ion mode | Compound detecteda | Molecular Formula |
|---|---|---|---|---|---|
| OsE | 594 | – | 595 | Vicenin 2 | C27H30O15 |
| 462 | 461 | 463 | Luteolin-7-O-glucuronide | C21H18O12 | |
| 448 | 447 | – | Isorientin, Orientin, Galuteolin | C21H20O11 | |
| 446 | 445 | 447 | Apigenin-7-O-glucuronide | C21H18O11 | |
| 432 | 431 | – | Vitexin, Isovitexin | C21H20O10 | |
| 360 | 359 | – | Rosmarinic acid | C18H16O8 | |
| 354 | 353 | – | Chlorogenic acid | C16H18O9 | |
| 340 | 339 | – | Aesculin | C15H16O9 | |
| 302 | 301 | 303 | Quercetin | C15H10O7 | |
| 286 | 285 | – | Luteolin | C15H10O6 | |
| 270 | 269 | – | Apigenin | C15H10O5 | |
| 180 | 179 | – | Caffeic acid | C9H8O4 | |
| OsB | 462 | 461 | 463 | Luteolin-7-O-glucuronide | C21H18O12 |
| 446 | 445 | – | Apigenin-7-O-glucuronide | C21H18O11 | |
| 360 | 359 | – | Rosmarinic acid | C18H16O8 | |
| 354 | 353 | 355 | Chlorogenic acid | C16H18O9 | |
| 286 | 285 | – | Luteolin | C15H10O6 | |
| 270 | 269 | – | Apigenin | C15H10O5 | |
| 180 | 179 | – | Caffeic acid | C9H8O4 | |
| OsR | 314 | 313 | – | Cirsimaritin | C17H14O6 |
aSamples tentatively identified by mass spectral data
The data depicted that polyphenols identified were ten flavones i.e. apigenin(1), vitexin(2), isovitexin(3), vicenin 2(4), apigenin-7-O-glucuronide(5), luteolin(6), orientin(7), isorientin(8), galuteolin(9), luteolin-7-O-glucuronide(10). Vicenin 2 was the only diglycoside derivative detected at m/z 595 (Table 4, Fig. 1). The sugar moiety present in mono- and di-glycosides was glucose. The glycosides might be either C-linked (6-C, 8-C) or O-linked (5-O, 7-O) either in the form of glucoside or glucuronide. The loss of 162 amu from the pseudomolecular is related to the sugar glucose and 194 amu to the glucuronic acid. The interesting fact is that traces of quercetin (12) (m/z 303), a flavonol, were also found in the OsE fraction. Previously quercetin has not reported in O. sanctum although detected in other Ocimum species (Gupta et al. 2015; Jain and Mehata 2017).
The other polyphenol detected was a coumarin glycoside i.e. aesculin (11) at m/z 339. The analysis of the OsB and OsE fractions of O. sanctum revealed the presence of three hydroxybenzoic acid derivatives; caffeic acid (13), rosmarinic acid (14) and chlorogenic acid (15), the last two being the derivatives of caffeic acid ester. The residue fraction OsR depicted the presence of high molecular mass compounds (supplementary Fig. 3a, b) which may be tannins, terpenes,saponins, glycosides or their polymers. A dimethoxy flavone i.e. cirsimaritin (16) could be identified at m/z 314 (Table 4, Fig. 3) in OsR. The major peak in the OsM spectra was characterized as rosmarinic acid confirmed by ESI–MS-MS analysis in negative ion mode and UV-spectra matching. The peak exhibited two absorptions, in the range of 230–240 nm of band-II and in the range of 307–354 nm of band-I. The result here indicated that this compound tentatively belongs to 3-substituted flavones or flavonol.
Fig. 3.
Structures of identified polyphenolic flavonoid compounds
The mass spectrum for rosmarinic acid exhibited the deprotonated molecule [M–H]− ion at m/z 359 (Hossain et al. 2010). The major fragment ions produced by CID analysis were at m/z 197 for 3,4-dihydroxyphenyllactic acid and 179 for caffeic acid revealing the constituents of rosmarinic acid before condensation (Supplementary Fig. 4a, b). These fragment ions by sequential loss of one or two water molecules form a dehydrated ion fragment at m/z 161. The fragment ion at m/z 179 produced at fragment ion m/z 135 by loss of CO2. Other fragment ions at m/z 123 and 73 unique to rosmarinic acid were also observed. Agarwal et al (2017) have reported that ethyl acetate fraction of Ocimum root extract is rich in flavonoids rosmarinic acid, caffeic acid and derivatives such as caffeoyldihydrophenylacetoyl tartaric acid (Agarwal et al. 2017). Caffeic acid, its derivatives and rosmarinic acid are major constituents of alcoholic extract of Ocimum leaves (Shan et al. 2005). Mondal et al. 2009 have reviewed the presence of apigenin, luteolin, apigenin7-O-glucoside, luteolin 7-O-glucoside, isoorientin, orientin, vitexin, aescutin, chlorogenic acid, caffeic acid in stem bark and leaf extracts (Mondal et al. 2009). Rosmarinic acid and ursolic acid were identified in ethanolic extract of O.sanctum leaves. Hakkim et al. 2007 have also identified rosmarinic acid as the major phenolic acid in field grown as well as in vitro callus culture of holy basil (Hakkim et al. 2007). To best of our knowledge this is the first report of fractionation of O. sanctum leaf extract exhibiting all phenolics and flavonoids reported so far in ethyl acetate and n-butanol fractions. Flavonol quercetin is observed first time in ethyl acetate fraction. Further the antioxidant properties of fractions were correlated to the phenolic and flavonoid content. The findings of the present study are in line with the reports for the extracts of other parts of this plant (Agarwal et al. 2017).
Flavonoid derivatives have shown a wide range of properties including anti-bacterial, anti-viral, anti-inflammatory, anti-cancer and anti-allergic agents (Montoro et al. 2005) while in other study Ocimum flavonoids have shown the protection against the damage caused by irradiations in animals (Uma Devi et al. 1999). In vivo antioxidant activity of the flavonoids from O. sanctum, was reported as a remarkable reduction in lipid peroxidation and protection of lymphocytes under oxidative stress induced by irradiation (Satyamitra et al. 2014; Uma Devi et al. 1999). Quercetin in polyherbal preparation including Ocimum is reported to protect liver against oxidative damage (Gupta et al. 2015). The extracts of leaves and stem of Ocimum with polyphenols cirsilineol, cirsimaritin, isothymusin, apigenin, rosmarinic acid and eugenol are reported to exhibit antioxidant activity (Mondal et al. 2009; Shan et al. 2005; Venuprasad et al. 2014). However, the extract was not fractionated to focus on polyphenolic-enriched preparation. The present study underlines the strong antioxidant potential of holy basil. A significant positive correlation between the free radical scavenging potential of OsE and OsB fractions of O. sanctum and the contents of phenolics or flavonoids indicated that polyphenols and flavonoids might be the major contributors for the antioxidant activity of these fractions. The non-significant correlation observed between the hydrogen peroxide radical scavenging and polyphenol content of fractions might be due to the presence of other biomolecules in these fractions such as sugars or ascorbic acid influencing the determination procedure. Moreover, different polyphenolics respond differently in the quantitating assays due to differences in their chemical structure (Saeed et al. 2012).
Conclusion
Our investigations on O. sanctum leaves extract and fractions revealed that the n-butanol and ethyl acetate solvents could extract high phenolic and flavonoid content and have strong antioxidant potential. The high antioxidant properties of these fractions may be due to the polyphenolic compounds, i.e. luteolin, apigenin, chlorogenic acid, rosmarinic acid, caffeic acid, quercetin and their derivatives, characterized by LC–MS data. The antioxidant potential of flavonoid enriched n-butanol and ethylacetate fractions strengthens their possible application against the damage caused by oxidative stress. Therapeutic and neutraceutical use of these fractions may be explored to incorporate the herbal antioxidants for implications in foods, nutrition and human health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The research work is fully supported by Haryana State Council for Science & Technology (HSCST/R&D/2015/2385), Panchkula, India. Authors are thankful to SAIF, Panjab University, Chandigarh (India) for LC–MS data.
Compliance with ethical standards
Conflict of interest
Authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agarwal K, Singh DK, Jyotshna J, Ahmad A, Shanker K, Tandon S, Luqman S. Antioxidative potential of two chemically characterized Ocimum (Tulsi) species extracts. Biomed Res Therapy. 2017;4:1574. doi: 10.15419/bmrat.v4i9.366. [DOI] [Google Scholar]
- Ali A, Ali M. New fatty acid derivatives from Ocimum sanctum L. Leaves Indian drugs. 2012;49:13–18. [Google Scholar]
- Ao C, Li A, Elzaawely AA, Xuan TD, Tawata S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control. 2008;19:940–948. doi: 10.1016/j.foodcont.2007.09.007. [DOI] [Google Scholar]
- Atoui A, Mansouria A, Boskoub G, Kefal P. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem. 2005;89:27–36. doi: 10.1016/j.foodchem.2004.01.075. [DOI] [Google Scholar]
- Babu BH, Shylesh BS, Padikkala J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia. 2001;72:272–277. doi: 10.1016/S0367-326X(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/s0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM. Tulsi—Ocimum sanctum: a herb for all reasons. J Ayurveda Integr Med. 2014;5:251–259. doi: 10.4103/0975-9476.146554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua A, Garg G, Mahajan R. Polyphenols, flavonoids and antimicrobial properties of methanolic extract of fennel (Foeniculum vulgare Miller) European. J Exp Biol. 2013;3:203–208. [Google Scholar]
- Gupta A, Sheth N, Pandey S, Yadav J. Determination of quercetin a biomarker in hepatoprotective polyherbal formulation through high performance thin layer chromatography journal of chromatography & separation. Techniques. 2015;06:2. doi: 10.4172/2157-7064.1000285. [DOI] [Google Scholar]
- Hakkim FL, Shankar CG, Girija S. Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J Agric Food Chem. 2007;55:9109–9117. doi: 10.1021/jf071509h. [DOI] [PubMed] [Google Scholar]
- Harichandan S, Sahu A, Gautam S, Nemani R. Phytochemical screening and antioxidant activity of methanolic extract of Ocimum sanctum Linn. Leaves. GSC Biol Pharm Sci. 2019;8:022–033. doi: 10.30574/gscbps.2019.8.2.0131. [DOI] [Google Scholar]
- Hossain MB, Rai DK, Brunton NP, Martin-Diana AB, Barry-Ryan C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J Agric Food Chem. 2010;58:10576–10581. doi: 10.1021/jf102042g. [DOI] [PubMed] [Google Scholar]
- Ilavarasana R, Mallikab M, Venkataramanc S. Anti-inflammatory and antioxidant activities of Cassia fistula linn bark extracts. Afr J Tradit Complement Altern Med. 2005;2:70–85. [Google Scholar]
- Iloki-Assanga SB, Lewis-Lujan LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL, Haines DD. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res Notes. 2015;8:396. doi: 10.1186/s13104-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep. 2017;7:15867. doi: 10.1038/s41598-017-15724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadian R, Milind P. Therapeutic potential and phytopharmacology of tulsi. Int J Pharm Life Sci. 2012;3:1858–1867. [Google Scholar]
- Kchaou W, Abbès F, Blecker C, Attia H, Besbes S. Effects of extraction solvents on phenolic contents and antioxidant activities of Tunisian date varieties (Phoenix dactylifera L.) Ind Crops Prod. 2013;45:262–269. doi: 10.1016/j.indcrop.2012.12.028. [DOI] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazandarani M. Effects of solvent type on phenolics and flavonoids content and antioxidant activities in Onosma dichroanthum Boiss. J Med Plants Res. 2012 doi: 10.5897/jmpr11.1460. [DOI] [Google Scholar]
- Mondal S, Mirdha BR, Mahapatra SC. The science behind sacredness of Tulsi (Ocimum sanctum Linn.) Indian J Physiol Pharmacol. 2009;53:291–306. [PubMed] [Google Scholar]
- Montoro P, Braca A, Pizza C, Detommasi N. Structure and antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005;92:349–355. doi: 10.1016/j.foodchem.2004.07.028. [DOI] [Google Scholar]
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes P, Silva F, Guedes R, Guedes DS, Almeida J, de Lima J, et al. Biolog Oxid Antioxid Act Nat Prod. 2012 doi: 10.5772/26956. [DOI] [Google Scholar]
- Ranjit P, Veneetha C, Giri A. Evaluation of Total phenolics flavonoids, and antioxidant activity of leaf extracts of Pimpenella tirupatensis. Int J Pharm Sci Rev Res. 2016;41:58–63. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarija K, Mohan S, Perumal N, Hashim K. In vitro antioxidant studies and phytochemical screening on the seeds of Corchorus trilocularis. Der Pharmacia Sinica. 2014;5:82–85. [Google Scholar]
- Satyamitra M, Mantena S, Nair C, Chandna S, Dwarakanath B, Uma Devi P. The antioxidant flavonoids, orientin and vicenin enhance repair of radiation-induced damage scholarena. J Pharm Pharmacol. 2014 doi: 10.18875/2375-2262.1.105. [DOI] [Google Scholar]
- Schafer F, Kelly E, Buettner G (2003) Oxidative stress and antioxidant intervention. In: Rodriguez RGCaH (ed) Critical Reviews of Oxidative Stress and Aging: Advances in Basic Science, Diagnostics and Intervention, vol II. World Scie N, pp 849–869
- Shafqatullah KR, Hassan W, Hussain A, Asadullah RK, Ali J. Development of HPLC method by UV-VIS detection for the quantification of phenolic acids in different Ocimum sanctum Linn. extracts. Pak J Pharm Sci. 2014;27:1271–1275. [PubMed] [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Sundaram R, Gowtham L, Ramanathan M, Manikandan P, Venugopal V, Kamalakannan D, Nayak B. Quantification of bioactive principles in indian traditional Herb Ocimum Sanctum Linn. (Holy Basil) leaves by High performance liquid chromatography. Asian J Biomed Pharm Sci. 2011;1:35–41. [Google Scholar]
- Uma Devi P, Ganasoundari A, Rao BS, Srinivasan KK. vivo radioprotection by ocimum flavonoids: survival of mice. Radiat Res. 1999;151:74–78. doi: 10.2307/3579750. [DOI] [PubMed] [Google Scholar]
- Umamaheswari M, Chatterjee TK. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Tradit Complement Altern Med. 2007;5:61–73. [PMC free article] [PubMed] [Google Scholar]
- Venuprasad MP, Kumar Kandikattu H, Razack S, Khanum F. Phytochemical analysis of Ocimum gratissimum by LC-ESI–MS/MS and its antioxidant and anxiolytic effects. S Afr J Bot. 2014;92:151–158. doi: 10.1016/j.sajb.2014.02.010. [DOI] [Google Scholar]
- Złotek U, Szymanowska U, Karaś M, Świeca M. Antioxidative and anti-inflammatory potential of phenolics from purple basil (Ocimum basilicum L.) leaves induced by jasmonic, arachidonic and β-aminobutyric acid elicitation. Int J Food Sci Technol. 2016;51:163–170. doi: 10.1111/ijfs.12970. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.